
Boron and Phosphorus Removal in Si Purification with Ca-Si
Alloy under Air Atmosphere
H R Cheng
1
, S S Zheng
1
and C Chen
1, 2, *
1
College of Energy, Xiamen University, Xiamen 361102, P. R. China
2
College of Physical Science and Technology, Xiamen University, Xiamen 361005,
P. R. China
Corresponding author and e-mail: C Chen, cchen@xmu.edu.cn
Abstract. To further discuss the reduction of phosphorus (P) in the purification of
metallurgical grade silicon (MG-Si), MG-Si alloyed with Ca-Si alloy first and then acid
leaching was employed to removal CaSi
2
phase in which P was enriched under air atmosphere
at 1723 K in this paper. The higher removal fraction of P can be obtained by adding more Ca-
Si alloy to Si. The P removal fraction is 95.45% when the mass ratio of Si to Ca -Si alloy is
0.5. However, B cannot be removed from Si by alloying with Ca-Si alloy due to the
concentration of B in the Si phase is higher than that in CaSi
2
phase.
1. Introduction
Solar energy which is converted into direct current electricity via the photovoltaic effect is
considered to be the main renewable energy source in the future due to its relatively infinite reserves.
Silicon (Si) will remain the dominant material for solar cells in the foreseeable future [1]. The purity
of Si used in manufacturing solar cells which is called solar grade Si (SoG-Si) is higher than 6N, thus
the total metal impurities content is < 1ppmw, P < 0.1ppmw, and B < 0.3ppmw [2].
It is wasteful that crystalline silicon made by the chemical method (mainly the improved Siemens
method) must dope boron (B) or phosphorus (P) to make solar cells. It is considered as a cost-
effective method to purity metallurgical grade Si (MG-Si) to SoG-Si by metallurgical technology due
to its simple equipment, low investment, low energy consumption and no pollution. The main
methods of removing B from Si by metallurgical method are plasma treatment [3] and slag refining
[4], while vacuum technology [5] and electron beam [6] for the removal of P. Alloy refining
combined with directional solidification can also be used to remove B and P from Si. Hu et al. [7]
claimed that P can be further reduced by adding Ca when using Sn to purify Si. The removal
efficiency of P in Si increased from 73.4% to 86.5% when Ca addition accounted for 4 mass percent
in the Sn-Si system and it was found that the mass ratio of P in the Si phase containing P after
purification was up to 17.8%. Johnston and Barati [8] achieved a P removal fraction of 98.8% by
adding 4% calcium as getter metal to Si containing 326 ppmw of P combined with acid leaching. He
et al. [9] studied the role of calcium oxide in the process of removing metal impurities from MG-Si
by acid leaching, they stated that acid insoluble Si-Fe-based precipitates transferred into acid soluble
Si-Ca-based alloys, thus promoting the effect of acid leaching. Meteleva-Fischer et al. [10] believed
Si alloying with Ca resulted in the agglomeration of inclusions and better segregation of impurity
Cheng, H., Zheng, S. and Chen, C.
Boron and Phosphorus Removal in Si Purification with Ca-Si Alloy under Air Atmosphere.
In Proceedings of the International Workshop on Materials, Chemistry and Engineering (IWMCE 2018), pages 127-132
ISBN: 978-989-758-346-9
Copyright © 2018 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
127

phases. They pointed out that the FeSi
2
Ti phase became enriched with P in the Ca-alloyed samples,
however, no P content was detected in CaSi
2
phase in their work.
Slag treatment using CaO-based slag coupled with acid leaching is also applied in removing metal
impurities and P elimination from Si. In our previous study [11], we found that part of P was likely
reduced to Ca-Si-P compounds uniformly distributed into the CaSi
2
phase among Si grains after a
short period of slag refining using CaO-SiO
2
-CaF
2
system under air atmosphere, and the scattered
distributed CaSi
2
phase contributed to the removal of Fe, Al and Ca impurities from MG-Si. And, it
is found that the removal effect had a positive correlation with the content of Ca in Si.
However, research on the effect on the removal of B from Si by slag treatment using CaO-based
slag or by alloying with Ca has not been conducted so far. In the present paper, the result of slag
refining using CaO-based slag is simulated, a certain amount of Ca-Si alloy is molten with MG-Si to
be refined in medium frequency induction, which results in a certain amount of Ca in Si. And the
distribution of B and P in Si and CaSi
2
, the removal efficiency of B and P will be discussed.
2. Experiments
2.1. Alloy refining
The alloy refining experiments was conducted in a laboratory scale medium frequency induction
furnace. Si-Ca alloy used in this study was provided by Shaanxi Shenghua metallurgical Co. Ltd.,
and its element analysis was shown in Table 1. The composition of MG-Si to be refined in the
present paper was shown in Table 2. Samples of MG-Si and Ca-Si alloy were placed in a high-purity
dense graphite crucible (99.99%, 45 mm outer diameter, 30 mm inner diameter, 120 mm height, and
105 mm depth). Thereafter, the crucibles with the samples were placed in medium frequency
induction furnace. After melting and holding at 1723 K ± 20 K for 10 min, the samples were cooled
to 1273 K at 10 K/min for solidification refining. After cooling to room temperature, the samples
were cut into pieces, and some parts were polished to examine the morphology and microarea
composition by SEM-EDS.
Table 1. Contents of main elements in Ca-Si alloy.
Elements
Ca
Al
Si
C
S
P
B
Content (wt%)
30.22
1.24
60.74
0.52
0.035
0.021
0.0079
Table 2. Content of main impurities in MG-Si analyzed by ICP-AES.
Elements
Fe
Al
Ca
B
P
Content (ppmw)
3960
2156
904
20.24
89.92
2.2. Acid leaching
Samples after alloy refining were crushed into powder to be leached with aqua regia (HCl:HNO
3
=
3:1 by volume, diluted by deionized water by volume ratio of 3:2) and HF (diluted by deionized
water by volume ratio of 1:1) successively at room temperature for 1 hour, respectively. Finally, the
concentrations of impurities in the leached Si powder was analyzed by inductively couple plasma-
atomic emission spectroscopy (ICP-AES, Thermo Fisher iCAP 6300).
3. Results and discussions
According to the content of Ca and Si in CaSi
2
listed in Table 1, the raw material Ca-Si alloy can be
considered as a mixture of CaSi
2
and Si. In our previous study [11], it’s found that CaSi
2
phase was
produced among the silicon grains after slag treatment by CaO-SiO
2
-CaF
2
system. After solvent
refining, CaSi
2
will be produced by a eutectic reaction at 1301 K during the cooling of Ca-Si melt
IWMCE 2018 - International Workshop on Materials, Chemistry and Engineering
128

according to the Ca-Si phase diagram. The content of B and P in Si changed after alloy refining and
the following acid leaching, as listed in Table 3 and shown in Figure 1. This is due to segregation of
B and P impurities between CaSi
2
and Si phases. It is difficult for B or P in liquid Si to volatilize
under air atmosphere, so the B or P concentration in CaSi
2
can be calculated based on the
conservation of mass.
The distribution ratio of B or P in CaSi
2
and Si is calculated according to the following equation,
2
B or P in CaSi
B or P
B or P in final Si
C
=
C
L
(1)
Table 3. Experiment results of Si after alloy refining and acid treatment.
Mass ratio of Si to Ca-Si alloy
0.5
1
2
4
8
16
Content in mixture (ppmw)
B
59.41
49.62
39.83
31.99
26.77
23.70
P
169.97
149.96
129.95
113.94
103.26
96.98
Yiled during acid leaching (%)
44.80
58.90
74.00
85.00
90.90
93.00
Content after
acid leach ing
(ppmw)
Si
B
45.66
39.19
30.49
26.31
22.10
19.92
P
4.09
6.88
11.14
22.35
42.77
65.42
CaSi
2
B
70.58
64.57
66.41
64.22
73.45
73.93
P
304.60
355.00
468.10
632.94
707.55
516.37
L
B
1.55
1.65
2.18
2.44
3.32
3.71
L
P
74.39
51.58
42.03
28.32
16.54
7.89
3.1. Removal of phosphorus
P content in raw Si, Ca-Si alloy, mixture of Si and Ca-Si alloy, solvent refined Si, CaSi
2
and yield of
Si during acid leaching as a function of mass ratio of Si to Ca-Si alloy in starting material are shown
in Figure 1.
Generally, the content of P in commercial Ca-Si alloy is higher than that in MG-Si. It is obvious
that the P content in the mixture is between Si and Ca-Si alloy after smelting Si and Ca-Si alloy with
different mass ratios. And with the increase of Si ratio, the P content in the mixture is more and more
close to that in Si. However, after acid leaching, the content of P in purified Si and that in the mixture
after smelting are different with the change of the mass ratio of Si to Ca-Si alloy in the initial raw
material: Even though the P content in mixture is higher than that in MG-Si, but P content in the
purified silicon is lower than that in MG-Si after acid leaching instead. This indicates that the
addition of Ca-Si alloy to MG-Si is beneficial to the removal of P from Si. This is because the CaSi
2
has aggregation effect to P, which results in P from MG-Si into CaSi
2
phase. Therefore, Ca-Si alloy
can be employed to remove P from Si, and the higher removal fraction of P can be obtained by
adding more Ca-Si alloy to Si.
After alloy refining experiment, P content in CaSi
2
is shown in Figure 1. The content of P in CaSi
2
is much higher than that of P in refined Si after acid leaching. The distribution ratio of P between
CaSi
2
and Si, and the Si yield during the acid leaching, with the mass ratio of Si to Ca-Si alloy in the
initial material are shown in Figure 2. As can be seen from Figure 2, L
P
increases with the increase of
Ca-Si alloy content. L
P
between CaSi
2
and Si reaches 74.39 and the P removal fraction is 95.45%
when the mass ratio of Si to Ca-Si alloy is 0.5, which means effect of P removal is obvious. However,
it can also be seen from Figure 2 that the Si yield during acid leaching decreases rapidly while the P
removal fraction is high. Therefore, it is important to find an optimal mass ratio to reduce the P
content in Si with Si yield to be considered in industrial production.
Boron and Phosphorus Removal in Si Purification with Ca-Si Alloy under Air Atmosphere
129
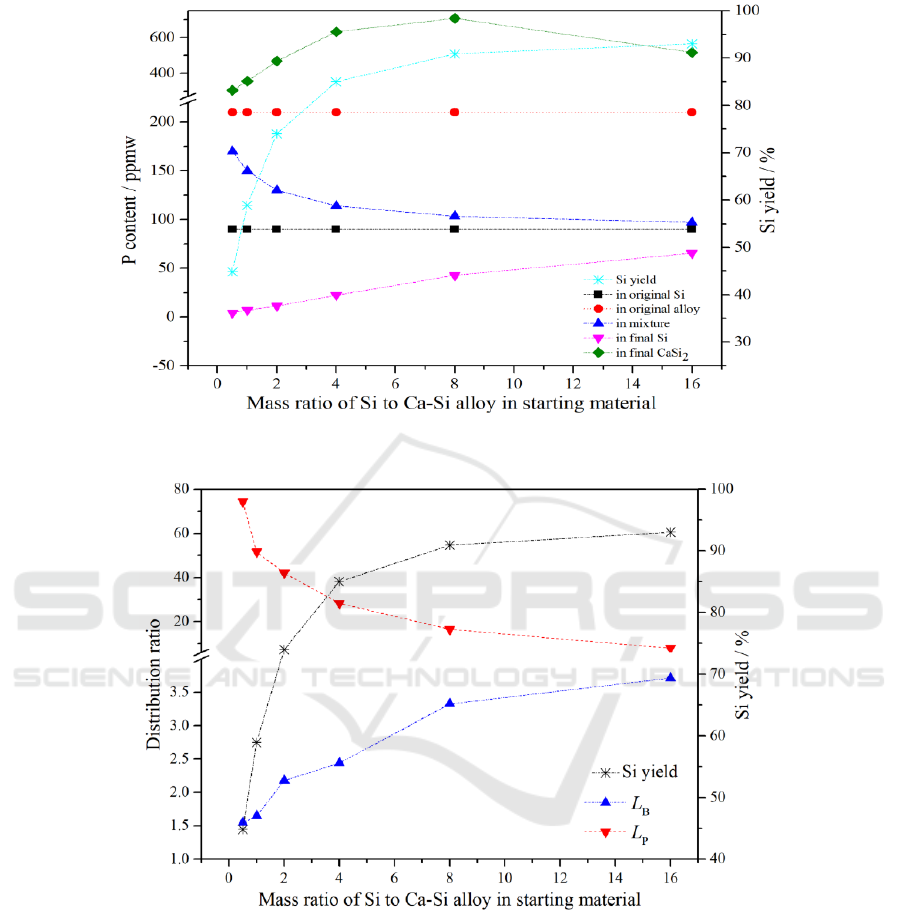
Figure 1. Content of P and Si yield during acid leaching as a function of mass ratio of Si to Ca-Si
alloy in starting material.
Figure 2. L
P
, L
B
and Si yield as a function of mass ratio of Si to Ca-Si alloy in starting material.
3.2. Removal of boron
B content in raw Si, Ca-Si alloy, mixture of Si and Ca-Si alloy, solvent refined Si, CaSi
2
and Si yield
during acid leaching as a function of mass ratio of Si to Ca-Si alloy in starting material are shown in
Figure 3.
Apparently, the B content in the mixture reduces with the increase of mass ratio of Si to Ca-Si
alloy because the B content in commercial Ca-Si alloy is also higher than that in MG-Si. The content
of B in refined Si decreased slightly after acid leaching which indicated that acid leaching had a
positive effect on removal of B from Si after alloying with Ca, and the change trend of B content in
Si was the same before and after acid leaching. The B content in CaSi
2
is slightly lower than that in
the Ca-Si alloy, which may be due to the diffusion of B from the Ca-Si alloy into the MG-Si. The
IWMCE 2018 - International Workshop on Materials, Chemistry and Engineering
130
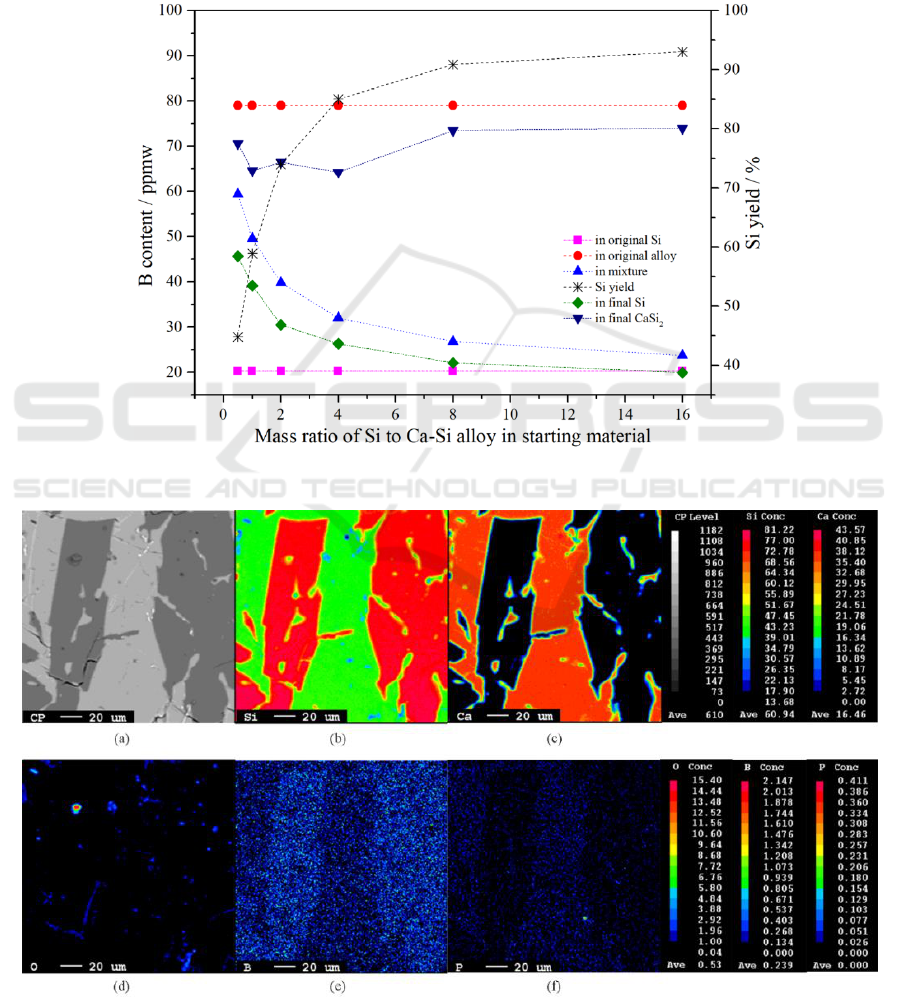
distribution ratio of B between CaSi
2
and Si, and Si yield during the acid leaching changed with the
mass ratio of Si to Ca-Si alloy in the starting material shown in Figure 3. L
B
increases with the
increase of the mass ratio of Si to Ca-Si alloy in raw material, from 1.55 at the ratio of 0.5, to 3.71 at
16. Despite the large L
B
, the B content in refined Si couldn’t be reduced from the view of B
concentration in refined Si compared to raw MG-Si. This could be illustrated by an EPMA diagram
of a Si sample containing a CaSi
2
phase. It is can be observed that the content of B in Si phase is
higher than that in CaSi
2
phase from the EPMA-WDS analysis of a Si sample containing CaSi
2
phase
shown in Figure 4. Therefore, B cannot be removed from Si alloyed with Ca or Ca-Si alloy.
Figure 3. Content of B and Si yield as a function of mass ratio of Si to Ca-Si alloy in starting
material.
Figure 4. EPMA-WDS elements mapping of Si containing CaSi
2
.
Boron and Phosphorus Removal in Si Purification with Ca-Si Alloy under Air Atmosphere
131

4. Conclusions
Ca-Si alloy was employed to refined MG-Si in the intermediate frequency furnace under air
atmosphere at 1723 K. The removal of B and P was discussed, and the main conclusions were as
follows:
1. Ca-Si alloy can be employed to remove P from Si, and the higher removal fraction of P can be
obtained by adding more Ca-Si alloy to Si. L
P
between CaSi
2
and Si reaches 74.39 and the P removal
fraction is 95.45% when the mass ratio of Si to Ca-Si alloy is 0.5.
2. Si yield during acid leaching decreases rapidly while the P removal fraction is high.
3. B cannot be removed from Si by alloying with Ca-Si alloy due to the concentration of B in the
Si phase is higher than CaSi
2
phase.
Acknowledgment
This work was supported by funding from the ShangNan ZhongJian Industrial Co., Ltd. In Shaanxi
Province.
References
[1] Tao M 2008 The Electrochemical Society Interface 17 30-5
[2] Kato Y, Hanazawa K, Baba H, Nakamura N, Yuge N, Sakaguchi Y, Hiwasa S and Aratani F
2000 Tetsu-to-Hagané 86 717-24
[3] Suzuki K, Kumagai T and Sano N 1992 ISIJ Int. 32 630-634
[4] Li Y, Wu J and Ma W 2014 Sep. Sci. Technol. 49 1946-1952
[5] Zheng S, Engh T A, Tangstad M and X Luo 2011 Sep. Purif. Technol. 82 128-137
[6] Jiang D, Tan Y, Shi S, Dong W, Gu Z and Zou R 2012 Mater. Lett. 78 4-7
[7] Hu L, Wang Z, Gong X, Guo Z and Zhang H 2013 Sep. Purif. Technol. 118 699-703
[8] Johnston M D and Barati M 2013 Sep. Purif. Technol. 107 129-34
[9] He F, Zheng S and Chen C 2012 Metall. Mater. Trans. B 43 1011-8
[10] Meteleva-Fischer Y V, Yang Y, Boom R, Kraaijveld B and Kuntzel H 2012 Intermetallics 25
9-17
[11] Cheng H, Zheng S and Chen C 2012 Sep. Purif. Technol. 201 60-70
IWMCE 2018 - International Workshop on Materials, Chemistry and Engineering
132
