
Schiff-base Complexes Immobilized on the Fe
3
O
4
@SiO
2
for
Selective Oxidation of Benzyl Alcohol
F H Li
1
, S S Jia
1
, Y W Fang
1
and Y B Song
1, *
1
College of Science,Shantou University, Shantou, Guangdong, 515063, China
Corresponding author and e-mail: Y B Song, ybsong@stu.edu.cn
Abstract. Efficient and highly selective heterogeneous catalysts were prepared through
grafting the metal-salen on the modified magnetic nanoparticles. Fe
3
O
4
nanoparticles were
prepared was hydrothermal synthesis, after the Fe
3
O
4
@ SiO
2
was amino-functionalized using
3-aminopropyltriethoxysilane and then Fe
3
O
4
@ SiO
2
-NH
2
was obtained by the reaction.
Finally, Fe
3
O
4
@ SiO
2
[(EtO)
3
Si-L
2
]/Mn were successfully synthesized. These
surface-modified nanoparticles were using various characterize techniques such as XRD,
FT-IR and SEM. The heterogeneous catalyst showed high conversion and selectivity in the
reaction of oxidation of benzyl alcohol. Furthermore, the catalysts were easily separated by
the external magnetic field after the reaction, reused at least 8 consecutive cycles without
significant loss catalysis activity.
1. Introduction
Metal Schiff-base complexes have a wide range of applications in catalysis. Schiff-base complexes
coordinate with transition metal easily, and form steadily metal Schiff-base complexes with different
valence state. The metal Schiff-base complexes could catalysis a lot kinds of chemical reactions, and
prepare essential chemical intermediates, such as polymerization reaction [1], oxidation reaction
[2-3], epoxidation reaction [4], reduction reaction [5], Michael addition reaction [6], cyclopropane
reaction [7-8], ring opening reaction [9]. Homogeneous metal Schiff-base complexes have high
catalytic activity. However it is difficult to separate them from the products, they are not conducive to
reuse, which greatly limits the catalyst of reuse. In addition, in the process of the catalysis reaction,
polymerization phenomenon can also lead to deactivation of catalyst. So the heterogenization of the
homogeneous catalyst research increasingly draws people attention. Heterogenization not only can
keep the structure of the homogeneous catalyst and the excellent properties, but also can avoid the
disadvantage of recycling hard. At the same time, Heterogenization could show some synergistic
effect, which can improve the catalyst of performance.
At present, the supporting according to their material can be divided into inorganic supporting,
organic synthetic polymer supporting, natural high polymer supporting. Inorganic supports mainly
including carbon nanotubes, carbon nanofibers, SiO
2
particles, zeolites. These supports with high
specific surface area to disperse the metal Schiff-base complex greatly improve the catalytic
performance. Guangxing Li encapsulated Co(salen) in NaY type molecular sieve to catalyze the
oxidative carbonylation reaction of benzene ammonia under the condition of 170°C , and high
conversion and selectivity were obtained [10]. Priti Sharma grafted Mn Schiff-base complexes on the
modified SBA-15 to catalyze the oxidation of sulfide [11]. Organic synthetic polymer carrier contains
Li, F., Jia, S., Fang, Y. and Song, Y.
Schiff-Base Complexes Immobilized on the Fe3O4@SiO2 for Selective Oxidation of Benzyl Alcohol.
In Proceedings of the International Workshop on Materials, Chemistry and Engineering (IWMCE 2018), pages 409-415
ISBN: 978-989-758-346-9
Copyright © 2018 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
409

polyacrylic acid polymer material, polypropylene, polyvinyl alcohol and polyethylene imine. Kim et
al. prepared heteropoly nuclear complexes by inserting the metal Schiff-base complexes into the
structure of the polymer to catalyze ring-opening reaction of epoxide. Alan J.McCue reported [12]
epoxidation reaction catalyzed by one kind of chiral metal Schiff- base immobilized onto PAMAM
polymer.
Ferroferric with the spinel structure oxide belongs to the cubic crystal system. It has good
magnetic properties that make it useful in the field of magnetic storage, microwave absorbing
materials, coatings with special function, drug targeting, magnetic multifunctional composite
materials, biological engineering [13]. The catalyst immobilized on the magnetic materials can be
recycled by the extra magnetic field, which can reduce the cost of reaction and avoid the organic
solvents pollution to the environment. Magnetic nanoparticles may be synthesized by several typical
methods, including coprecipitation [14], microemulsion synthesis [15], hydrothermal synthesis [16],
thermal decomposition [17], sol-gel [18], and others [19]. The morphology and properties of the
product vary from preparation process. People can select the suitable preparation methods to achieve
the desired goal according to the need.
2. Experiments
2.1. Materials and characterization
Ferric chloride (FeCl
3
·6H
2
O), 3-Aminopropyltriethoxysilane, tetraethyl orthosilicate (TEOS),
ethylene glycol,3,5-Dinitro-2-hydroxybenzaldehyde , metal acetate, ammonia (25 wt.%) were
purchased from commercial sources and used for the reaction without further purification. Thermo
scientific Nicolet Magna 750 Research FT-IR Spectrophotometer. X-Ray power diffraction (XRD)
pattern were carried out utilizing a Brucker D8 Advance diffractor meter equipped with Cu-Kα
radiation (λ=0.15418nm) at 40 kV and 40 mA. The diffraction data were revealed under 2θ between
10~70°. The surface morphology and microstructure of these samples was exhibited utilizing a
Scanning Electron Microscope (SEM).
2.1.1. Preparation of ligand and magnetic nanoparticles (Fe
3
O
4
). The ligand was prepared according
to the reported method [20-21]. The preparation of magnetic nanoparticles specific experimental
steps: 1.35 g of ferric chloride hexahydrate was dissolved in 60 ml of ethylene glycol with stirring to
form a clear solution. Then surface active agent and anhydrous sodium acetate (6.12 g) was added
under magnetic stirring to form a uniform system. Next transferred into a Teflon-lined stainless-steel
autoclave with a capacity of 100 ml, heated to 200 ºC for 10h. After cooling to room temperature the
resultant solid was filtered, and the black solid products were collected and washed with ethanol and
distilled water some times, respectively. Finally the products were dried in vacuum for 10 h at 60 ºC .
2.1.2. Preparation of magnetic Fe
3
O
4
@SiO
2
nanoparticles. Ferroferric oxide nanoparticles (1 g)
were distributed and ultrasonic in the mixture of ethanol 35 ml and deionized water 10 ml for 15 min.
TEOS 1.5 ml was added slowly to the dispersion and ultrasonic for another 10 min. Then aqueous
ammonia (10 %, 1.4 ml) was added slowly over 10 min under mechanical stirrer. The reaction
continued for 12 h at 40°C . After the reaction, the silica coated magnetic nanoparticles was separated
from the mixture using the extra magnetic field and washed several times with distilled water and
ethanol. The products were vacuum dried for 10 h at 60°C . At last obtained the silica coated
ferroferric oxide nanoparticles Fe
3
O
4
@SiO
2
.
2.1.3. Preparation of Fe
3
O
4
@ SiO
2
[(EtO)
3
Si-L
2
]/Mn. For the modification step: 2 g of Fe
3
O
4
@SiO
2
were dispersed in 100 ml of toluene. After 15 min ultrasonic processing 2 ml
3-Aminopropyltriethoxysilane were added into the suspension under nitrogen atmosphere. Then the
IWMCE 2018 - International Workshop on Materials, Chemistry and Engineering
410
mixture was refluxed for 12 h. After the result solid cooled to room temperature, the products were
separated by the magnetic field, and washed with a certain volume of ethanol and deionized water
several times, respectively. Last the products were vacuum dried for 10 h at 100 ºC. For the grafting
step: The modified material was dispersed into 100 ml of acetonitrile ultrasonic for 10 min. Added
3.2 mmol 3,5-Dinitro-2-hydroxybenzaldehyde and 0.5 ml triethylamine into the suspension, then the
resulted mixture was refluxed for 12 h. When it cooed room temperature, the products were removed
by the extra magnetic field, washed with a certain volume of ethanol several times. The final
products dried vacuum 100 ºC for 10 h. The above products dispersed into the solution of 50 ml of
ethanol solution containing 1.5 mmol of metal acetate. After ultrasonic for 10 min, the resulted
mixture was refluxed for 10 h. While the reaction cooled room temperature, the final products were
removed by the extra magnetic field, and then washed with ethanol several times and vacuum dried
for 10 h at 100 ºC.
2.2. Test the catalytic performance
The oxidation reaction was carried out in a 50 ml round bottomed flask equipped with a condenser
and a stirrer. H
2
O
2
was used as oxidation. Benzyl alcohol 0.28 mmol and 30 ml toluene, catalyst 100
mg was added into the flask. The flask was immersed into an oil bath to keep the reaction
temperature at 60 °C . The reaction continued for 4 h. Then use the gen GC 800 to detect the reaction
conversion and selectivity.
3. Results and discussion
3.1. Preparation and characterization of the modified Fe
3
O
4
@ SiO
2
catalyst.
As illustrated in Figure 1 the overall procedure for the preparation of the catalyst contains three steps.
Firstly, Fe
3
O
4
nanoparticles were synthesized via a solvothermal method according to the previous
report [22-23].
Secondly, a thin silica layer was coated on the surface of the prepared Fe
3
O
4
nanoparticles to form Fe
3
O
4
@SiO
2
composites through adding TEOS and aqueous ammonia (10 %).
Thirdly, the Fe
3
O
4
@SiO
2
composites were modified with ammonia propyl triethoxy silane to
increase the functional group. The groups were used to grafting the metal Schiff-base complexes.
Figure 1. Catalyst preparation process: (A) Fe
3
O
4
(B)Fe
3
O
4
@ SiO
2
(C) Fe
3
O
4
@ SiO
2
-NH
2
(D)
Fe
3
O
4
@ SiO
2
(EtO)
3
Si-L
2
(E) Fe
3
O
4
@ SiO
2
[(EtO)
3
Si-L
2
]/Mn.
Schiff-Base Complexes Immobilized on the Fe3O4@SiO2 for Selective Oxidation of Benzyl Alcohol
411
3.2. Characterization of the catalyst
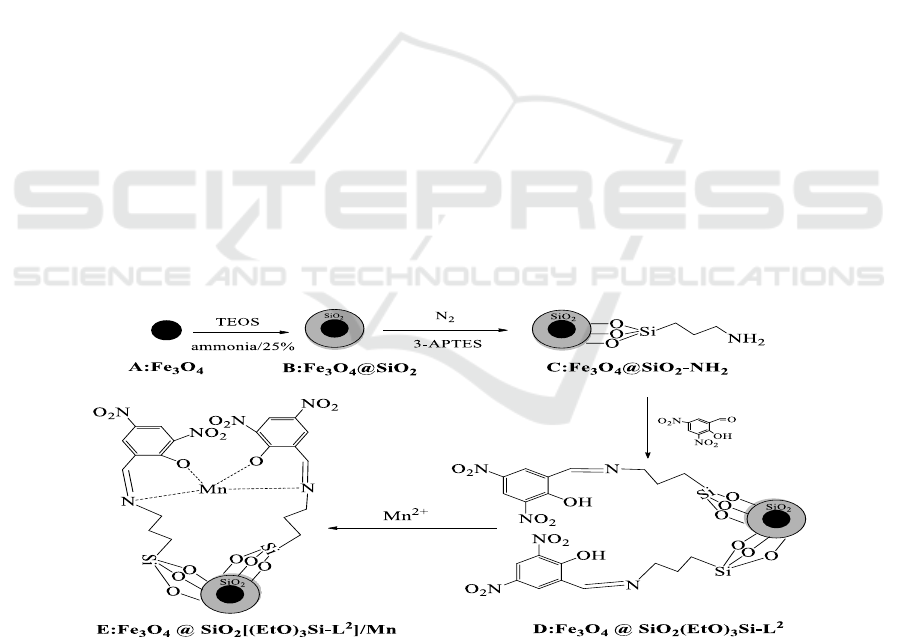
3.2.1. FT-IR. The strong absorb bands related to Si–O–Si stretching vibrations was observed in all
spectra at 1000-1100 cm
-1
shown in Figure 2. It is suggest that silica shell is successfully formed on
the magnetic nanoparticles surface. The spectrum of Fe
3
O
4
@SiO
2
-NH
2
(Figure 1c) showed several
signals appeared in the area of 1450-1560 cm
-1
and 2250-2345 cm
-1
which are related to C-H
stretching modes of the propyl groups. These bands conformed that the Fe
3
O
4
@SiO
2
was by
3-Aminopropyltriethoxysilane successfully modified. In the spectrum a band at 1630 cm
-1
associated
to C=N stretching vibration [24], and some weak bands at 3066-3040 cm
-1
and 1400-1500 cm
-1
assigned to stretching vibrations of aromatic rings were observed in Figure1(d). These bands
confirmed the successful anchoring of Schiff base ligand. The band at 1615 cm
-1
(Figure 1d)
indicated the coordination of C=N group of complexes with Mn. Also, a new absorption band at 529
cm
-1
was assigned to Mn-N band [25].
4000 3600 3200 2800 2400 2000 1600 1200 800 400
Wavenumber(cm
-1
)
Transmittance(a.u.)
(a)
(b)
(c)
(d)
1615
3066
1091
Figure 2. The FT-IR spectrum of (a)Fe
3
O
4
(b)Fe
3
O
4
@SiO
2
(c)Fe
3
O
4
@SiO
2
-NH
2
and (d) Fe
3
O
4
@
SiO
2
[(EtO)
3
Si-L
2
]/Mn.
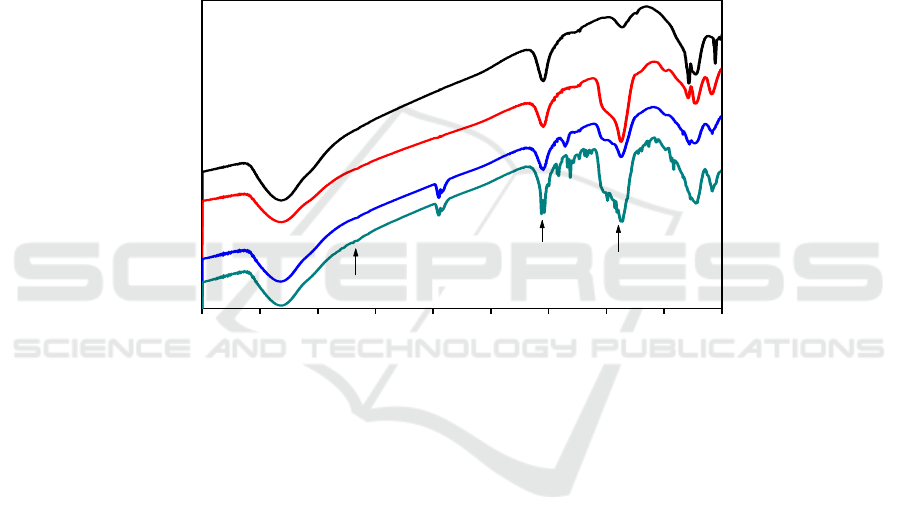
3.2.2. XRD. From the XRD spectra of the samples in Figure 3, the characteristic diffraction peaks of
the Fe
3
O
4
nanoparticles could be observed at 2θ=29.91, 35.41, 43.11, 57.21, and 62.81°, which can
be assigned to cubic spinel phase of Fe
3
O
4
[26]. However the main peaks of Fe
3
O
4
@ SiO
2
and Fe
3
O
4
@ SiO
2
[(EtO)
3
Si-L
2
]/Mn exhibit no obvious changes compared to Fe
3
O
4
, which confirmed that the
coating and grafting process didn’t induce any phase change of the Fe
3
O
4
.
IWMCE 2018 - International Workshop on Materials, Chemistry and Engineering
412
10 20 30 40 50 60 70
2/degree
(a)
(b)
(c)
29.91
35.41
43.11
57.21
62.81
Intensity(a.u.)
Figure 3. The X-ray diffraction patterns of (a) Fe
3
O
4
(b) Fe
3
O
4
@SiO
2
(c) Fe
3
O
4
@
SiO
2
[(EtO)
3
Si-L
2
]/Mn.
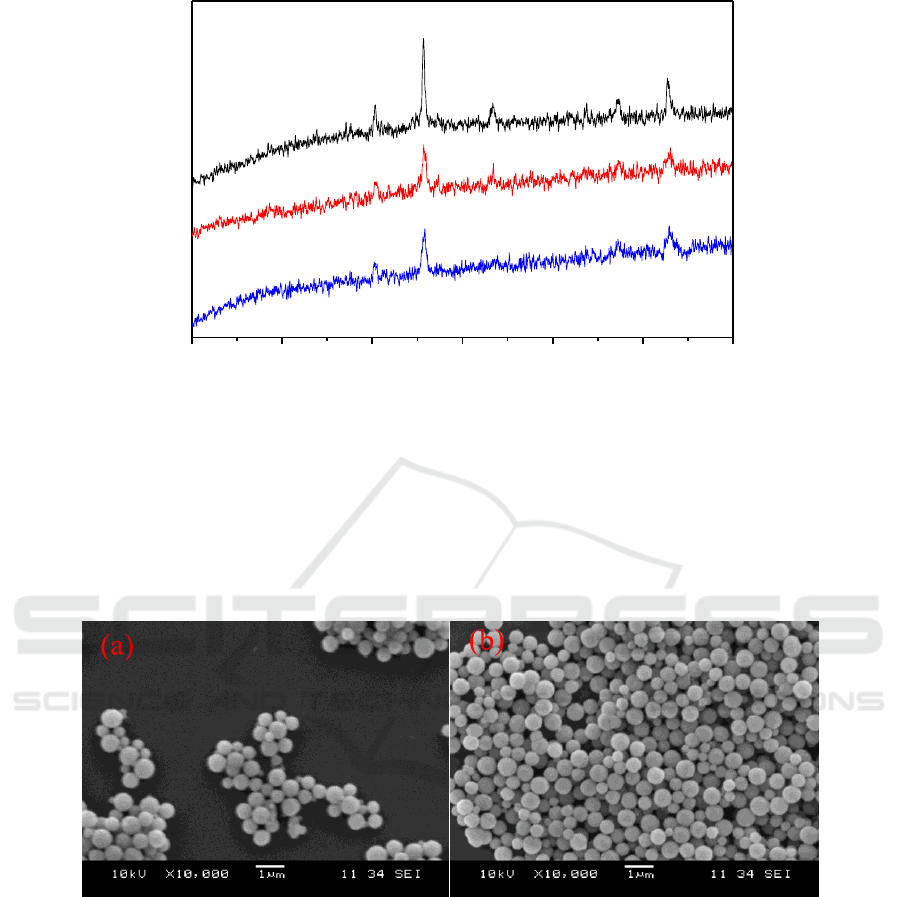
3.2.3. SEM. The image in Figure 4 showed the morphology change in the modification process. The
image of the production after coating showed less smoothing than before, which may cause by the
coating process. This could confirm the coating modification process might be successful. And after
coating showed some agglomeration piece that might be the SiO
2
.
Figure 4. SEM images of Fe
3
O
4
before (a) and after coating modification (b).
3.3. Catalyst activity tests
Table 1 showed performance of homogeneous catalyst and heterogeneous catalyst in selectivity
oxidation of benzene methanol. From the blank experiment, the influence of the 1, 2, 3 to the catalyst
could be excluded. Through comparing the entries 4 with 5, the little reduction of performance
happened, which might be related with the loading amount of homogeneous catalyst. In the
modification process, the loading amount was limited, so the catalysis effect decreased. Compared
with other reports literature, The reaction conversion rate relatively lower. We will continue to study
the catalyst performance.
Schiff-Base Complexes Immobilized on the Fe3O4@SiO2 for Selective Oxidation of Benzyl Alcohol
413
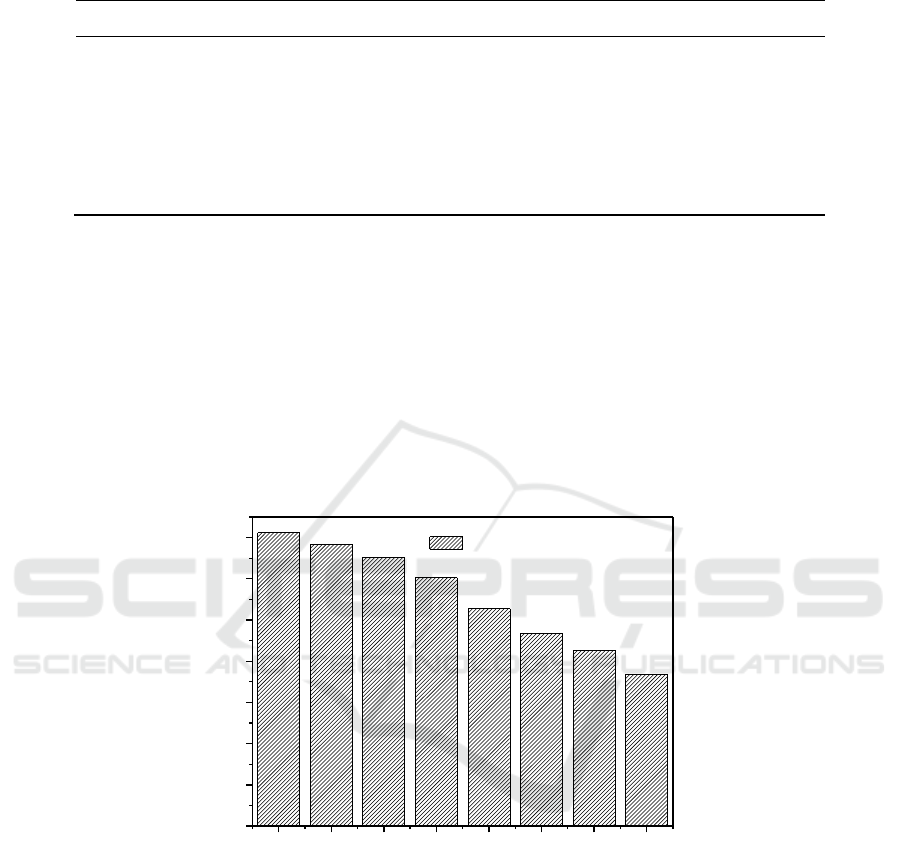
Table 1.Test of catalysis performance.
Entry
Catalyst
Conversion (%)
Selectivity (%)
1
L
2
0.78
96.0
2
Fe
3
O
4
@SiO
2
1.2
97.2
3
Mn-acetate
7.5
95.3
4
Mn-L
2
74.1
98.6
5
Fe
3
O
4
@ SiO
2
[(EtO)
3
Si-L
2
]/Mn
71.2
97.5
3.4. Catalyst life tests
Figure 5 shows the catalyst life in the recycle process. In the experiment, the recovered
heterogeneous catalyst can be reused for at least 5 times at the same reaction conditions without great
loss of activity. After 5 times reused, the damage of coating layer and the small part of
polymerization of the Schiff base complexes may cause the decrease gradually. However the
performance of the homogeneous catalyst reduced apparently in the recycle process, and they almost
deactivated after the 4 times. So the immobilization can efficiently enhance the recycle capacity of
the catalyst. Although catalyst can be reused eight times, and closed to reported literature, but the
catalytic performance of falling fast, we will continue to further research.
1 2 3 4 5 6 7 8
0
10
20
30
40
50
60
70
Conversion (%)
Recycle times
Fe
3
O
4
@ SiO
2
[(EtO)
3
Si-L
2
]/Mn
Figure 5. Catalyst life test.
4. Conclusions
In this work we have shown that heterogeneous catalyst made by anchoring the metal Schiff-base on
the magnetic nanoparticles coated by silica have excellent performance in the reaction of selective
oxidation of benzyl alcohol. The catalyst can be reused for at least 8 times without great loss of
activity. Due to the magnetic nature of the support, the catalyst was separated simply by applying an
external magnet. It is not only reduced the pollution of organic solvent used in the recycle process,
also is good to the practical application in industry.
IWMCE 2018 - International Workshop on Materials, Chemistry and Engineering
414

Acknowledgement
Financial supports from the Science and technology planning project of Guangdong Province (No.
2014A020216045, 2013KJCX0081) are gratefully acknowledged.
References
[1] Matsui S and Fujita T 2001 Catal. Today 66 63-73
[2] Willingh V, Gavin, Abbo H S and Titinchi S J J 2014 Catal.Today 227 96-104
[3] Farzaneh F, Majidian M and Ghandi M 1999 J. Mol. Catal. A: Chem. 148 227-233
[4] Musie G, Wei M, Subramaniam B, and Busch D H 2001 Coord. Chem. Rev. 219
789-820
[5] Fehring V and Selke R 1998 Angew. Chem. Int. Ed. 37 1827-30
[6] Suga H, Fudo T, and Ibata T 1998 Synlett. 1998 933-935
[7] Che C M, Kwong H L, Chu W C, Cheng K F, Lee W S, Yu H.S and Cheung K K 2002 Eur. J.
of Inorg.Chem. 2002 1456-63
[8] Kowalak S and Balkus K J 1992 Collect. Czech. Chem. Commun. 57 774-780
[9] Lee K Y, Kawthekar R B and Kim G J 2007 Bull Korean Chem. Soc. 28 1553-61
[10] Li G, Chen L, Bao J, Li T and Mei F 2008 Appl.Catal. A: General 346 134-139
[11] Sharma P, Lazar A and Singh A P 2012 Appl. Catal. A: General 439 101-110
[12] McCue A J, Urgast D S, Wells R P, and Anderson J A 2014 Catal. Commun. 43 159-163.
[13] Zhang L, Wang T, Liu P 2012 Chem. Eng. J. 187 372-379
[14] Lee S J, Jeong J R, Shin S C, Kim J C and Kim J D 2004 J. Magn. Magn.Mater. 282
147-150
[15] Chin A B and Yaacob I I 2007 J. Mater. Process.Technol. 191 235-237
[16] Wan J, Chen X, Wang Z, Yang X and Qian Y 2005 J.Cryst. Growth 276 571-576
[17] Miguel-Sancho N, Bomati-Miguel O, Roca A G, Martinez G, Arruebo M and Santamaria J
2012 Ind. Eng. Chem. Res. 51 8348-57
[18] Niederberger M. Acc. 2007 Chem. Res. 40 793-800
[19] Kim E H, Lee H S, Kwak B K, and Kim B K 2005 J. Magn. Magn.Mater. 289 328-330
[20] Zhu D, Mei F, Chen L, Li T, Mo W and Li G 2009 Energy Fuels 23 2359-2363
[21] Reddy G R, Balasubramanian S and Chennakesavulu K 2014 J. Mater. Chem. 2 15598-15610
[22] Chen D, Xu R 1998 Mater. Res. Bull 33 1015-21
[23] Wan J, Chen X, Wang Z, Yang X and Qian Y 2005 J. Cryst.Growth 276 571-576
[24] Arshadi M and Ghiaci M 2011 Appl. Catal. A: General 399 75-86
[25] Chen L, Li B and Liu D 2014 Catal.Lett. 144 1053-61
[26] Kong L, Lu X, Bian X, Zhang W and Wang C 2010 ACS Appl. Mat.Interfaces 3 35-42
Schiff-Base Complexes Immobilized on the Fe3O4@SiO2 for Selective Oxidation of Benzyl Alcohol
415
