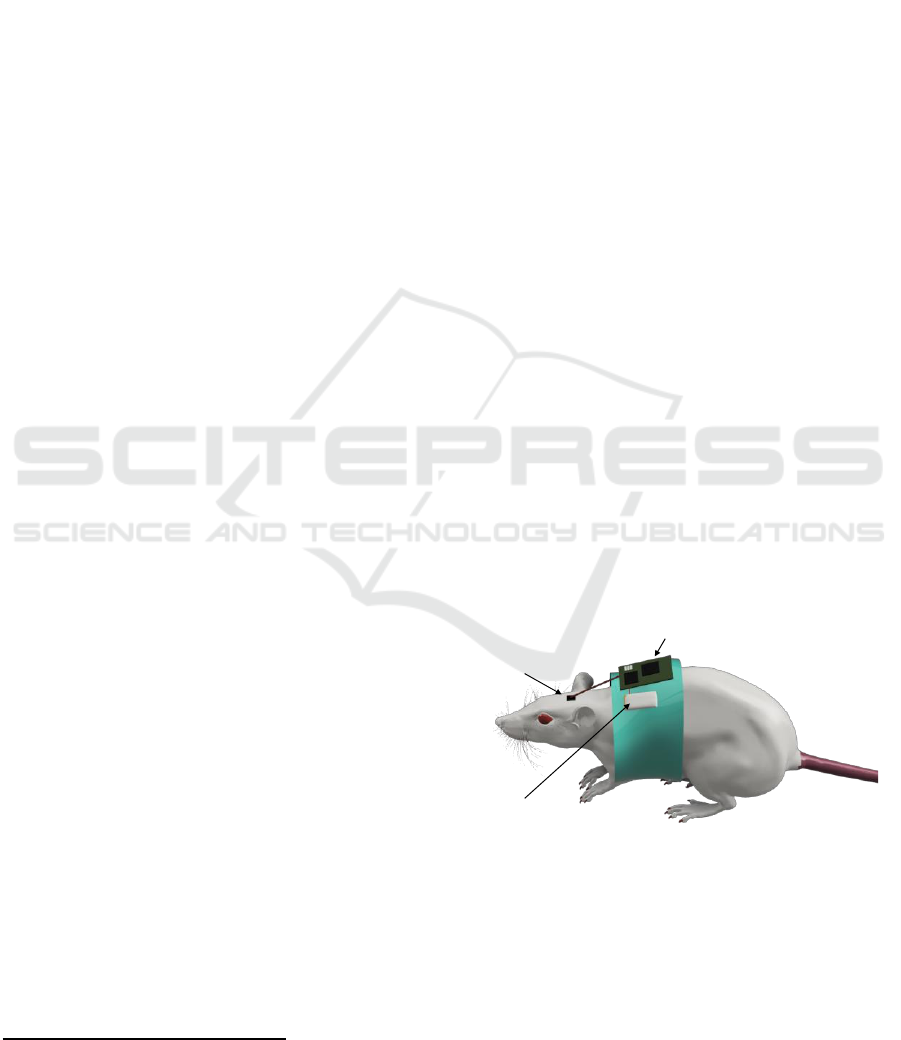
Wireless Thermal Neuromodulator for Long-term in Vivo Cooling
Performance Assessment
A. M. Miranda
*
, C. Silva
*
, V. Silva
*
and P. M. Mendes
CMEMS, University of Minho, 4800-058 Guimarães, Portugal
Keywords: Implantable Device, Thermal Neuromodulator, Biomedical Wireless Device, Focal Cooling.
Abstract: Focal cooling is considered a potential solution to stop or control epileptic activity. However, despite the
available proof of concept studies, such approach requires further validation before being used in humans.
One hindering factor is the lack of suitable devices to enable large-scale validation of such methodology. This
paper presents a wireless thermal neuromodulator that can wirelessly record the rat’s brain electrical activity
and temperature. At the same time, the temperature is reduced without the need to use cumbersome liquid
pipes. The proposed device has two modules: one headstage with a cooler and sensors, and one backpack with
acquisition electronics and wireless communication capability. It is possible to record the brain temperature,
the EEG at 16 kbps, and to control the cooler’s temperature, with an autonomy of 1 day.
1 INTRODUCTION
Thermal neuromodulation has been proposed as a
solution to handle medication resistant neurological
disorders, being epilepsy one of such diseases. It has
been demonstrated that focal cooling is a potential
solution to stop epileptic activity (Fujii, 2010).
Despite such validation in controlling neuronal
activity, and before becoming an effective solution,
further tests are required to demonstrate the large
scale and long-term efficacy of focal cooling on
epilepsy control. However, the available cooling
devices are not suitable to perform the required large
scale, long-term testing in vivo. Such tests may be
performed using rats and will require the
development of a suitable device to control the rats’
brain temperature in specific spots (Fernandes, 2018).
To be placed on a rats’ brain, the cooler must satisfy
size and biocompatibility constraints (Mata, 2005).
To be transported by the rodent, the system must be
wireless and must not be oversized or overweighed.
In this paper, the design of a wireless, small, low-
power thermal neuromodulator is presented in order
to reduce the rodent’s brain temperature, while EEG,
and focus temperature is being recorded.
*
These authors contributed equally to this paper
2 COOLER ARCHITECTURE
The proposed cooling device uses two modules to
reduce at maximum the weight and volume on top of
the rats’ head. Placed in contact with the brain, is the
thermal neuromodulator. This component is
connected to the unit of acquisition which is inserted
in a bag on the rat’s back. Fig.1 shows the proposed
system architecture.
Figure 1: Wireless neuromodulator system overview.
The two modules were designed taking into
account their specific constraints. Their design will be
explained next.
THERMAL
ACTUATOR
ELECTRONICS
BATTERY
Miranda, A., Silva, C., Silva, V. and Mendes, P.
Wireless Thermal Neuromodulator for Long-term in Vivo Cooling Performance Assessment.
DOI: 10.5220/0006936100670072
In Proceedings of the 6th International Congress on Neurotechnology, Electronics and Informatics (NEUROTECHNIX 2018), pages 67-72
ISBN: 978-989-758-326-1
Copyright © 2018 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
67

3 ACQUISITION AND CONTROL
SYSTEM
The acquisition and control system on the rats’ back
should communicate wirelessly with the outside
world using a low power solution. The proposed
device is shown below, in Fig.2.
Figure 2: Block diagram of the proposed system.
The system has two fundamental parts. The first
one, placed on the freely moving rat, and the second
one attached to the PC. The rats’ module integrates a
small and thin battery, a Bluetooth 5 (BL5) module
(ISP1507), a low-power electrophysiology signal
acquisition chip (RHD2216) and a Peltier module
used to cool down the epileptic focus.
Since it must sit on the back of a rat, this device
must be lightweight, and have a small volume, so it
will not become uncomfortable for the rodent. If so,
the animal will try to remove the device with its paws.
According to literature, a rat heavier than 250 g (an
adult rodent often is heavier than this) can carry 60 g
on its back and move freely without any
inconvenience on locomotion or motivation
(Hampson, 2009). Hence, the rat can have, positioned
on its back, a board with a size of 2.5x5 cm
2
without
any disturbance. Nevertheless, the size and the weight
should be reduced to the maximum possible
(Ativanichayaphong, 2008).
Our device will have near 10 g and will occupy a
volume of 4 x 4 x 0.3 cm
3
.
The second part uses also a BL5 module, which
will be connected to the PC to record and process the
data sent wirelessly from the rats’ device.
BL5 was selected, since it offers improvements in
numbers of bytes that can be sent in each connection,
allowing higher speed without compromising energy
efficiency (DiMarco, 2017). The Bluetooth 5 module
ISP1507 consumes only 7,5 mA on tx with 4 dBm or
5,3 mA with 0 dBm and 5,4 mA when working on rx.
It allows, also, for better range. BL5 grants a data
packet to be sent at a rate of 2 Mbps between 2
devices and communication in a range of 200 m
outdoors and 40m indoors (Collotta, 2017). However,
the ISP1507 reports only 100 m free open space
which is more than necessary for this application
since we only need a range of 3 to 5 meters for the
system to work correctly.
The main disadvantage of using this type of
communication standard is the difficulty to achieve
high data rate communications. However, since we
want to record an EEG at sampling rates between 250
and 2000 Hz the data rate is enough. Since we have
an 8-bit resolution ADC, we only need a data rate of
8*(sampling rate) bits per second (bps). For instance,
if we select a sampling rate of 2000 Hz, the data rate
of 16 kbps will be feasible with BL5.
The ISP1507 is from Insight Sip and it is based on
nRF52832 integrated with decoupling and loading
capacitors, 2 crystals (32 MHz and 32,768 kHz) and
a RF matching circuit and antenna. The integration of
the antenna is important, since it allows for full
system size reduction (8x8x1 mm
3
).
3.1 System Main Features
The implemented device allows to record both the
temperature and the EEG, and to control Peltier
temperature. Since the device runs on batteries, it also
sends the battery levels to recharge or to replace it.
Temperature and EEG will be described next with
more details.
3.1.1 Temperature Control
When desired, a command can be sent from the PC to
the device, to turn on the Peltier positioned on the
rat’s brain. It activates one PWM unit that digitally
encodes an analog signal level. This modulation
technique uses a square wave in which the time the
signal is on (high) or off (low) can be controlled. To
characterize the amount of time the signal is on we
can set the duty cycle to the percentage aimed. For
instance, if the duty cycle is set to 75%, then the
output from the PWM would be a square wave with
high voltage 75% of the time. If, for example, the
supply is 3 V, the resulting analog signal would be of
2.25 V (Barr, 2001). The frequency of the square
wave can also be controlled. Finally, since the output
current from the PWM generated by the chip is low
and not enough to power the peltier, it was amplified
using a Darlington pair connected between the PWM
and the Peltier. A Darlington pair acts as a single
transistor and generates a high current gain. This way,
the Peltier can be turned on and off wirelessly using
the BL5 module.
BATTERY LEVEL
EEG SIGNAL
PWM
(DUTTYCYCLE
FREQUENCY)
BATTERY
NRF52832
RHD2216
PELTIER
DARLINGTONPAIR
ELECTRODES
RAT
NEUROTECHNIX 2018 - 6th International Congress on Neurotechnology, Electronics and Informatics
68

3.1.2 EEG Acquisition
To detect when the rat is having a seizure, its
frequency and duration, it is necessary to record its
electroencephalogram (EEG). This is done using 3
electrodes placed on its cortical surface. These
electrodes will be connected to the electrophysiology
chip on the device. The selected chip was the
RHD2216 from Intan Technologies. It integrates 16
amplifiers, analog and digital filters and a
multiplexed analog-to-digital converter (ADC). Its
miniature size (4.8x4.1 mm
3
) and low-power make it
ideal for this application. It can sample 16 differential
amplified channels at a maximum data rate of
30 kSamples/s each.
This chip communicates with the BL5 module
over a digital Serial Peripheral Interface bus (SPI).
Each digital signal sent over this bus is transmitted on
a single wire. So, this communication consists of 4
standard signals. An active-low chip select (𝐶𝑆
̅
̅
̅
̅
) and
a serial data clock (SCKL) provided by the BL5
module (master device); a MOSI (master out, slave
in) data line in which a 16-bit command word flows
from the BL5 module to the Intan chip and a MISO
(master in, slave out) data line where the response
from the Intan chip (also a 16-bit word) flows to the
BL5 module.
3.2 Software Description
There are two possible network configurations in BL5
specification: connection (bidirectional
communication), and broadcast (unidirectional
communication). Since we need both BL5 modules to
communicate with each other, a connection topology
will be implemented.
In connection mode, a peripheral device sends
advertising packets. The central device receives the
advertisement and accepts the connection. Once this
is established, the peripheral stops advertising and
both devices start trading data (Collotta, 2017).
The peripheral’s data can be classified in services
and characteristics. The service is a collection of
characteristics describing a function of the peripheral.
A peripheral can have multiples, or only one service,
and a service can, also, have only one or multiple
characteristics. Each service and characteristic uses
an UUID (universally unique identifier) to identify
itself.
Only two services were created on the peripheral
device, the Biosignals service and the Battery service.
The Biosignals service has the EEG level
characteristic and the Temperature characteristic. The
Battery service has the Battery level characteristic.
The first one is responsible of sending both the data
acquired with the Intan chip and the temperature
values and receiving the configuration of the PWM.
The second one sends a notification of low battery
when its levels reach 2V.
On the central device it was created the service
PWM with the characteristic PWM values. This
characteristic is responsible for sending the frequency
and the duty cycle for the PWM. These values are set
on the PC side and the sent over BL5.
The peripheral device will send the data as a
notification which the central device will be able to
read immediately as it changes (Hortelano, 2017).
This attribute data has a Maximum Transmission Unit
of 247 bytes, i.e., for each notification, 247 bytes are
transferred from the peripheral to the central device.
After a connection between both BL5 modules is
established, the BL5 module connected to the PC
receives the data from the rat and sends it to the PC
using the universal asynchronous receiver-transmitter
(UART).
4 MICROCOOLER DESING
The previous sections describe the system’s overall
concept, as well as the control hardware. This section
will explain the cooler and heatsink design process.
To be able to perform the required thermal
neuromodulation the cooler must guarantee that the
neuronal cells temperature does not exceed 43 ºC, to
ensure cellular integrity (Yarmolenko, 2011). On the
other hand, the cold side must reach temperatures
lower than 30 ºC to suppress epileptic seizures.
Hou et al. (2011) present the project and
development of a thermal neuromodulator to epilepsy
control. In this work, the chip permits the EEG
acquisition so that the epilepsy event can be detected
and the neuromodulator can actuate and suppress it.
The chip’s dimensions are 1.4 x 0.95 mm
2
. So, we can
deduce the possible dimensions of an implantable
device on the brain.
So that the cooling in situ is able to interfere with
the cerebral activity a solid-state cooler using a Peltier
component (Micropelt MPC-D403) was selected. The
device is only 2x2x1 mm
3
in volume and allows to
reach a net cooling up to ~50 K, depending on current
and heat loading.
4.1 Heatsink and Packaging Design
It is important to refer that once the device is
implanted in the brain, it is in direct contact with the
neuronal cells. Thus, triggering an electric current in
Wireless Thermal Neuromodulator for Long-term in Vivo Cooling Performance Assessment
69
the Peltier results in a temperature gradient being
generated between the cold and the hot sides of the
Peltier device. Therefore, the cold side enables neural
cooling while the hot side is responsible for
dissipating the heat that is being generated to cool
down the brain.
However, it is necessary to control the
temperatures reached at the hot side of the Peltier, to
ensure the thermal modulation of the brain does not
induce irreversible damage to the cells near the hot
plate. Moreover, the continuous increase of the
temperature on the Peltier's hot side causes a
consequent increase in the temperatures reached at
the cold end after a certain time, due to the Joule
effect. So, it is necessary to include in the heat
modulating device a heatsink that needs to be in direct
contact with the hot end side of the Peltier. The reason
to choose aluminum as the heatsink was because,
despite having a lower thermal conductivity than
copper, it is a viable and a low-cost alternative in the
manufacture of heatsinks.
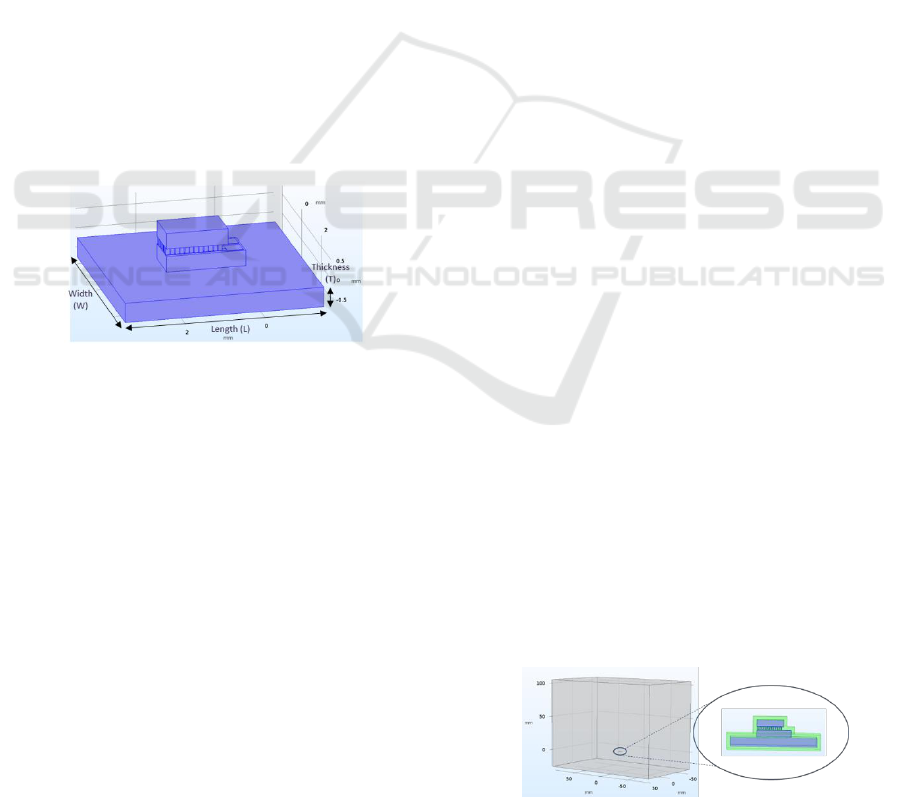
For heatsink design, shown in Fig.3, special
constraints were considered: the volume required to
efficiently dissipate the heat being generated; the
need to miniaturize the device dimensions without
compromising heat dissipation.
Figure 3: Model used to simulate the microcooler (Peltier
and heatsink).
Is also important to ensure the safety and viability
of the implantable device. To do so, it is important to
meet certain requirements, namely concerning its
biocompatibility. Biocompatibility is an essential
feature that ensures the device’s long-term
implantation, avoiding the induction of irreversible
damage. Therefore, the device was isolated with a
biocompatible material. Several options like flexible
polymer substrates such as polydimethylsiloxane
(PDMS), polyimide (PI) or parylene (Patil, 2016),
ensure that the device can be embedded without
compromising its structure or mode of operation.
Among the options presented, PDMS is a silicone
elastomer with excellent physicochemical properties
that make it an attractive and promising material in
the development of MEMS and numerous
components in biomedical applications. PDMS has
been traditionally used as a biomaterial in catheters
and other drainage tubes, ear and nose implants and
also as insulation material in pacemakers (Mata,
2005).
In addition, PDMS handling and manipulation is
rather easy and since it is a non-toxic and
biocompatible material it can be implanted in vivo.
Also, its elastic properties permit this polymer to
recover its initial shape after undergoing long cycles
of deformation, with the particularity of standing
strong strain deformations before breaking.
Concerning thermal properties, this material is
thermally stable, acting as a thermal insulation
(thermal conductivity of 0.2 W/mK), since it does not
allow resistive heat dissipation (Mata, 2005).
Hence, in order to simulate the PDMS embedding
device, a tri-dimensional CAD model for the PDMS
structure was planned and designed.
This structure, besides protecting the cold side of
the Peltier, which is in direct contact with brain cells,
also fills the spaces between the Peltier tellurium
bismuth pellets and covers the hot side plate of the
PDMS that is not in direct contact with heatsink.
Furthermore, since the heatsink is also coated with
PDMS, the PDMS structure should be adapted to the
dimensions and shape of the heatsink.
In the next set of tests, the PDMS thickness
relationship on the thermal performance of the device
was subsequently tested (Mata, 2005).
4.2 Temperature Control Modelling
To understand how the brain temperature changes
with the control current it was necessary to model the
cooling system behavior. Such behavior will be
highly dependent on the heatsink and its dimensions.
The temperature control modelling was studied using
COMSOL, which allows modelling all systems
previously described
A tri-dimensional block of 180x110x130 mm
3
was used to model the brain, since studies state that
the human brain volume ranges from 650 cm
3
to 1260
cm
3
(Lahr, 2004). Thus, a parallelepiped with the
reported dimensions was designed and the previously
presented Peltier device was included inside,
simulating an implantable neuronal device, as can be
seen in Fig. 4.
Figure 4: Simulated brain block with implanted device.
NEUROTECHNIX 2018 - 6th International Congress on Neurotechnology, Electronics and Informatics
70
The Peltier device was simulated considering the
materials that compose the semiconductor pellets
(bismuth telluride Bi
2
Te
3
), the conductive bond-pads
(copper Cu) and the materials that compose the cold
and hot sides of the plates of the device (alumina or
aluminum oxide Al
2
O
3
) above mentioned.
4.3 Heatsink and PDMS Cover
Performance Assessment
In this section are presented simulation results for
several heatsink dimensions and PDMS thicknesses.
Initially, it was only simulated the Peltier device
and the heatsink when implanted inside the brain. The
main goal of this study is to find the minimum
dimensions of heatsink that allow maximum heating
dissipation without the temperature in cerebral cells
being higher than the threshold of 43 °C. In order to
analyze the maximum temperature (T
max
) and the
minimum temperature (T
min
) resulting from heat
transfer between the device and the brain, different
combinations of L, W and T values (L, W, T) were
considered for the heatsink dimensions. These form
the parallelepiped seen in table 1.
The simulation time was set to 20 minutes, where
only from 10 s<t≤1200 s was applied a 50 mA
current. Furthermore, it should be noted that the
initial temperature of the device considered for all
simulations (at t=0 s) was 37 °C. The reason to do it
so was since this temperature is the basal temperature
of the human body and the device is totally integrated
in the brain tissue.
From the simulation results for the 18 different
combinations tested, the following table was
obtained.
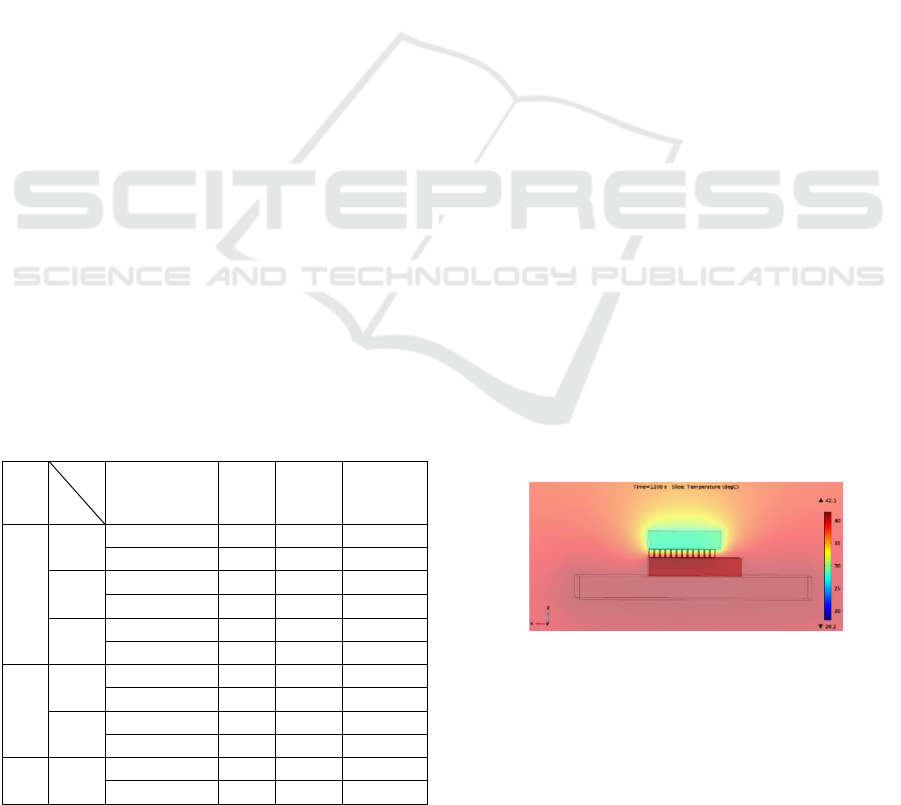
Table 1: Results of simulations with different dimensions
of heatsink (length unit is mm).
L
T
W
0.5
1
1.5
3
3
T
min
(°C)
30.4
29.8
29.3
T
max
(°C)
44.9
44.1
43.5
4
T
min
(°C)
29.7
29.1
28.7
T
max
(°C)
43.9
43.3
42.8
5
T
min
(°C)
29.1
28.7
28.3
T
max
(°C)
43.3
42.7
42.3
4
4
T
min
(°C)
29.0
28.6
28.3
T
max
(°C)
43.1
42.6
42.2
5
T
min
(°C)
28.6
28.2
27.9
T
max
(°C)
42.6
42.1
41.8
5
5
T
min
(°C)
28.2
27.9
27.6
T
max
(°C)
42.1
41.7
41.4
From the previous results, one can conclude that
increasing L and W values lead to a decrease in the
T
min
and T
max
values. These results went according to
what was expected, since a larger heatsink area
implies a greater efficiency when heat dissipation is
occurring and, consequently, the temperatures
recorded will be lower.
Regarding the thickness relationship, it has been
observed that an increase in the thickness of the
heatsink (T), for fixed values of L and W, results in
decreasing temperature values for T
min
and T
max
, as
expected, since larger thickness values lead to a
higher volume of heat dissipation. Concluding, the
larger the heatsink (5x5x1.5 mm
3
), the lower the
reached temperature is.
However, since it is desirable to miniaturize the
device, the smallest heatsink should be used, while
still respecting the requirements for T
min
and T
max
temperatures that do not compromise the integrity of
neuronal cells. Since the device behavior is being
simulated, it is advisable to ensure a margin of safety
for the maximum temperature that can be reached
with respect to the critical temperature, T
max
, of 43 °C.
It was, therefore, chosen a safety margin of
approximately 1 ºC.
By observing the simulation results presented in
Table 1, it is possible to conclude that 5x5x0.5 mm
3
heatsink results in a T
max
of 42.1°C. Compared with
other feasible dimensions that achieved
approximately the same critical temperature, these are
the dimensions for which the volume of heat
dissipation is lower and therefore were the chosen
dimensions for the heatsink.
In Fig. 5, it is possible to observe the temperature
profile of the device being tested for the chosen
heatsink dimensions. In the coloring bar presented at
the right side of the image are the maximum and
minimum temperatures registered after the simulation
has been performed.
Figure 5: Temperature profile of the device with the
5x5x0.5 mm
3
heatsink.
The 5x5x0.5 mm
3
heatsink was tested with
different PDMS thicknesses to analyze the influence
of the referred thicknesses in achieved temperatures.
Followingly are presented the device’s temperature
Wireless Thermal Neuromodulator for Long-term in Vivo Cooling Performance Assessment
71

profiles for PDMS thicknesses of 20, 50 and 100 µm,
respectively.
Figure 6: Temperature profile of the device with the
5x5x0.5 mm
3
heatsink for PDMS thicknesses: a) 20 µm, b)
50 µm, c) 100 µm.
It was observed that increasing PDMS thickness
results in an increase T
max
, which is explained by the
thermal insulation behavior of PDMS. It was also
noted that T
min
value decreased with increasing
PDMS thickness. However, lower T
min
values was
observed specially on the cold plate of Peltier and not
on the brain. It should also be referred that with the
increasing PDMS thickness the neuronal cells cooling
region is closer to the Peltier’s cold end.
It is also noteworthy that, ideally, the thinnest
PDMS thickness that guarantees biocompatibility is
desirable.
5 CONCLUSIONS
This work presents the design and implementation of
wireless thermal neuromodulator, small and light
enough to be used for long-term in-vivo testing on
rats. The proposed device records brain temperature,
EEG, and allows to control the current that will
switch the cooling element on and off. The electronics
were fully assembled and tested, while the heatsink
and cooler were fully designed and are undergoing
testing in laboratorial conditions.
ACKNOWLEDGEMENTS
This work is supported by Foundation for Science and
Technology (FCT) project PTDC/EEI-
TEL/5250/2014, by FEDER funds through POCI-01-
145-FEDER-16695 and Projecto 3599—Promover a
Produção Científica e Desenvolvimento Tecnológico
e a Constituição de Redes Temáticas.
REFERENCES
Fujii, M., Fujioka, H., Oku, T., Tanaka, N., Imoto, H.,
Maruta, Y., Nomura, S., Kajiwara, K., Saito, T.,
Yamakawa, T. and Yamakawa, T., 2010. Application
of focal cerebral cooling for the treatment of intractable
epilepsy.Neurologia medico-chirurgica, 50(9), pp.839-
844.
Fernandes, J., Vendramini, E., Miranda, A.M., Silva, C.,
Dinis, H., Coizet, V., David, O. and Mendes, P.M.,
2018. Design and Performance Assessment of a Solid-
State Microcooler for Thermal Neuromodulation.
Micromachines, 9(2), p.47.
Mata, A., Fleischman, A.J. and Roy, S., 2005.
Characterization of polydimethylsiloxane (PDMS)
properties for biomedical micro/nanosystems.
Biomedical microdevices, 7(4), pp.281-293.
Hampson, R.E., Collins, V. and Deadwyler, S.A., 2009. A
wireless recording system that utilizes Bluetooth
technology to transmit neural activity in freely moving
animals. Journal of neuroscience methods, 182(2),
pp.195-204.
Ativanichayaphong, T., He, J.W., Hagains, C.E., Peng,
Y.B. and Chiao, J.C., 2008. A combined wireless neural
stimulating and recording system for study of pain
processing. Journal of neuroscience methods, 170(1),
pp.25-34.
Di Marco, P., Skillermark, P., Larmo, A., Arvidson, P. and
Chirikov, R., 2017. Performance Evaluation of the Data
Transfer Modes in Bluetooth 5. IEEE Communications
Standards Magazine, 1(2), pp.92-97.
Collotta, M., Pau, G., Talty, T. and Tonguz, O.K., 2017.
Bluetooth 5: a concrete step forward towards the IoT.
arXiv preprint arXiv:1711.00257.
Barr, M., 2001. Pulse width modulation. Embedded
Systems Programming, 14(10), pp.103-104.
Hortelano, D., Olivares, T., Ruiz, M.C., Garrido-Hidalgo,
C. and López, V., 2017. From sensor networks to
internet of things. Bluetooth low energy, a standard for
this evolution. Sensors, 17(2), p.372.
Yarmolenko, P.S., Moon, E.J., Landon, C., Manzoor, A.,
Hochman, D.W., Viglianti, B.L. and Dewhirst, M.W.,
2011. Thresholds for thermal damage to normal tissues:
an update. International Journal of Hyperthermia,
27(4), pp.320-343.
Hou, K.C., Chang, C.W., Chiou, J.C., Huang, Y.H. and
Shaw, F.Z., 2011. Wireless and batteryless biomedical
microsystem for neural recording and epilepsy
suppression based on brain focal cooling. IET
nanobiotechnology, 5(4), pp.143-147.
Lahr, M.M. and Foley, R., 2004. Palaeoanthropology:
Human evolution writ small. Nature, 431(7012),
p.1043.
Patil, A.C. and Thakor, N.V., 2016. Implantable
neurotechnologies: a review of micro-and
nanoelectrodes for neural recording. Medical &
biological engineering & computing, 54(1), pp.23-44.
NEUROTECHNIX 2018 - 6th International Congress on Neurotechnology, Electronics and Informatics
72
