
Preparation of Nano-silica Dioxide Modified Cellulose Acetate (CA)
Membranes for Enhanced Performance in Forward Osmosis Process
Haiyang Jin
1,2,3
, Li Lin
1,2,*
, Min Liu
1,2
, Qingyun Li
1,2
, Zhuo Huang
1,2
, Xianqiang Tang
1,2
and Ping Yu
3
1
B
asin Water Environmental Research Department, Changjiang River Scientific Research Institute, Wuhan, Hubei, 430010,
People's Republic of China
2
Key Lab of Basin Water Resource and Eco-Environmental Science in Hubei Province, Wuhan 430010,
China
3
College of Chemistry and Molecular Sciences, Wuhan University, Wuhan, Hubei, 430072 People's
Republic of China
Email: linli1229@hotmail.com
Keywords: Forward osmosis, membrane modification, nano silica dioxide, cellulose acetate
Abstract: Nano-silica dioxide (nano-SiO
2
) modified cellulose acetate (CA) membranes for forward osmosis (FO)
were prepared by phase inversion via immersion precipitation technique. Nano-SiO
2
particles were added
into the casting solution of CA, 1, 4-dioxane, acetone, lactic acid and methanol to improve the FO
membranes performance. Different percentage of nano-SiO
2
(2, 4 and 6 wt. %) of CA composite
membranes were cast. The modified membranes were characterized by various methods to probe the
membranes structure and surface properties. The FO performances were also evaluated for the modified
membranes compared to the non nano-SiO
2
membranes. The surface hydrophilicity, porosity and tensile
strength of nano-SiO
2
CA membranes were improved with the increment of the percentage of nano-SiO
2
added to the CA. The morphological studies showed that the addition of nano-SiO
2
significantly changed
the surface properties of the CA membranes. The FO performance was evaluated using 1M NaCl solution as
feed solution and purified water as draw solution. The nano-SiO
2
CA membranes showed better water flux
and reverse salt flux in the range of 2–6 wt. % nano-SiO
2
content than original CA membranes. These
encouraging results suggested that nano-SiO
2
CA membranes displayed potential to be further developed for
FO applications.
1 INTRODUCTION
Forward Osmosis (FO) is the transportation of water
across a selectively permeable membrane from a
region with higher water chemical potential to a
region with lower water chemical potential. This
process is driven by a difference in solute
concentrations across the membrane which allows
passage of water but rejects most solute molecules
or ions. Due to its inherent advantages, such as low
energy expenditure, low membrane fouling, high
water recovery, simple configuration and equipment
and etc, forward osmosis process was extensively
studied by scientists for a variety of applications in
science and engineering (
Cath et al., 2006;
Shuaifei et al., 2012
). Now, its applications have
showed potential values in seawater desalination,
wastewater treatment, food and pharmaceuticals
processing, controlling drug release, and electrical
power generation (
Shuaifei et al., 2012; Achilli et
al., 2009
), etc.
However, there are some problems which limit
the use of FO, such as flux decline caused by
internal concentration polarization, lack of proper
draw solute and effective recovery. Recent works
have focused on reducing internal concentration
polarization and seeking optimal draw solute. The
internal concentration polarization which occurs
within the support layer is the major limiting factor
causing the decline of water flux (
McCutcheon and
Elimelech, 2006
). The influence of internal
concentration polarization on FO water flux has
been investigated by different modeling techniques
and the solution-diffusion theory (
Shuaifei et al.,
2012
). A variety of draw solutes/solutions,
including magnetic and/or hydrophilic nanoparticles
Jin, H., Lin, L., Liu, M., Li, Q., Huang, Z., Tang, X. and Yu, P.
Preparation of Nano-silica Dioxide Modified Cellulose Acetate (CA) Membranes for Enhanced Performance in Forward Osmosis Process.
In Proceedings of the International Workshop on Environment and Geoscience (IWEG 2018), pages 163-169
ISBN: 978-989-758-342-1
Copyright © 2018 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
163
and organic compounds (Kim et al., 2012;
McCutcheon et al., 2005
), have been investigated
in FO process.
In the early study, reverse osmosis (RO)
membranes were tested for FO process. But they
showed low water flux due to the internal
concentration polarization caused by the porous
support layer. Hence, a perfect FO membrane should
consist of a single active layer without any support
layer (
Wang et al., 2010). However, the lack of
mechanical strength may limit its applicability.
Chung’s group developed flat-sheet CA-based
membranes comprised double- skinned layers that
were able to eliminate internal concentration
polarization (Zhang et al., 2010;
PhuongNgaNguyen et al., 2013
). However, the
membranes showed low water flux due to the
additional resistance to water transport. Thi Phuong
Nga Nguyen et al. prepared CTA/CA FO
membranes to improve water flux (
Smitha et al.,
2004
), but relatively high reverse solute flux (RSF)
limits the application of the membranes. It is
necessary to prepared FO membranes with high
water flux and salt rejection for practical application.
Introducing inorganic particles into membrane
materials has shown potential to improve the
permeability and selectivity of membrane (Sairam
et al., 2011
). Nano-silica dioxide (nano-SiO
2
) is
one of the most important new high-tech ultra-fine
inorganic materials and has lots of particular
characteristics: the particle size of 20±5 nm, the high
specific surface area of 640-700m
2
/g and superior
thermal and chemical stability. Besides, the rich
hydroxyl groups on the surface of nano-SiO
2
can
form hydrogen bonds with the hydroxyl groups of
polymer chains. In a word, nano-SiO
2
plays an
irreplaceable role in many subjects due to its
particular characteristics (
Liu et al., 2004)].
In this research, nano-SiO
2
are added into the CA
casting solution of 1,4-dioxane and acetone with
additives of lactic acid and methanol to prepare the
new nano-SiO
2
modified cellulose acetate (CA)
membranes for FO application. Membranes are
prepared by phase inversion. Subsequently, the new
nano-SiO
2
modified CA membranes are
characterized in different ways and compared with
CA membranes without nano-SiO
2.
The influences
of the content of nano-SiO
2
on the membrane
performance are also discussed.
2 MATERIAL AND METHODS
2.1 Materials
CA(54.5 ~ 56.0wt. ﹪ acetyl) were purchased from
Sinopharm Chemical Reagent Co., Ltd and used
without further purification. 1, 4-dioxane
(≥99.5%purity), acetone (≥99% purity), lactic acid
(≥99%purity) and methanol (≥99.8% purity) were
obtained from Sinopharm Chemical Reagent Co.,
Ltd. Sodium chloride (NaCl, 99%purity) and
deionized water were used for membrane
performance testing. Nano-silica dioxide (7nm) was
from Sinopharm Chemical Reagent Co., Ltd and
used for modifying. Disodium carbonate (Na
2
CO
3
,
Sinopharm Chemical Reagent Co., Ltd) was used as
an effluent for ion chromatography (ICS-900,
Dionex, CA, USA).
2.2 Preparation of Flat-sheet CA-based
Membrane and Nano-SiO
2
Modified CA Membrane
Flat-sheet membranes were prepared by phase
inversion. The casting solution contained CA
polymers dissolved into 1, 4-dioxane and acetone
with additives of lactic acid and methanol (
Sairam
et al., 2011
). The solution was kept in a round flask
sealed with a glass stopper to prevent evaporation of
the solvents. The solution was homogenized by
using a mechanical stirrer (JJ, Yitong Electron Co.,
Ltd, China). The casting solution was then cast on a
glass plate by using an 100mm thick casting knife in
a constant temperature (set up at 25℃) and humidity
(70% relatively humidity) room. After evaporation
of the solvent for 30s, the casting film together with
the glass plate was immersed in a coagulation bath
of 1±0.3℃ deionized water. The membrane was
then stripped off the glass plate and kept in a bath of
deionized water at room-temperature (20℃), which
was changed every 4h for 24h to wash out the
solvents. Then the membrane was stored in
deionized water for performance testing.
The compositions of casting solution were of
13.4 wt. % CA, 53.2 wt. % 1, 4-dioxane, 18.4 wt. %
acetone, 6.8 wt. % lactic acid and 8.2 wt. %
methanol. The nano-SiO
2
were added into the
solution in different contents (2, 4 and 6 wt. %) and
were dispersed by stirring for 8h at room
temperature. The resultant solution was prepared for
phase inversion after eliminating air bladder by
IWEG 2018 - International Workshop on Environment and Geoscience
164

sonic oscillating. The nano-SiO
2
modified CA
membranes are denoted as CAN-2, CAN-4 and
CAN-6 assigned to their nano-SiO
2
content.
2.3 Characterization of Membrane
Structure and Surface Properties
Scanning electron microscopy (SEM) was used to
acquire the cross section and top surface imaging of
membranes to determine the morphological
properties. The SEM analysis was performed using
FEI Quanta 200 from Holland and operated at 15
KV. Every membrane sample was dried under
vacuum for 24h to dehydrate it for preparing SEM
samples. Membranes were fractured in liquid
nitrogen to clearly scan the cross-section image. The
membranes were coated with a gold layer for
observation by a sputter coater. Furthermore, energy
dispersive spectrometer (EDS) was used to collect
the elemental composition of membranes.
Attenuated total reflectance spectroscopy (ATR-
FTIR) was used to analyze the chemical changes of
the membranes in this study. Nicolet AVATAR 360
FTIR Spectrophotometer together with an ATR
accessory (ZnSe crystal) was used to collect the
spectra. The incidence angle was 45
o
and each
spectrum was recorded using 32 scans at a resolution
of 4cm
−1
in the region between 400 and 4000 cm
−1
.
OMNIC 8.2 software was used to record the spectra,
correct their baselines, normalize the spectra and
find the peaks.
Contact angle measurements were conducted by
dynamic contact angle instrument (DSA100,
KRŰSS GmbH, Hamburg, Germany). Contact angle
indicates the hydrophilicity and the smaller angle
indicates the better hydrophilicity. Every membrane
sample was dried under vacuum for 24h before
measurement. Two replicates were used, and five
drops per replicate were measured.
Membranes porosity(Ɛ) was calculated from the
equations below (
Wang et al., 2010):
wet dry w
wet dry w dry p
(m - m )/ρ
ε 100
(m - m )/ρ (m /ρ )
=×
+
(1)
Where m
wet
and m
dry
are the mass of the hydrated
and dried samples, ρ
w
and ρ
p
are the density of water
and polymers at a dried state respectively. The
relative density of the CA polymers used is 1.31
g/cm
3
according to the reagent instructions.
Mechanical properties (e.g., tensile strength) of
the CA membranes and modified nano-SiO
2
CA
membranes were measured using a universal tensile
testing machine (AGS-J, Shimazu, Japan). Every
membrane sample was dried under vacuum for 24h
before measurement. The elongation velocity was 2
mm/min with an initial gauge length of 15 mm.
2.4 Performance Testing
The prepared membranes were tested in FO mode
using a test cell with 30.25cm
2
effective area (width,
6.5 cm; length, 6.5 cm). Either feed solution or draw
solution was kept in a 2L tank. Both solutions were
circulated at a rate of 15 L·h
−1
in a closed loop by
using two diaphragm pumps (PLD-2203, China).
The draw solution was placed on a weighing balance
and the feed solution was placed on a platform at the
same height to eliminate any gravitational effects.
Feed solution was Purified water from a Milli-Q
system(18M Ω cm) and draw solution was 1M
sodium chloride (NaCl). Both of the volume of the
solutions was 1L. Membrane performances were
assessed by measuring permeate water flux (J
w
) and
reverse salt flux (J
s
).
The permeate water flux (J
w
) was calculated
through the increment of the weight of the draw
solution because of the water permeated over the
membrane during the FO testing. The change of the
weight was obtained by the weighting balance which
the draw solution was placed on. Corresponding, the
feed solution was put on a platform at the same
height to eliminate any gravitational effects. The
calculation equation of water flux was as follows.
Where is the change of the weight of the
draw solution, is the water density, Am is the
effective membrane area and is the time of
testing.
RSF (reverse salt flux) was defined as the mass
of NaCl diffusing from the draw solution to the feed
solution per unit time per unit membrane area during
FO testing. The mass of NaCl was obtained through
the concentration of chloride ions in each solution
which was determined by ion chromatography. 10
mL samples of both solutions were collected for
determination before and after the testing. The
calculation equation of RSF was as follows:
()
(
2
w
w
J /h or LMH2)
Am t
ρ
Δ
=⋅
××Δ
lm
wΔ
ρ
tΔ
2
s-NaCl
w
J = (g/m h) (3)
Am t
⋅
×Δ
Preparation of Nano-silica Dioxide Modified Cellulose Acetate (CA) Membranes for Enhanced Performance in Forward Osmosis Process
165
Where w is the mass of NaCl, Am is the
effective membrane area and is the time of
testing.
The membrane performances were tested in AL-
FS (active layer facing feed solution) orientation.
Data were recorded after running of the system for 5
min to stabilize water flux and the testing time was 1
h. All the tests were conducted at 25℃and repeated
three times to minimize experimental error.
3 RESULTS AND DISCUSSIONS
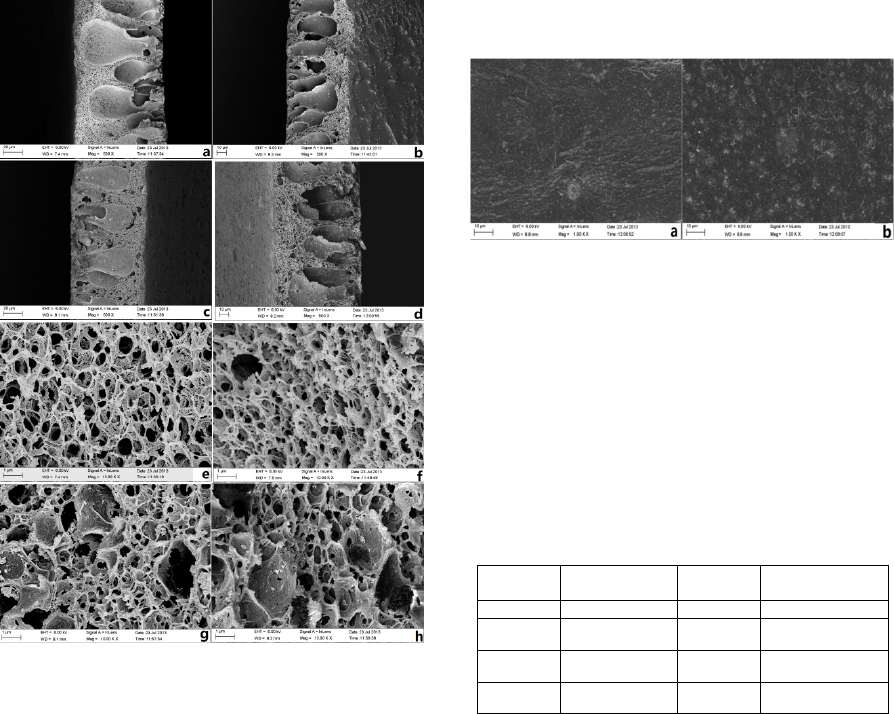
3.1 FO Membrane Morphologies
Figure 1: SEM cross sectional micrographs of CA
membranes prepared with: (a) 0 wt. % nano-SiO
2
, (b) 2
wt. % nano-SiO
2
, (c) 4 wt. % nano-SiO
2
and (d) 6 wt. %
nano-SiO
2
and amplified SEM cross sectional micrographs
of CA membranes prepared with: (e) 0 wt. % nano-SiO
2
,
(f) 2 wt. % nano-SiO
2
, (g) 4 wt. % nano-SiO
2
and (h) 6
wt. % nano-SiO
2
。
In the text, Figure 1 (a) to Figure 1 (d) shows the
cross-section morphology of CA membranes
prepared with different content of nano-SiO
2
in the
casting solution. The SEM images indicated that the
asymmetric structure of the membranes was not
changed by the presence of nano-SiO
2
. All the
membranes show the asymmetric structure of
compact cortical layer and porous support player
containing the finger-like structures. The Figure 1
(e) to Figure 1 (h) shows the amplified images of the
finger-like structure of the membranes and show that
the large pores appear gradually on the surface of the
support layer increase with the increment of the
content of nano-SiO
2.
The reason is that the addition
of nano-SiO
2
delays the process of the phase
separation during the preparation of membranes,
which plays an important effect on the membrane
structure.
Figure 2: SEM micrographs displaying the top surfaces of
CA membranes prepared with: (a) 0 wt. % nano-SiO2 and
(b) 2 wt. % nano- SiO2.
Figure 2 shows the surface morphologies of CA
membrane (a) and modified CA membranes
prepared with 6wt. % nano-SiO
2
in the casting
solution (b). The particles can be clearly seen on the
surface of the membrane when the nano-SiO
2
are
added in the casting solution.
Table 1: Contact angle, porosity and mechanical strength
of prepared FO membranes.
Membrane Contact angle (°) Porosity (%) Tensile strength
(MPa)
CA 67.1±0.72 47.52 6.17
CAN-2 54.1±0.83 52.20 7.79
CAN-4 49.3±0.21 57.57 8.63
CAN-6 43.6±0.54 61.64 9.22
Table 1 indicates the surface contact angles,
porosity and tensile strength of CA membranes
prepared with different content of nano-SiO
2
(0, 2, 4
and 6 wt. %). The contact angle of membranes
tΔ
IWEG 2018 - International Workshop on Environment and Geoscience
166
declined with the increase of the content of nano-
SiO
2
, suggesting that the hydrophilicity of CA
membrane surface was improved with the increment
of the content of nano-SiO
2
. This is due to the
silicon hydroxyl groups on the membrane structure
by the presence of nano-SiO
2
in the CA polymer
system. Meanwhile, the porosity of membranes
increased with the increase of the content of nano-
SiO
2
. This is because pores were formed when the
nano-SiO
2
added in the casting solution filtered out
of the main body of the membrane during the phase
inversion (
Lin et al., 2012). The tensile strength of
the membranes was also improved with the increase
of the content of nano-SiO
2.
3.2 ATR-FTIR Spectra and EDS of CA
and Nano-SiO
2
Modified CA
Membranes
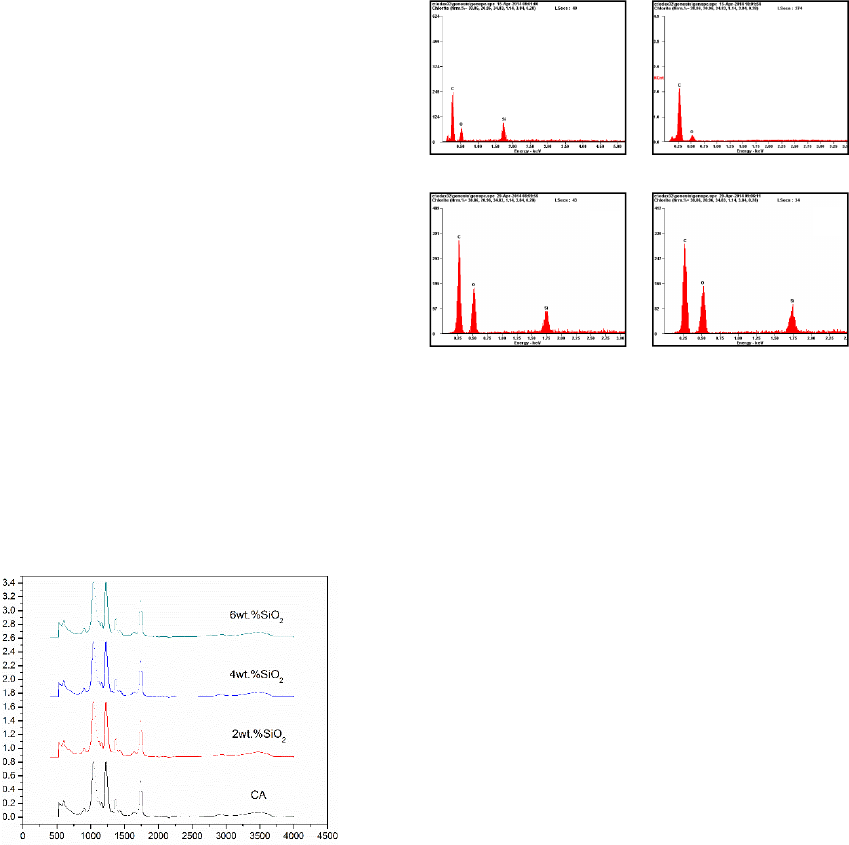
Figure 3 shows the ATR FT-IR spectra of the CA
membranes prepared with different content of nano-
SiO
2
(0, 2, 4 and 6 wt. %). The appearance of peaks
at 1739cm
-1
corresponds to the carbonyl (C=O) of
CA. The appearance of peaks at 1040 cm
-1
and 1228
cm
-1
is assigned to the ether group (C-O-C) of CA.
The appearance of peaks at 3466 cm
-1
corresponds to
the hydroxyl (-OH). There are no characteristic
peaks in relation to silica oxygen group (Si-O-Si)
and silicon carbon group (Si-O-C). It is also
suggested that there is no silicon hydroxyl group (Si-
OH) since the intensity of the peak of hydroxyl (-OH)
is weak.
Figure 3: ATR FT-IR spectra of the CA membranes
prepared with different content of nano-SiO2 (0, 2, 4 and 6
wt. %).
Figure 4 shows the EDS of the CA membranes prepared
with different content of nano-SiO2 (0, 2, 4 and 6 wt. %).
It clearly demonstrates that silicon element exists in the
membrane after modification by nano-SiO2. In addition,
the intensity of the peak of silicon in the EDS increases
with the increase of the content of nano-SiO2. With the
FT-IR results, it can be concluded that nano-SiO2 did not
react with CA to form a new chemical bond. The addition
of nano-SiO2 plays a role of physical blending and nano-
SiO2 consists in the CA organic system as an additive.
Figure 4: EDS of the CA membranes prepared with
different content of nano-SiO2 (0, 2, 4 and 6 wt. %).
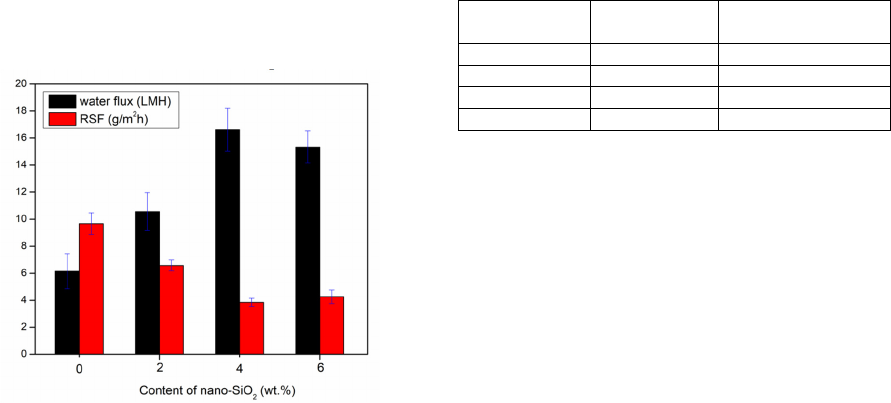
3.3 Determination of FO Performance
Figure 5 shows the FO performance of CA
membranes prepared with different content of nano-
SiO
2
(0, 2, 4 and 6 wt. %) including water flux,
reverse salt flux and salt rejection and the detailed
data is listed in Table 2. It is worthwhile to note that
the nano-SiO
2
modified CA membranes for FO
exhibits superior separation properties than
unmodified CA membrane. The water permeability
and salt rejection were improved with the increase of
the content of nano-SiO
2
in the CA membrane
structure in the range of 0–4 wt. %. When the
content of nano-SiO
2
increased from 0 wt. % to 4
wt. %, the water flux was improved from 6.15 LMH
to 16.62 LMH and the reverse salt flux declined
from 9.67 to 3.85 g NaCl/m
2
h. The improvement in
water flux of nano-SiO
2
modified CA membranes
could be attributed to the existence of nano-SiO
2
that
forms nanochannels in the top surface of the
membranes. Both inner cores of nanotubes (internal
nanochannels) and the interfacial gap between nano-
SiO
2
and CA polymer at the interface of CA layer
(external nanochannels) create additional passage for
solvent transfer. The latter plays a dominant role
a b
c
d
Preparation of Nano-silica Dioxide Modified Cellulose Acetate (CA) Membranes for Enhanced Performance in Forward Osmosis Process
167
since the internal nanochannels are too thin to
extract water without applying pressure (FO mode).
Moreover, the external nanochannels create direct
and appropriate path in comparison with the inner
cores (
Ma et al., 2012). Also, the presence of nano-
SiO
2
increases the surface hydrophilicity of FO
membranes, which causes improvement of the water
permeation through the modified FO membranes.
The higher salt rejection is caused by the
improvement in surface hydrophilicity, which
promotes the water preferential adsorbing and
decreases reverse salt flux. Reverse salt flux is
proportional to the salt rejection coefficient R (
Cath
et al., 2006
) and the lower reverse salt flux
represents the higher salt rejection (
Ng et al., 2006).
It is worthwhile to note that both of the water flux
and reverse salt flux of the modified membrane
decrease when the content of nano-SiO
2
further
increases from 4 wt. % to 6 wt. %, which might be
attributed to the defect of nano-SiO
2
agglomeration
in the phase inversion process. Hence, the excessive
addition of nano-SiO
2
may not be advisable for FO
applications. In summary, these results specified that
the nano-SiO
2
modified CA membranes exhibit
improved salt rejection as well as water permeability
than unmodified membrane in FO process.
Figure 5: Water flux and reverse salt flux of the CA
membranes prepared with different content of nano-SiO2.
4 CONCLUSIONS
Nano silica dioxide (nano-SiO
2
) modified cellulose
acetate (CA) membranes were prepared. The FO
performance of the modified membranes were
evaluated and compared with those membranes
without nano-SiO
2
. The addition of nano-SiO
2
does
not change the asymmetric structure of CA
membrane. The hydrophilicity, porosity and tensile
strength of the membranes were improved with the
increase of the content of nano-SiO
2
in the CA
polymer system. The nano-SiO
2
did not react with
the CA but consisted in the CA organic system as an
additive according to the FTIR results. The smart
selection of the nano-SiO
2
in order to use in FO
membranes structure caused the formation of
membranes with sufficient separation properties and
FO performance. The water flux and salt rejection
were simultaneously improved in the nano-SiO
2
modified CA FO membranes. However, excessive
addition of nano-SiO
2
might decreased the
permeability and selectivity of the membranes.
Finally, it can be concluded that nano-SiO
2
modified
CA membranes have potential for practical FO
application as a result of their enhanced structural
and separation properties.
Table 2: Water flux and reverse salt flux of prepared FO
membranes.
membrane Water flux J
w
(LMH)
Reverse salt flux J
s
-
N
aCl (g NaCl/m
2
h)
CA 6.15 9.67
CAN-2 10.56 6.58
CAN-4 16.62 3.85
CAN-6 15.34 4.26
REFERENCES
Achilli A, Cath T Y and Childress A E 2009 Power
generation with pressure retarded osmosis: an
experimental and theoretical investigation, Journal of
Membrane Science 343 42–52
Cath T Y, Childress A E and Elimelech M 2006 Forward
osmosis: principles, applications, and recent
developments, Journal of Membrane Science 281 70–
87
Kim T-W, Kim Y, Yun C, Jang H, Kim W and Park S
2012 Systematic approach for draw solute selection
and optimal system design for forward osmosis
desalination, Desalination 284 253–260
Lin W, Zhu T, Li Q, Yi S and Li Y 2012 Study of
pervaporation for dehydration of caprolactam through
PVA/nano silica composite membranes, Desalination
285 39–45
Liu Y-L, Hsu C-Y, Su Y-H, et al. 2004 Chitosan−silica
complex membranes from sulfonic acid functionalized
silica nanoparticles for pervaporation dehydration of
ethanol−water solutions, Biomacromolecules 6(1) 368-
373
IWEG 2018 - International Workshop on Environment and Geoscience
168
Ma H, Burger C, Hsiao B S and Chu B 2012 Highly
permeable polymer membranes containing directed
channels for water purification, ACS Macro Letters
1723–726
McCutcheon J R and Elimelech M 2006 Influence of
Concentrative and Dilutive Internal Concentration
Polarization on Flux Behavior in Forward Osmosis.
Journal of Membrane Science 284(1-2) 237–247
McCutcheon J R, McGinnis R L and Elimelech M 2005 A
novel ammonia–carbon dioxide forward (direct)
osmosis desalination process, Desalination 174 1–11.
Ng H Y, Tang W, Wong W S 2006 Performance of
forward (direct) osmosis process: membrane structure
and transport phenomenon, Environmental Science &
Technology 40 2408–2413.
PhuongNgaNguyen Thi, Eun-TaeYun, In-ChulKim,
Young-NamKwon 2013 Preparation of cellulose
triacetate/cellulose acetate (CTA/CA)-based
membranes for forward osmosis, Journal of Membrane
Science 433 49–59
Sairam M, Sereewatthanawut E, Li K, Bismarck A and
Livingston A G 2011 Method for the preparation of
cellulose acetate flat sheet composite membranes for
forward osmosis—Desalination using MgSO4 draw
solution, Desalination 273 299–307
Shuaifei Zhaoa, Linda Zoua, Chuyang Y. Tangb and
Dennis Mulcahya 2012 Recent developments in
forward osmosis: Opportunities and challenges,
Journal of Membrane Science 396 1– 21
Smitha B, Suhanya D, Sridhar S, et al. 2004 Separation of
organic–organic mixtures by pervaporation—a review,
Journal of Membrane Science 241(1) 1-21
Wang K Y, Ong R C and Chung T-S 2010 Double-
skinned forward osmosis membranes for reducing
internal concentration polarization within the porous
sublayer, Industrial & Engineering Chemistry
Research 49 4824–4831
Wang R, Shi L, Tang C Y, Chou S, Qiu C, Fane A G 2010
Characterization of novel forward osmosis hollow fiber
membranes, Journal of Membrane Science 355 158–
167
Zhang S, Wang K Y, Chung T-S, Chen H, Jean Y C, Amy
G 2010 Well-constructed cellulose acetate membranes
for forward osmosis: minimized internal concentration
polarization with an ultra-thin selective layer, Journal
of Membrane Science 360 522–535
Preparation of Nano-silica Dioxide Modified Cellulose Acetate (CA) Membranes for Enhanced Performance in Forward Osmosis Process
169
