
The Effect of Reaction Temperature on Fluorescence
Properties of Carbon Dots
K Yang, C L Wang, S Ding, F Li and F Tian
*
Institute of Medical Equipment, Academy of Military Medical Sciences, Tianjin,
300161, PR China.
Corresponding author and e-mail: F Tian, tianfeng62037@163.com
Abstract. In the present study, highly fluorescence carbon dots (CDs) were synthesized by
one step hydrotherma l method using citric acid (CA) as carbon source and 1,2-
ethylenediamine (EDA) as passivation agent. The samples of CDs under different reaction
temperature were prepared and evaluated by FT-IR, UV-vis and fluorescence detection to
estimate and summa rize the forming rules and fluorescence mechanism of CDs. Results
indicated the optima l reaction temperature was 200
o
C, the quantum yie ld were measured to
be 79.7%. The fluorescence of CDs was determined by carbon core and surface state and its
forming process mainly included the steps of cracking, po ly merization, carbonization and
growth of nuclei. The as-prepared CDs had excellent water solubility and biocompatibility,
which held a great potential in the fields of cell targeting and drug delivery.
1. Introduction
CDs are a new group of zero-dimension carbon nanomaterials or nanoparticles, the size of most CDs
are less than 10 nm [1-2].
In the broad sense, the chemical structure of CDs are consisted of two parts,
one is carbon core which is arised from sp
2
/sp
3
carbon, the other is surface state that derives from the
oxygen/nitrogen based groups or polymeric on the surface of CDs [3]. There are so many routes to
prepare CDs which is divided into top-down and bottom-up two methods [4], and the hydrothermal
method is commonly used due to the uniform heating, simple and safe process, high quantum yield
and excellent solubility of CDs.
In recent years, nitrogen-doped CDs (N-CDs) have drawn great attention due to the enhancement
of fluorescence [5], and one-step hydrothermal method using CA/EDA to synthesize N-CDs with
great photoluminescence and water solubility were common used in previous work [6]. However,
few reports were focused on preparing CDs with the best performance and summarizing the
formation rules and analysed fluorescence mechanism of CDs. Herein, in this paper, we took the
CA/EDA typical N-CDs as the research object and explored the effect of reaction temperature on the
fluorescence properties, discussed the formation rules, and finally evaluated the cytotoxicity of CDs.
Yang, K., Wang, C., Ding, S., Li, F. and Tian, F.
The Effect of Reaction Temperature on Fluorescence Properties of Carbon Dots.
In Proceedings of the International Workshop on Materials, Chemistry and Engineering (IWMCE 2018), pages 229-235
ISBN: 978-989-758-346-9
Copyright © 2018 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
229

2. Reagents and apparatus
2.1. Reagents
Acrylic acid (99%) and 1, 2-ethylenediamine (EDA, 98%) were purchased from Alfa. Aesar. Quinine
sulfate (98%, suitable for fluorescence) was supplied by Fluka (New York, USA). All other reagents
were of analytical grades and used without further purification.
2.2. Apparatus
UV-vis absorption was measured by TU-1810 UV-vis Spectrophotometer (PERSEE, China).
Photoluminescence emission measurements were performed on Fluorolog 3 fluorometer (HORIBA,
USA). Fourier Transform Infrared Spectroscopy (FT-IR) spectra were conducted on a Nicolet 380
spectrometer (Thermo Fisher Scientific) within a range of 500-4000 cm
-1
.
3. Experimental
3.1. Synthesis and characterizations of CDs
Typically, 2.1 g acrylic acid was dissolved in 60 ml deionized water, and then 2.68 ml EDA were
added and the mixed solution was stirred to form a colourless, transparent and homogeneous solution.
Then the solution was treated with a hydrothermal procedure for 5 h. The reaction temperature was
160
o
C, 200
o
C, 240
o
C, 280
o
C, respectively, corresponding to CD-EDA-T1, CD-EDA-T2, CD-
EDA-T3, CD-EDA-T4 four groups. Subsequently, the obtained products were cooled down to room
temperature and dialyzed for 48 h. The CDs were examined by FT-IR, UV-vis and fluorescence
detection to analyze the properties of CDs.
3.2. Measurement of fluorescence quantum yields
The quantum yield of the CDs was determined by a comparative method [7]. Quinine sulfate in 0.1
M H
2
SO
4
(literature quantum yield: 54%) was selected as a standard sample to calculate the QY of
test sample (i.e. CDs) which was dissolved in deionized water at different concentrations. All the
absorbance values of the solutions at the excitation wavelength were measured with UV-Vis
spectrophotometer. Fluorescence emission spectra of all the sample solutions were recorded by
Fluorolog 3 fluorometer at an excitation wavelength of 400 nm. The integrated fluorescence intensity
is the area under the PL curve in the wavelength range from 500 to 700 nm. Then a graph was plotted
using the integrated fluorescence intensity against the absorbance and a trend line was added for each
curve with intercept at zero. Absolute values of the fluorescence quantum yield were calculated using
the following equation:
2
2
ST
X
ST
X
STX
Grad
Grad
(1)
Where the subscripts ST and X denote standard and test respectively, QY is the fluorescence
quantum yield, G is the gradient from the plot of integrated fluorescence intensity vs absorbance, and
η is the refractive index of the solvent. In order to minimize re-absorption effects, absorbance in the
10 mm fluorescence cuvette should never exceed 0.12 at the excitation wavelength.
3.3. Cytotoxicity of CDs
100 μl of HepG2 hepatoma cells were seeded in 96-well plates, and the cell density was maintained
at 10000/well, and cultured in the incubator for 24 h. Remove the culture solution and add the fresh
medium. At the same time, different concentrations of CDs (40, 80, 120, 160, 200, 240, 280, and 320
μg/ml) were added and incubated for another 24 h at 37 ºC. The medium was removed and washed
with PBS in multiple times, then, 200 μl of serum-free fresh medium containing 20 μl MTT (5 mg/ml
IWMCE 2018 - International Workshop on Materials, Chemistry and Engineering
230
dissolved in PBS) was added to each well and incubated for 4 h. Discard the well solution, add 150 μl
of DMSO, shake for 10 min and determine the absorbance at 590 nm to calculate the cell viability.
4. Results and discussion
As shown in table 1, the fluorescence quantum yield of CDs samples under different reaction
temperature (CD-EDA) were calculated according to equation 1, CD-EDA-T2 had best performance
of fluorescence, the quantum yield was determined to be 79.7%, indicating their superior PL
performance to most other CDs reported[8, 9].
Table 1.The fluorescence quantum yield of CD-EDA
Sample
CD-EDA-T1
CD-EDA-T2
CD-EDA-T3
CD-EDA-T4
QY (%)
56.1
79.7
61.9
35.4
Same concentration (0.2 mg/ml) of samples were prepared and used fluorescence spectrometer to
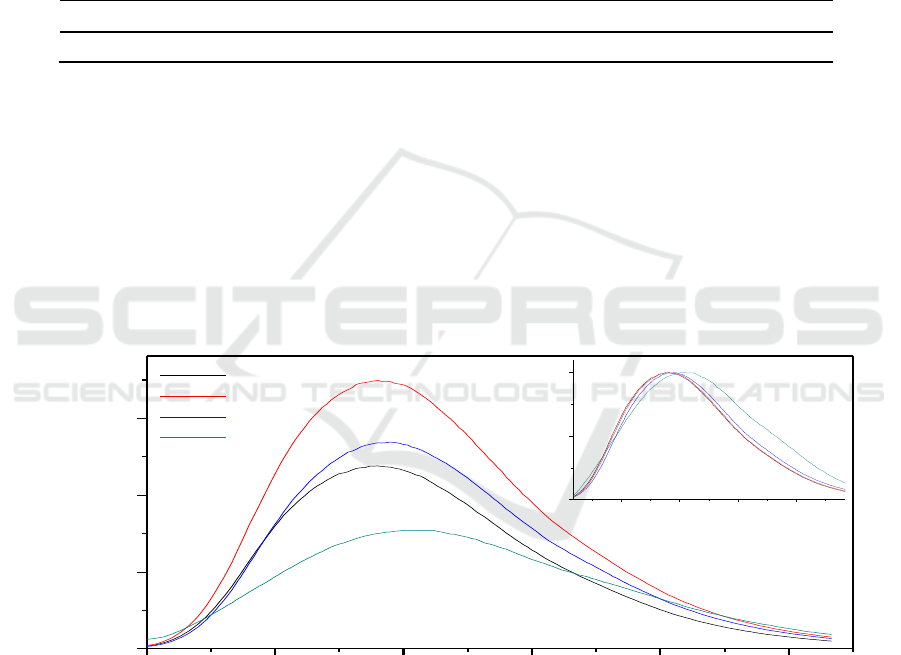
measure CDs emission intensity under 380 nm excitation. Figure 1 exhibited CD-EDA-T2 had the
strongest emission spectra, followed by CD-EDA-T3, CD-EDA-T1, CD-EDA-T4 samples, which
were consistent with the calculation results of fluorescence quantum yield. The inset in Figure 1
indicated the locations of emission spectra were red-shift to longer wavelength, which is probably
attributed to carbonation degree of carbon nuclear. With increasing heating temperature, more and
more molecular fragment was carbide to form the carbon nuclei. Meanwhile, the higher reaction
temperature, the larger red-shift wavelength in four CDs samples. Therefore, we considered the red
shift emission spectra in Figure 1 came from the increasing carbonization degree of carbon core.
390 420 450 480 510 540
0
800
1600
2400
420 450 480 510
0.0
0.5
1.0
Wavelength (nm)
FL Intensity (a.u.)
Wavelength (nm)
T1
T2
T3
T4
Normalized FL
Figure 1. The emission spectra of CD-EDA (λ
ex
=380 nm), the inset is normalized
photoluminescence.
The Effect of Reaction Temperature on Fluorescence Properties of Carbon Dots
231
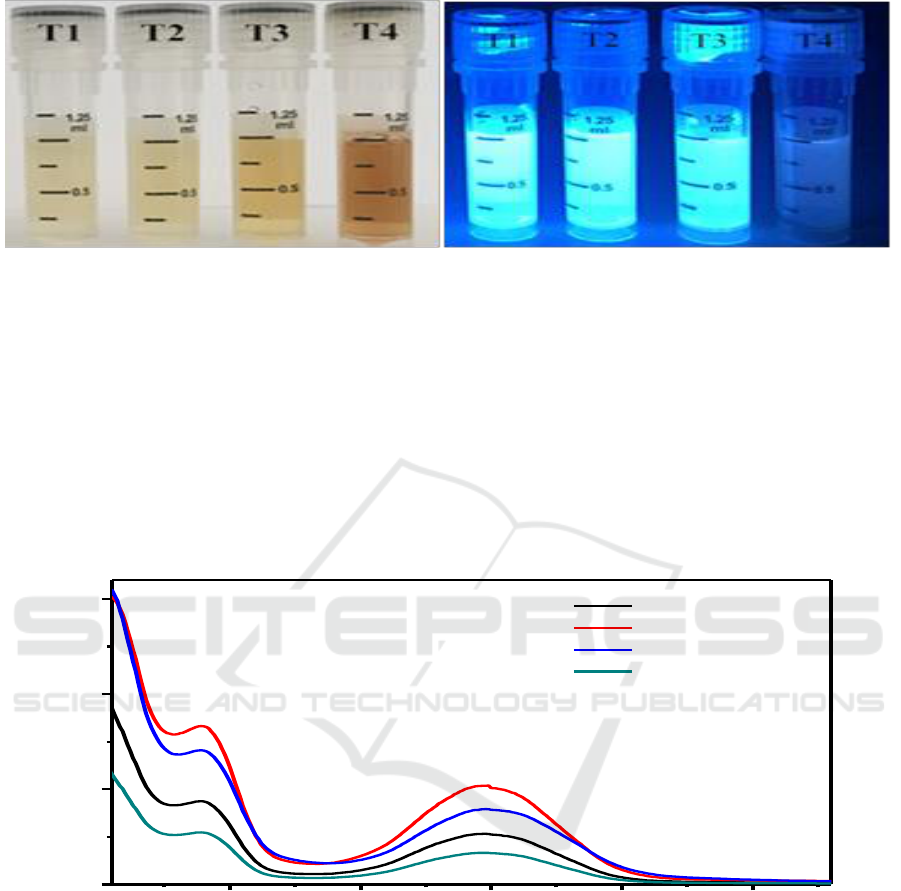
Figure 2. The images of CD-EDA solutions (the concentration is 1 mg/ml, the left is irradiated at
daylight, the right is excited at 365 nm of UV light).
To demonstrate the temperature contributing to the formation of carbon core, 1mg/ml CD-EDA
samples were prepared to observe. As shown in Figure 2, the CD-EDA had excellent solubility.
Although the CD-EDA had the same concentration, the four samples presented different colors at the
daylight, which varied from light yellow to yellow, and finally to dark brown. We inferred the color
in CD-EDA represented the degree of carbonization, the deeper of the color, the more serious of
carbonization, and more developing of nuclei. However, the fluorescence of CD-EDA in UV light
was not corresponding to the degree of carbonization, indicating the fluorescence properties were
also affected by the surface state of CDs.
250 300 350 400 450
0
1
2
3
Absorbance
Wavelength (nm)
CD-EDA-T1
CD-EDA-T2
CD-EDA-T3
CD-EDA-T4
Figure 3. UV-vis absorption spectra of CD-EDA.
Figure 3 was the UV-vis absorbance spectra of CD-EDA, all CDs samples manifested the similar
shape in the range of 200-450 nm, the absorbance peaks at 230 nm and 350 nm were both due to the
π-π* (aromatic C=C) transition. [10] The CD-EDA-T2 had the highest absorbance in whole spectral
ranges, therefore presented the best flourescence performance. The absorbance intensity of CD-EDA
were consistence with fluorecence intensity.
IWMCE 2018 - International Workshop on Materials, Chemistry and Engineering
232
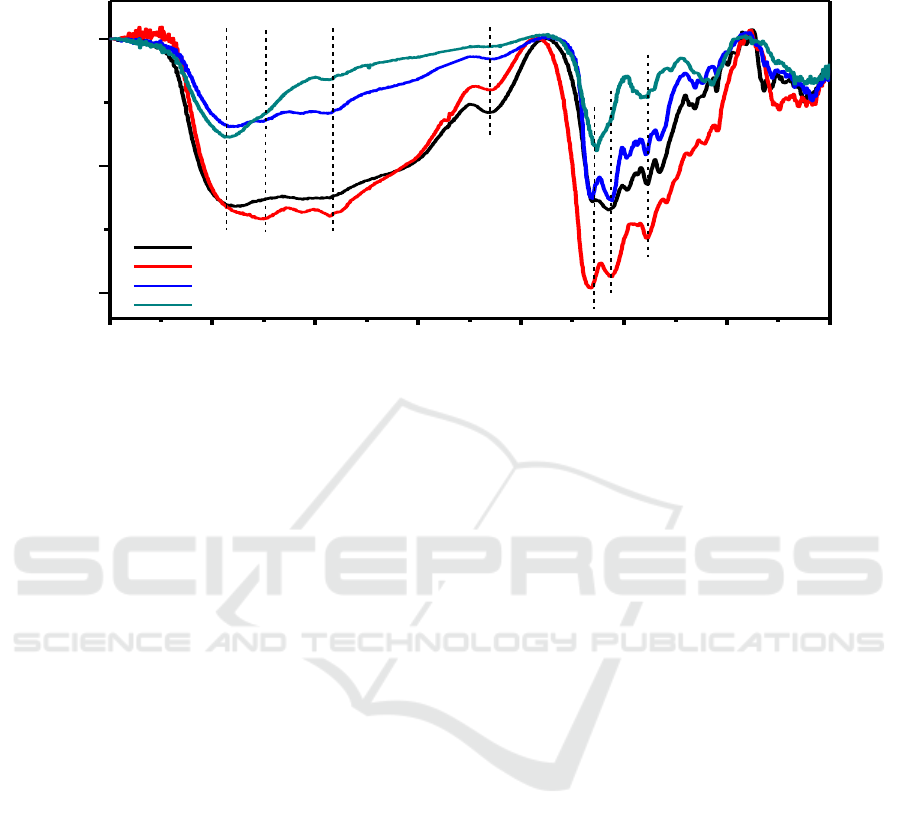
4000 3500 3000 2500 2000 1500 1000 500
C-N
N-H
-CO-NH-
C=N
CH
3
NH
2
-OH
Wavenumber(cm
-1
)
Transmittance(%)
CA-EDA-T1
CA-EDA-T2
CA-EDA-T3
CA-EDA-T4
Figure 4. FT-IR spectra of CD-EDA.
To analyze the surface states and the composition of the as-prepared CDs, FT-IR were then
performed. As shown in Figure 4, CD-EDA had many characteristic absorption bands, the strong
peaks of 3401, 2871, 1650, 1564 cm
-1
, 1108 cm
-1
were attributed to -OH, -NH
2
, -CH
3
, amide I C=O,
N-H and C-N respectively, which guaranteed the CD-EDA had excellent solubility in aqueous
solution. It is obvious the temperature affected the surface state of CDs, the absorption intensity of
functional groups in CD-EDA-T4 were greatly reduced, and the -OH and N-H groups were
disappeared completely. This phenomenon was very consistent with the high temperature destroying
surface organic functional groups. As for CD-EDA-T2 formed under 200
o
C, the characteristic
absorption peaks of various functional groups were highest in all samples, indicating a great number
of functional groups were passivated on the surface, and the surface of CD-EDA-T4 was very active.
It was noted that just a part not all functional groups on the surface were related to the fluorescence
performance of CDs. It had been demonstrated amino groups could greatly enhance fluorescence of
CDs, [11] the intensity of -NH
2
and C-N in Figure 4 were also corresponding to the fluorescence
quantum yield, which further proved this conclusion.
These above results reflected that the reaction temperature affected the degree carbonization of
carbon core and the surface state. It had been testified that the overall fluorescence of CDs was
formed by carbon core and surface states [12, 13]. Therefore, we inferred that the process of reaction
temperature affecting on the fluorescence properties of CDs was as following, the high energy of
reaction temperature cracked the molecular into small fragments, these fragments were further
carbonization and then formed carbon core through sp
2
hybridization, some exposed chemical groups
developed the surface state. When the temperature was 160
o
C, both carbon core and surface state are
in the formation process, hence as the temperature raised to 200
o
C, the carbon core was further
enlarged and the internal fluorescence was increased. At the same time, the surface state is active and
the organic fluorophore produced a strong fluorescence, which resulted in an excellent fluorescence
performance. When the temperature was above 200
o
C, although the fluorescence of carbon core was
further enhanced, the carbonization process sacrificed the surface organic fluorophore, which led a
dramatic decrease of surface intensity and the overall fluorescence was reduced at last. Therefore, the
temperature of 200
o
C is the best reaction conditions, and the fluorescence original from carbon core
The Effect of Reaction Temperature on Fluorescence Properties of Carbon Dots
233
and surface state in CD-EDA-T2 generally show the best intensity with the fluorescence quantum
yield of 79.7%.
0 40 80 120 160 200 240 280 320
0
40
80
120
Cell Viability (%)
Concentration (ug/ml)
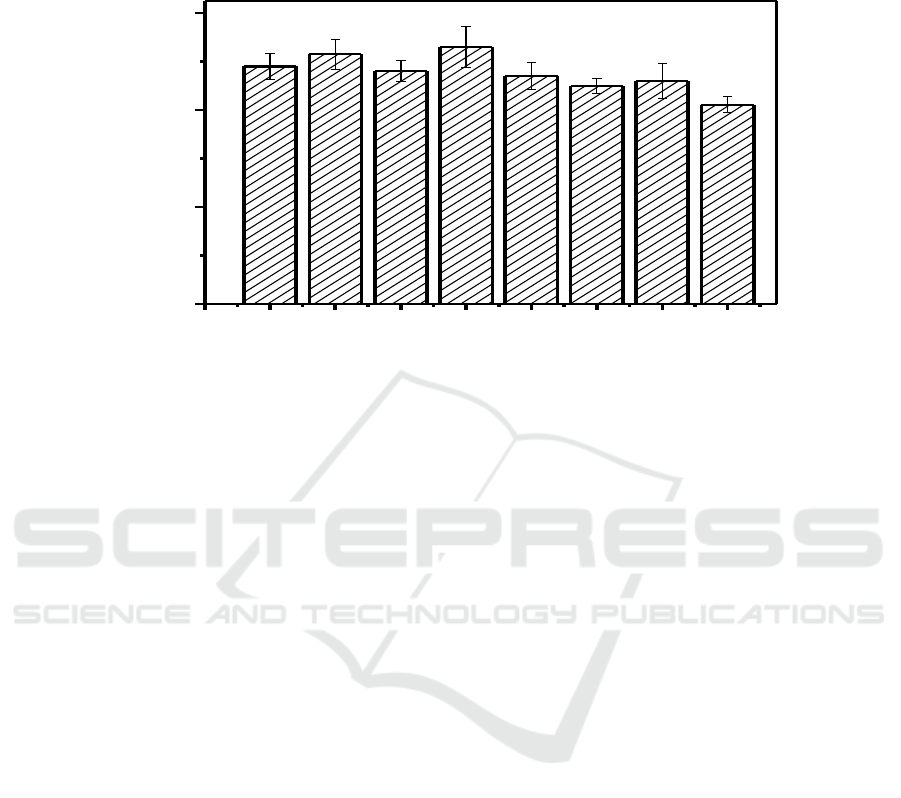
Figure 5. Cell viability of HepG2 treated with different concentration of CD-EDA-T2.
The basic requirement for application of CDs in cell labeling is low toxicity and good
biocompatibility. The cytotoxicity of CD-EDA-T2 were evaluated by MTT assay. As shown in
Figure 5, at a lower concentration, the carbon quantum dots had no effect on the cellular activity of
HepG2, and even the concentration of CD-EDA-T2 reached 320 μg/ml, the cell viability rate
remained at about 82%, which was satisfactory for cell imaging.
5. Conclusions
The fluorescence of CDs prepared by CA/EDA was determined by carbon core and surface state, a
suitable temperature, mot too low nor too high, was more beneficial to achieve the best fluorescence
performance. The CDs had excellent biocompatibility, the cell viability was more than 90% when the
concentration was less than 280 μg/ml, which held a great potential in bio-imaging and biomedical
applications.
Acknowledgments
The authors gratefully acknowledge the support for this work from the Natural Sciences Foundation
of China [No. 51502345] and Tianjin Sciences Foundation [16JCQNJC03100].
References
[1] Sun Y P, Zhou B, Lin Y and et al 2006 Quantum-sized carbon dots for bright and colorful
photoluminescence J. Journal of the American Chemical Society 128(24) 7756-7757
[2] Miao X, Yan X, Qu D and et al 2017 Red emissive sulfur, nitrogen codoped carbon dots and
their application in ion detection and theraonostics J. ACS applied materials & interfaces
9(22) 18549-18556
[3] Martindale B, Hutton G A M, Caputo C A and et al 2017 Enhancing Light Absorption and
Charge Transfer Efficiency in Carbon Dots through Graphitization and Core Nitrogen
Doping J. Angewandte Chemie International Edition 56(23) 6459-6463
[4] Yuan F, Li S, Fan Z and et al 2016 Shining carbon dots: Synthesis and biomedical and
optoelectronic applications J. Nano Today 11(5) 565-586
[5] Yang Z, Xu M, Liu Y and et al 2014 Nitrogen-doped, carbon-rich, highly photoluminescent
IWMCE 2018 - International Workshop on Materials, Chemistry and Engineering
234

carbon dots from ammonium citrate J. Nanoscale 6(3) 1890-1895
[6] Yan F, Zou Y, Wang M and et al 2014 Highly photoluminescent carbon dots-based fluorescent
chemosensors for sensitive and selective detection of mercury ions and application of
imaging in living cells J. Sensors and Actuators B: Chemical 192 488-495
[7] Zhou J, Booker C, Li R and et al 2007 An electrochemical avenue to blue luminescent
nanocrystals from multiwalled carbon nanotubes (MWCNTs) J. Journal of the American
Chemical Society 129(4) 744-745
[8] Vassilakopoulou A, Georgakilas V, Vainos N and et al 2017 Successful entrapment of carbon
dots within flexible free-standing transparent mesoporous organic-inorganic silica hybrid
films for photonic applications J. Journal of Physics and Chemistry of Solids 103 190~196
[9] Li H, He X, Kang Z and et al 2010 Water-soluble fluorescent carbon quantum dots and
photocatalyst design J. Angewandte Chemie International Edition 49(26) 4430-4434
[10] Mao Y, Bao Y, Han D and et al 2012 Efficient one-pot synthesis of molecularly imprinted
silica nanospheres embedded carbon dots for fluorescent dopamine optosensing J.
Biosensors and Bioelectronics 38(1) 55~60
[11] Zhai X, Zhang P, Liu C and et al 2012 Highly luminescent carbon nanodots by microwave-
assisted pyrolysis J. Chemical Communications 48(64) 7955-7957
[12] Yu P, Wen X, Toh Y R and et al 2012 Temperature-dependent fluorescence in carbon dots J.
The Journal of Physical Chemistry C 116(48) 25552~25557
[13] Krysmann M J, Kelarakis A, Dallas P and et al 2011 Formation mechanism of carbogenic
nanoparticles with dual photoluminescence emission J. Journal of the American Chemical
Society 134(2) 747~750
The Effect of Reaction Temperature on Fluorescence Properties of Carbon Dots
235
