
A Grid Cell Inspired Model of Cortical Column Function
Jochen Kerdels and Gabriele Peters
FernUniversitt in Hagen – University of Hagen,
Human-Computer Interaction, Faculty of Mathematics and Computer Science,
Universit
¨
atsstrasse 1 , D-58097 Hagen, Germany
Keywords:
Cortical Column, Autoassociative Memory, Grid Cells, Attractor Dynamics.
Abstract:
The cortex of mammals has a distinct, low-level structure consisting of six horizontal layers that are vertically
connected by local groups of about 80 to 100 neurons forming so-called minicolumns. A well-known and
widely discussed hypothesis suggests that this regular structure may indicate that there could be a common
computational principle that governs the diverse functions performed by the cortex. However, no generally
accepted theory regarding such a common principle has been presented so far. In this position paper we
provide a novel perspective on a possible function of cortical columns. Based on our previous efforts to model
the behaviour of entorhinal grid cells we argue that a single cortical column can function as an independent,
autoassociative memory cell (AMC) that utilizes a sparse distributed encoding. We demonstrate the basic
operation of this AMC by a first set of preliminary simulation results.
1 INTRODUCTION
The mammalian cortex has a remarkably regular,
low-level structure. It is organized into six hori-
zontal layers, which are vertically connected by lo-
cal groups of about 80 to 100 neurons (in prima-
tes) that form so-called minicolumns. These minico-
lumns are in turn connected by local, short-range ho-
rizontal connections forming cortical macrocolumns
(sometimes referred to as just columns or modules)
in a self-similar fashion (Mountcastle, 1978; Moun-
tcastle, 1997; Buxhoeveden and Casanova, 2002).
This general pattern of organization into layers and
(mini)columns suggests that there might be a com-
mon computational principle that governs the diverse
functions performed by the cortex. Theories regar-
ding such a common principle cover a wide range of
putative mechanisms and models including, among
others, an updated version of the hierarchical tem-
poral memory (HTM) model (Hawkins et al., 2017),
which postulates that individual columns can learn
predictive models of entire objects by combining sen-
sory data with a location input that indicates the spa-
tial origin of the respective data; a sparse distributed
coding model (Rinkus, 2017) that relates the functi-
ons of macro- and minicolumns by postulating that
minicolumns enforce the sparsity of a sparse distri-
buted representation stored and recognized by indivi-
dual macrocolumns; or a cortical column model based
on predictability minimization (Hashmi and Lipasti,
2009). Whether any of the existing hypothesis regar-
ding the potential function of cortical columns per-
tains, or if there is any common computational prin-
ciple at all remains controversial (Horton and Adams,
2005).
In this paper we approach the question whether
the columnar structure of the cortex reflects a com-
mon computational principle by focusing on a puta-
tive core function of the cortical minicolumn that is
implemented by a specific subset of neurons while le-
aving the overall function of the entire minicolumn
unspecified for now. Given the canonical connecti-
vity of cortical principal cells shown in figure 1, we
argue that groups of layer 2 /3 and upper layer 5 neu-
rons (L2/3 PC and L5 ITN in Fig. 1) form a local, au-
toassociative memory that builds the core functiona-
lity of a minicolumn while all other neurons depicted
in figure 1 serve supporting functions, e.g., commu-
nication to subcerebral targets (L5 SPN), communica-
tion to long-range cortical targets (L6 CC), modula-
tion of sensory input or input from lower-order cortex
(L6 CT), or modulation of the autoassociative memory
itself (L4 PC). We base this hypothesis on earlier work
directed at modeling and understanding of grid cells
located in layer 2 /3 and layer 5 of the medial entor-
hinal cortex (Fyhn et al., 2004; Hafting et al., 2005).
While grid cells are commonly viewed as part of a
specialized system for navigation and orientation (Ro-
204
Kerdels, J. and Peters, G.
A Grid Cell Inspired Model of Cortical Column Function.
DOI: 10.5220/0006931502040210
In Proceedings of the 10th International Joint Conference on Computational Intelligence (IJCCI 2018), pages 204-210
ISBN: 978-989-758-327-8
Copyright © 2018 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
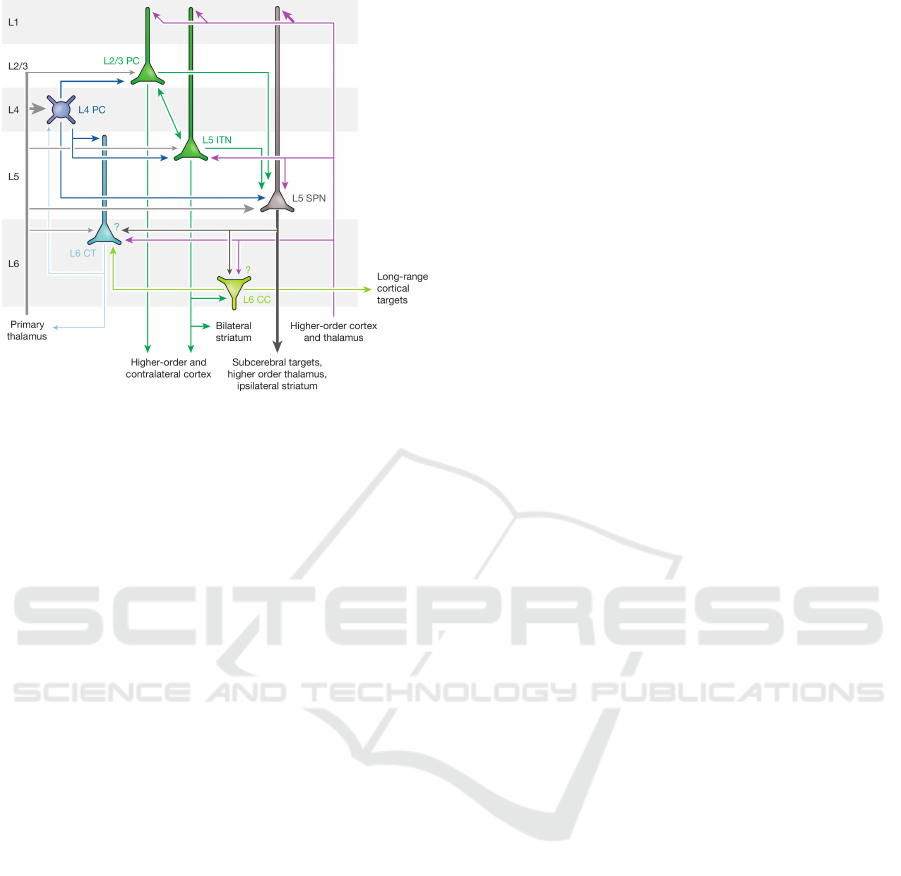
Figure 1: Canonical connectivity of cortical principal cells.
Reprinted with permission from (Harris and Mrsic-Flogel,
2013). PC: principal cell, ITN: intratelencephalic neuron,
SPN: subcerebral projection neuron, CT: corticothalamic
neuron, CC: corticocortical neuron. Line thickness repre-
sents the strength of a pathway, question marks indicate
connections that appear likely but have not yet been directly
shown.
wland et al., 2016), it can be demonstrated that their
behavior can also be interpreted as just one instance of
a more general information processing scheme (Ker-
dels and Peters, 2015; Kerdels, 2016). Here we ex-
tend this idea by generalizing and augmenting the pre-
vious grid cell model into an autoassociative memory
model describing the core function of a cortical mini-
column.
The next section revisits the grid cell model
(GCM) upon which the proposed autoassociative me-
mory model will be built. At its core the GCM is
based on the recursive growing neural gas (RGNG)
algorithm, which will also be a central component of
the new model. Section 3 introduces a first set of
changes to the original GCM on the individual neu-
ron level that improve the long-term stability of lear-
ned representations, and allow the differentiation be-
tween processes occurring in the proximal and distal
sections of the neuron’s dendritic tree. In section 4
these changes are then integrated into a full autoasso-
ciative memory model, which consists of two distinct
groups of neurons that interact reciprocally. Section 5
presents preliminary results of early simulations that
support the basic assumptions of the presented model.
Finally, section 6 discusses the prospects of the propo-
sed perspective on cortical minicolumns and outlines
our future research agenda in that regard.
2 RGNG-BASED GRID CELL
MODEL
Grid cells are neurons in the entorhinal cortex of the
mammalian brain whose individual activity correlates
strongly with periodic patterns of physical locations
in the animal’s environment (Fyhn et al., 2004; Haf-
ting et al., 2005; Rowland et al., 2016). This property
of grid cells makes them particularly suitable for ex-
perimental investigation and provides a rare view into
the behavior of cortical neurons in a higher-order part
of the brain. The most prevalent view on grid cells
so far interprets their behavior as part of a specialized
system for navigation and orientation (Rowland et al.,
2016). However, from a more general perspective the
activity of grid cells can also be interpreted in terms
of a domain-independent, general information proces-
sing scheme as shown by the RGNG-based GCM of
Kerdels and Peters (Kerdels and Peters, 2015; Ker-
dels, 2016). The main structure of this model can be
summarized as follows: Each neuron is viewed as part
of a local group of neurons that share the same set of
inputs. Within this group the neurons compete against
each other to maximize their activity while trying to
inhibit their peers. In order to do so, each neuron tries
to learn a representation of its entire input space by
means of competitive Hebbian learning. In this pro-
cess the neuron learns to recognize a limited number
of prototypical input patterns that optimally originate
from maximally diverse regions of the input space.
This learned set of input pattern prototypes then con-
stitutes the sparse, pointwise input space representa-
tion of the neuron. On the neuron group level the
competition among the neurons forces the individual,
sparse input space representations to be pairwise dis-
tinct from one another such that the neuron group as
a whole learns a joint input space representation that
enables the neuron group to work as a sparse distri-
buted memory (Kanerva, 1988).
The GCM uses the recursive growing neural gas
algorithm (RGNG) to simultaneously describe both
the learning of input pattern prototypes on the level
of individual neurons as well as the competition bet-
ween neurons on the neuron group level. In contrast
to the common notion of modeling neurons as sim-
ple integrate and fire units, the RGNG-based GCM
assumes that the complex dendritic tree of neurons
possesses more computational capabilities. More spe-
cifically, it assumes that individual subsections of a
neuron’s dendritic tree can learn to independently re-
cognize different input patterns. Recent direct obser-
vations of dendritic activity in cortical neurons sug-
gest that this assumption appears biologically plausi-
ble (Jia et al., 2010; Chen et al., 2013). In the model
A Grid Cell Inspired Model of Cortical Column Function
205

this intra-dendritic learning process is described by a
single growing neural gas per neuron, i.e., it is assu-
med that some form of competitive Hebbian learning
takes place within the dendritic tree. Similarly, it is
assumed that the competition between neurons on the
neuron group level is Hebbian in nature as well, and is
therefore modeled by analogous GNG dynamics. For
a full formal description and an in-depth characteri-
zation of the RGNG-based GCM we refer to (Kerdels
and Peters, 2016; Kerdels, 2016). In the following
section we focus on describing our modifications to
the original RGNG-based GCM.
3 EXTENDED NEURON MODEL
Although the neuron model outlined in the previous
section was conceived to describe the activity of en-
torhinal grid cells, it can already be understood as a
general computational model of certain cortical neu-
rons, i.e., L2/3 PC and L5 ITN in figure 1. However, to
function as part of our cortical column model we mo-
dified the existing GCM with respect to a number of
aspects:
3.1 Ensemble Activity
In response to an input ξ the GCM returns an en-
semble activity vector a where each entry of a cor-
responds to the activity of a single neuron. Given a
neuron group of N neurons, the ensemble activity a
is calculated as follows: For each neuron n ∈ N the
two prototypical input patterns s
n
1
and s
n
2
that are clo-
sest to input ξ are determined among all prototypical
input patterns I
n
learned by the dendritic tree of neu-
ron n, i.e.,
s
n
1
:= argmin
v∈I
n
kv − ξk
2
s
n
2
:= argmin
v∈I
n
\{s
n
1
}
kv − ξk
2
.
The ensemble activity a := (a
0
,...,a
N−1
) of the
group of neurons is then given by
a
i
:= 1 −
ks
i
1
− ξk
2
ks
i
2
− ξk
2
, i ∈ {0,.. .,N − 1}, (1)
followed by a softmax operation on a. Thus, the acti-
vity of each neuron is determined by the relative dis-
tances between the current input and the two best ma-
tching prototypical input patterns. If the input is about
equally distant to both prototypical input patterns the
activity is close to zero, and if the input is much clo-
ser to the best pattern than the second best pattern the
activity approaches one. The competition among the
neurons on the group level ensures that for any given
input only a small, but highly specific (Kerdels and
Peters, 2017) subset of neurons will exhibit a high
activation resulting in a sparse, distributed encoding
of the particular input.
Compared to the original GCM (Kerdels and Pe-
ters, 2016; Kerdels, 2016) we modified the activation
function (Eq. 1) to allow for a smoother response to
inputs that are further away from the best matching
unit s
1
than the distance between s
1
and s
2
, and we
normalized the ensemble output using the softmax
operation.
3.2 Learning Rate Adaptation
The original GCM uses fixed learning rates ε
b
and ε
n
,
which require to find a tradeoff between learning ra-
tes that are high enough to allow for a reliable adapta-
tion and alignment of initially random prototypes and
learning rates that are low enough to ensure a relati-
vely stable long-term representation of the respective
input space. We modified the original GCM in that re-
gard by introducing three learning phases that shape
the learning rates used by the GCM. During the initial
learning phase both ε
b
and ε
n
are kept at their initial
values (e.g., ε
b
= ε
n
= 0.01). The duration t
1
(measu-
red in # of inputs) of this first phase depends on the
maximum number of prototypes M and the interval λ
with which new prototypes are added to the model
1
,
i.e., t
1
= 2Mλ. This duration ensures that the RGNG
underlying the GCM has enough time to grow to its
maximum size and to find an initial alignment of its
prototypes. The second learning phase is a short tran-
sitional phase with a duration t
2
= Mλ in which both
learning rates ε
b
and ε
n
are reduced by one order of
magnitude to allow the initial alignment to settle in
a more stable configuration. Up to this point the pro-
totypes learned by the individual neurons of the model
are likely to be similar as primary learning rate ε
b
and
secondary learning rate ε
n
are equal so far
2
. Begin-
ning with the last learning phase, which is open-ended
in its duration, only the secondary learning rate ε
n
is
reduced once more by two orders of magnitude. This
asymmetric reduction of ε
n
initiates a differentiation
process among the individual neurons that allows the
prototypes of each neuron to settle on distinct locati-
ons of the input space.
1
Both M and λ are parameters of the RGNG used by the
original GCM.
2
See (Kerdels, 2016) for details on how primary and se-
condary learning rates relate within an RGNG.
IJCCI 2018 - 10th International Joint Conference on Computational Intelligence
206

3.3 Handling of Repetitive Inputs
Since the GCM is a continuously learning model it
has to be able to cope with repetitive inputs in such
a way that its learned representation is not distorted
if it is exposed to a single repeated input. Without
adaptation a repeated input would correspond to an
artificial increase in learning rate. To handle such a
situation we adjust the learning rates ε
b
and ε
n
by an
attenuation factor ζ:
ζ = 0.1
|s
1
|/10
,
with |s
1
| the number of successive times prototype s
1
was the best matching unit. The attenuation factor ζ
is applied when |s
1
| > 1.
3.4 Integration of Feedback Input
In order to use the GCM as part of our cortical co-
lumn model, we modified the existing GCM to allow
the integration of feedback connections. In the cor-
tex such feedback connections originate from higher-
order regions (violet arrows in Fig. 1) and predomi-
nantly terminate in layer 1 on the distal dendrites of
layer 2/ 3 and layer 5 neurons. To integrate these feed-
back connections into the model we added a secon-
dary GNG to the description of each neuron. With this
extension the dendritic tree of each neuron is now mo-
deled by two GNGs: a primary GNG that represents
the proximal dendrites that process the main inputs
to the neuron, and a secondary GNG that represents
the distal dendrites that process feedback inputs. Both
GNGs can independently elicit the neuron to become
active when either GNG receives a matching input.
If both GNGs receive inputs simultaneously, the out-
put o
p
of the primary GNG (determined by the ratio
shown in Eq. 1) is modulated by the output o
s
of the
secondary GNG depending on the agreement between
outputs o
p
and o
s
:
a
∗
:=
1
1 + e
−
(
o
p
−φ
)(
1+γ
(
1−|o
p
−o
s
|
))
,
with parameter γ determining the strength of the mo-
dulation and parameter φ determining at which acti-
vity level (Eq. 1) of o
p
the output is increased or de-
creased, respectively. Typical values are, e.g., γ = 10
and φ = 0.5.
A second important relation between the primary
and the secondary GNG concerns learning of new
prototypical input patterns. The secondary GNG can
only learn when the primary GNG has received a ma-
tching input that resulted in an activation of the neu-
ron, i.e., the learning rates ε
b
and ε
n
of the secondary
GNG are scaled by the output of the primary GNG in
response to a current main input to the neuron:
ε
∗
b
:= ε
b
o
p
,
ε
∗
n
:= ε
n
o
p
,
or by zero if there is no main input present. As a con-
sequence, the secondary GNG learns to represent only
those parts of the feedback input space that correlate
with regions of the main input space that are directly
represented by the prototypical input patterns of the
primary GNG. This way, the secondary GNG learns to
respond only to those feedback signals that regularly
co-occur with those main input patterns that were le-
arned by the primary GNG. One possible neurobiolo-
gical mechanism that could establish such a relation
between proximal and distal dendrites is the back-
propagation of action potentials (Stuart et al., 1997;
Waters et al., 2005).
Together, the primary and secondary GNG allow
a modeled neuron to learn a representation of its main
input space while selectively associating feedback in-
formation that may help to disambiguate noisy or dis-
torted main input or substitute such input if it is mis-
sing.
4 CORTICAL COLUMN MODEL
So far the extended neuron model describes a single
group of neurons that is able to learn a sparse, dis-
tributed representation of its input space and is able
to integrate additional feedback input. As such, the
group acts as a local, input-driven transformation that
maps input signals to output signals without maintai-
ning an active state, i.e., if no input is present, no
output will be generated. However, if two groups
share the same main input space but also receive the
group activity of each other as an additional input sig-
nal, both groups would be able to maintain a stable
active state even when the main input signal vanis-
hes. Instead of being stateless input-output transfor-
mations the two groups would form an active autoas-
sociative memory cell (AMC) (Kanerva, 1988; Ka-
nerva, 1992). Given the canonical connectivity of
cortical principal cells shown in figure 1 we argue
that L2/3 PC and L5 ITN constitute such a pair of reci-
procally connected neuron groups forming an AMC.
Both groups share a main input space via connecti-
ons from the primary thalamus and local L4 PC, while
they also both receive feedback input at their distal
dendrites in layer 1 from higher-order cortex and the
thalamus
3
.
3
These feedback connections motivated the extension
described in section 3.4.
A Grid Cell Inspired Model of Cortical Column Function
207

The other neuron groups shown in figure 1 can be
interpreted as providing support for the AMC: The
outputs of both L2/3 PC and L5 ITN connect locally to
L5 SPN, which projects to subcerebral targets, e.g.,
motor centres, and non-locally to higher-order parts
of the cortex. The output of the lower group L5 ITN
makes an additional local connection to L6 CC, which
projects to long-range cortical targets, but also locally
to L6 CT, which in turn connects back to L4 PC and the
primary thalamus.
We propose that the described neural structure
constitutes a single cortical minicolumn. At its core
the minicolumn hosts a single autoassociative me-
mory cell (L2/3 PC / L5 ITN) that is supported by neu-
rons regulating its activity (L4 PC), its input (L4 PC /
L6 CT) and its output (L5 SPN / L6 CC).
A key property of the described AMC is its ability
to feed its output back into itself via the reciprocal
connections between the two neuron groups ( L2/3 PC
/ L5 ITN). This local feedback loop does not only allow
the AMC to stay active when the main input vanis-
hes. It also enables a form of attractor dynamics (Ka-
nerva, 1988; Kanerva, 1992) where an initial, poten-
tially noisy or ambiguous input pattern can be itera-
tively refined to approach one of the stable, prototy-
pical input patterns learned by the AMC. In addition,
this iterative process can be supported or modulated
by the feedback input integrated via the distal den-
drites of both neuron groups. On a cortex-wide level
these attractor dynamics might play an important role
to dynamically bind subsets of cortical minicolumns
into joint, temporarily stable attractor states.
5 PRELIMINARY RESULTS
As a first step towards implementing the cortical co-
lumn model outlined in the previous section we simu-
lated a single AMC consisting of two neuron groups
a and b, which correspond to the two neuron groups
L2/3 PC and L5 ITN of figure 1. Each neuron group was
modeled by the extended GCM described in section 3
containing 25 neurons with 16 dendritic prototypes
each. Further parameters are given in table 1. As in-
put both groups received samples from the MNIST
database of handwritten digits (Lecun et al., 1998) re-
duced to a resolution of 16 × 16 pixels concatenated
with the current ensemble activity of the other group.
Each MNIST input was repeated 10× in a row combi-
ned in each case with the updated ensemble activity of
the respective other neuron group allowing for the co-
occurrence of the original input with the other group’s
reaction to that input. In total, the simulated AMC
was presented with 20 million inputs corresponding
Figure 2: Sixteen prototypes learned by a single neuron of
neuron group a. Each prototype consists of a 16 × 16 re-
presentation corresponding to the MNIST part of the input
(shown in gray) and a 5×5 representation corresponding to
the group b ensemble activation part of the input (visuali-
zed as color gradient from blue (low activation) to red (high
activation).
to about 33 full presentations of the MNIST training
data set to the system.
Figure 2 shows the 16 dendritic prototypes lear-
ned by a single neuron of group a after a total of
20 million inputs. Like the input patterns the pro-
totypes consist of two parts: a 16 × 16 representa-
tion corresponding to the MNIST portion of the in-
put and a 5 × 5 representation corresponding to the
ensemble activation of neuron group b co-occurring
with the particular MNIST inputs. The MNIST part
of the prototypes indicate that the neuron is, as ex-
pected, learning a sparse, pointwise representation of
its entire input space. While some prototypes exhi-
bit clear and distinct shapes that indicate that these
prototypes have already settled at stable locations in
input space, a few prototypes have less distinct shapes
indicating that these prototypes have not yet reached
such stable locations. One important aspect to note is
that this neuron will exhibit a high activity for any of
the learned input patterns. As a consequence, looking
just at the activity of this individual neuron it is not
possible to tell whether the input was a, e.g., “0” or
“2” or any of the other patterns represented by one of
the neuron’s prototypes.
A disambiguation of the input becomes only pos-
sible when observing the ensemble activity of an en-
tire neuron group, i.e., the representation of the input
is distributed across the entire group. No individual
neuron encodes for just a single input. Such ensem-
ble activities are captured by the ensemble part of the
prototypes and show, in this case, the average ensem-
ble activity of group b in response to the respective
MNIST input captured by the MNIST part of the pro-
totypes. The ensemble activities of group b shown in
figure 2 show that each input pattern evokes a distinct
pattern of ensemble activity allowing to disambiguate
the different input patterns. In case of already stable
IJCCI 2018 - 10th International Joint Conference on Computational Intelligence
208

Figure 3: Twenty examples of learned prototypes from dif-
ferent neurons of group a that represent numbers of similar
shape. Visualization as in Fig. 2.
prototypes it can also be seen, that the ensemble acti-
vity tends to be sparse with only a few neurons exhi-
biting a strong activation in response to a particular
input.
One interesting question regarding such a sparse,
distributed representation is the degree of variation in
this ensemble code if very similar inputs are proces-
sed. Figure 3 shows twenty examples of dendritic pro-
totypes from different neurons of group a that repre-
sent numbers of similar shape. The captured ensem-
ble activities of group b indicate that the similarity of
activation patterns appears to match well with the si-
milarity of the corresponding MNIST patterns. Even
in cases with an overall low activation like the respon-
ses to the number “8” (third row), the corresponding
ensemble codes appear similar.
These first tentative results indicate, that the pro-
posed model appears to be able to learn a sparse, dis-
tributed representation of its main input space (here
the MNIST set), while simultaneously learning the
ensemble codes of an accompanying group of neu-
rons that operates on the same input space.
6 CONCLUSION
The cortical column model outlined in this paper is
still in a very early stage. Yet, it combines multiple,
novel ideas that will guide our future research. First,
the RGNG-based, unsupervised learning of a sparse,
distributed input space representation utilizes a clas-
sic approach of prototype-based learning in a novel
way. Instead of establishing a one-to-one relation be-
tween a learned representation (prototype) and a cor-
responding region of input space, it learns an ensem-
ble code that utilizes the response of multiple neurons
(sets of prototypes) to a given input. The presented
preliminary results indicate that learning such an en-
semble code appears feasible. Our future research in
this regard will focus on improving our understanding
of the resulting ensemble code, as well as improving
the RGNG algorithm in terms of learning speed and
robustness w.r.t. a continuous learning regime.
Second, the idea of reciprocally connecting two
neuron groups that process a shared input space ena-
bles the creation of an autoassociative memory cell
(AMC) that is able to maintain an active state even in
the absence of any input. In addition, such an AMC
may exhibit some form of attractor dynamics where
the activities of the two, reciprocally connected neu-
ron groups self-stabilize. A precondition for such an
AMC is the groups’ ability to learn the ensemble code
of the respective other group. The presented prelimi-
nary results indicate that this is possible. Our future
research will focus on understanding the characteris-
tics of the dynamics of such an AMC, e.g., in response
to various kinds of input disturbances.
Third, the outlined cortical column model sug-
gests that the cortex may consist of a network of
autoassociative memory cells that influence each ot-
her via feedback as well as feedforward connections
while trying to achieve locally stable attractor states.
In addition, further cortical circuitry may modulate
the activity of individual cortical columns to facilitate
competition among cortical columns or groups of cor-
tical columns on a more global level, which may then
lead to the emergence of stable, global attractor states
that are able to temporarily bind together sets of corti-
cal columns. In this context our research is still in its
infancy and will focus on implementing a first version
of a full cortical column model that can then be tested
in small hierarchical network configurations, e.g., to
process more complex visual input.
REFERENCES
Buxhoeveden, D. P. and Casanova, M. F. (2002). The
minicolumn hypothesis in neuroscience. Brain,
125(5):935–951.
Chen, T.-W., Wardill, T. J., Sun, Y., Pulver, S. R., Rennin-
ger, S. L., Baohan, A., Schreiter, E. R., Kerr, R. A.,
Orger, M. B., Jayaraman, V., Looger, L. L., Svoboda,
K., and Kim, D. S. (2013). Ultrasensitive fluores-
cent proteins for imaging neuronal activity. Nature,
499(7458):295–300.
Fyhn, M., Molden, S., Witter, M. P., Moser, E. I., and Mo-
ser, M.-B. (2004). Spatial representation in the entor-
hinal cortex. Science, 305(5688):1258–1264.
Hafting, T., Fyhn, M., Molden, S., Moser, M.-B., and Mo-
ser, E. I. (2005). Microstructure of a spatial map in
the entorhinal cortex. Nature, 436(7052):801–806.
A Grid Cell Inspired Model of Cortical Column Function
209

Harris, K. D. and Mrsic-Flogel, T. D. (2013). Cortical con-
nectivity and sensory coding. Nature, 503:51.
Hashmi, A. G. and Lipasti, M. H. (2009). Cortical columns:
Building blocks for intelligent systems. In 2009 IEEE
Symposium on Computational Intelligence for Multi-
media Signal and Vision Processing, pages 21–28.
Hawkins, J., Ahmad, S., and Cui, Y. (2017). Why does
the neocortex have columns, a theory of learning the
structure of the world. bioRxiv.
Horton, J. C. and Adams, D. L. (2005). The cortical co-
lumn: a structure without a function. Philosophical
Transactions of the Royal Society of London B: Biolo-
gical Sciences, 360(1456):837–862.
Jia, H., Rochefort, N. L., Chen, X., and Konnerth, A.
(2010). Dendritic organization of sensory input to cor-
tical neurons in vivo. Nature, 464(7293):1307–1312.
Kanerva, P. (1988). Sparse Distributed Memory. MIT Press,
Cambridge, MA, USA.
Kanerva, P. (1992). Sparse distributed memory and related
models [microform] / Pentti Kanerva. Research In-
stitute for Advanced Computer Science, NASA Ames
Research Center.
Kerdels, J. (2016). A Computational Model of Grid Cells
based on a Recursive Growing Neural Gas. PhD the-
sis, FernUniversit
¨
at in Hagen.
Kerdels, J. and Peters, G. (2015). A new view on grid cells
beyond the cognitive map hypothesis. In 8th Confe-
rence on Artificial General Intelligence (AGI 2015).
Kerdels, J. and Peters, G. (2016). Modelling the grid-like
encoding of visual space in primates. In Proceedings
of the 8th International Joint Conference on Compu-
tational Intelligence, IJCCI 2016, Volume 3: NCTA,
Porto, Portugal, November 9-11, 2016., pages 42–49.
Kerdels, J. and Peters, G. (2017). Entorhinal grid cells may
facilitate pattern separation in the hippocampus. In
Proceedings of the 9th International Joint Conference
on Computational Intelligence, IJCCI 2017, Funchal,
Madeira, Portugal, November 1-3, 2017., pages 141–
148.
Lecun, Y., Bottou, L., Bengio, Y., and Haffner, P. (1998).
Gradient-based learning applied to document recogni-
tion. Proceedings of the IEEE, 86(11):2278–2324.
Mountcastle, V. B. (1978). An organizing principle for ce-
rebral function: The unit model and the distributed
system. In Edelman, G. M. and Mountcastle, V. V.,
editors, The Mindful Brain, pages 7–50. MIT Press,
Cambridge, MA.
Mountcastle, V. B. (1997). The columnar organization of
the neocortex. Brain, 120(4):701–722.
Rinkus, G. J. (2017). A cortical sparse distributed coding
model linking mini- and macrocolumn-scale functio-
nality. ArXiv e-prints.
Rowland, D. C., Roudi, Y., Moser, M.-B., and Moser, E. I.
(2016). Ten years of grid cells. Annual Review of
Neuroscience, 39(1):19–40. PMID: 27023731.
Stuart, G., Spruston, N., Sakmann, B., and Husser, M.
(1997). Action potential initiation and backpropaga-
tion in neurons of the mammalian cns. Trends in Neu-
rosciences, 20(3):125 – 131.
Waters, J., Schaefer, A., and Sakmann, B. (2005). Back-
propagating action potentials in neurones: measure-
ment, mechanisms and potential functions. Progress
in Biophysics and Molecular Biology, 87(1):145 –
170. Biophysics of Excitable Tissues.
APPENDIX
Parameterization
The neuron groups modeled by the extended GCM
described in section 3 use an underlying, two-layered
RGNG. Each layer of an RGNG requires its own set
of parameters. In this case we use the sets of parame-
ters θ
1
and θ
2
, respectively. Parameter set θ
1
controls
the inter-neuron level of the GCM while parameter
set θ
2
controls the intra-neuron level. Table 1 sum-
marizes the parameter values used for the simulation
runs presented in this paper. For a detailed charac-
terization of these parameters we refer to (Kerdels,
2016).
Table 1: Parameters of the RGNG-based, extended GCM
used for the preliminary results presented in section 5. For
a detailed characterization of these parameters we refer to
the appendix and (Kerdels, 2016).
θ
1
θ
2
ε
b
= 0.04 ε
b
= 0.01
ε
n
= 0.04 ε
n
= 0.01
ε
r
= 0.01 ε
r
= 0.01
λ = 1000 λ = 1000
τ = 100 τ = 100
α = 0.5 α = 0.5
β = 0.0005 β = 0.0005
M = 25 M = 16
IJCCI 2018 - 10th International Joint Conference on Computational Intelligence
210
