
Semantic Representation of Neuroimaging Observations: Proof of
Concept based on the VASARI Terminology
Emna Amdouni
1,2
and Bernard Gibaud
1,2
1
E-health department, B-com Institute of Research and Technology, Rennes, France
2
LTSI Inserm 1099, Universit
´
e de Rennes 1, Rennes, France
Keywords:
Medical Domain Ontology, Knowledge Representation, Ontology Reuse and Alignment.
Abstract:
The main objective of this work is to facilitate the identification, sharing and reasoning about cerebral tumors
observations via the formalization of their semantic meanings in order to facilitate their exploitation in both
the clinical practice and research. We have focused our analysis on the VASARI terminology as a proof of
concept, but we are convinced that our work can be useful in other biomedical imaging contexts. In this paper,
we propose (1) a methodology, a domain ontology and an annotation tool for providing unambiguous formal
definitions of neuroimaging data, (2) an experimental work on the REMBRANDT dataset to demonstrate the
added value of our work over existing methods, namely DICOM SR and the AIM model.
1 BACKGROUND AND
SIGNIFICANCE
In literature, an ontology is defined as ”a formal and
an explicit specification of a shared conceptualiza-
tion” (Gruber, 1993). Ontologies define the formal
semantics of vocabularies by specifying axioms, ex-
pressed in a logic-based language, that constrain and
structure relationships between terms. The main pur-
pose of ontologies is to enable knowledge integration
and semantic data querying. In the medical field, se-
mantic web technologies are used to standardize, for-
malize and share the medical data (coming form both
the clinical and research context) is very important
(Scheuermann et al., 2009; Seifert et al., 2010; Ober-
kampf et al., 2012). In this paper, we focused our
interest on the domain of cerebral tumors.
In oncology clinical practice, neuroimaging fea-
tures/phenotypes play an important role; in particu-
lar they help clinicians in making their diagnosis, se-
lecting the appropriate treatment and monitoring the
therapeutic response to an intervention as for exam-
ple the Response Evaluation Criteria in Solid Tumors
(RECIST) (Eisenhauer et al., 2009). Many radiology-
pathology correlation studies have been conducted
on cerebral tumors and show that some neuroima-
ging features are associated to genetic alterations and
gene expression (Gutman et al., 2013; Grams et al.,
2014). Therefore, suitable management of neuroima-
ging phenotypes is needed to facilitate their use and
reuse in multiple studies regarding imaging biomar-
kers (Levy et al., 2012; M
¨
oller et al., 2009; ESR,
2011; Rubin et al., 2014a).
Currently, neuroimaging features can be recorded
and stored in a formalized format such as the DICOM
(Digital Imaging and Communications in Medicine)
SR (Structured Report) (Clunie, 2000; Clunie, 2007)
and the Annotation and Imaging Markup (AIM) mo-
del (Channin et al., 2010): the DICOM SR forma-
lizes the representation of radiological observations
by introducing a set of rules that constrain concepts
organization and a vocabulary (i.e. codes and asso-
ciated code meanings) covering the domain of ima-
ging observations. DICOM SR includes measure-
ments and qualitative assessments, their relationships
with image evidence and with the clinical interpreta-
tion of the clinician. The AIM model is an informa-
tion model and an XML-based file format to describe
the minimal information necessary to record image
annotations. This information model has introduced
the most relevant entities used in image annotation.
These standard formats enable the description of
the content of medical images. Unfortunately, they
are not suitable to support logic-based reasoning;
these formats are based on coded terms and most of
them have no semantic axioms to specify their mea-
nings and the relationships between them. As a con-
sequence, only searches based on keywords can be
handled on decision making tools that exploit these
standard formats. Rubin et al., Levy MA and many
Amdouni, E. and Gibaud, B.
Semantic Representation of Neuroimaging Observations: Proof of Concept based on the VASARI Terminology.
DOI: 10.5220/0006931100630074
In Proceedings of the 10th International Joint Conference on Knowledge Discovery, Knowledge Engineering and Knowledge Management (IC3K 2018) - Volume 2: KEOD, pages 63-74
ISBN: 978-989-758-330-8
Copyright © 2018 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
63

others researchers have promoted the use of formal
ontologies (Rubin et al., 2009; Levy et al., 2009;
Kahn J.R. et al., 2011; Van Soest et al., 2014) for au-
tomatic reasoning on these data models and overcome
the current limitations listed above.
This work is based on the assumption that the use
of semantic web technologies, in particular ontologies
as well as reasoning capabilities, can make the mea-
ning of neuroimaging assessments more explicit and
facilitate their advanced exploitation and interpreta-
tion. We are convinced that this approach can be be-
neficiary to the whole domain of neuroimaging, and
beyond to the whole domain of biomedical imaging.
However, this work focuses on a limited domain, na-
mely the domain covered by the VASARI termino-
logy (for Visually Accessible Rembrandt Images ter-
minology)
1
, used as a proof of a concept.
The VASARI terminology is a controlled vocabu-
lary that describes thirty observations of high grade
cerebral gliomas (glioblastoma multiform or GBM)
in conventional Magnetic Resonance Imaging (MRI)
images. Its main objective consists in standardizing
brain tumors description and facilitating their inter-
pretation by neuro-radiologists. The VASARI termi-
nology was developed by experts in neuro-radiology
who have considered the majority of possible asses-
sments based on MRI. The validation of this corpus
of imaging features was realized by eight experienced
neuro-radiologists from distinct institutions.
Our work has three main contributions. First, ma-
king the meaning of VASARI features explicit via
the design and the implementation of a specialized
ontology, called VASARI ontology. Second, provi-
ding a semantic annotation tool that automatically
translates VASARI features into instances of the VA-
SARI ontology. This tool was applied to a VASARI
corpus called REMBRANDT data set (Repository of
Molecular Brain Neoplasia Data) (Madhavan et al.,
2009). Third, performing semantic queries and rea-
soning tasks on these data to show how this semantic
description of VASARI features can facilitate the in-
terpretation of the content of medical images.
The remaining of this article is organized as fol-
lows: in Section Material and Methods, we describe
the methodology for the design of the VASARI onto-
logy. Section Results provides, first, an overview of
the architecture and development details of both the
developed ontology and the semantic annotation tool.
Second, it demonstrates how we can automatically
store and semantically manipulate RDF (Resource
Description Framework) data of the REMBRANDT
data set. In Section Discussion, we explain some mo-
1
https://wiki.cancerimagingarchive.net/display/Public/
VASARI+Research+Project
deling choices that we have made regarding ontology
design and implementation, list accomplished work
and enumerate remaining problems.
2 MATERIAL AND METHODS
2.1 Design of the VASARI Ontology
The VASARI ontology was designed according to
the realism-based approach proposed by Ceusters and
Smith (Ceusters and Smith, 2005; Smith, 2006; Smith
and Ceusters, 2010). Our modeling methodology is
composed of five main steps that can be outlined as
follows. First, we analyzed for each VASARI fea-
ture Fi the meaning of the studied aspect and sorted
its possible configurations to establish the list of pos-
sible values allowed for each criterion. Second, we
identified and described the key real entities that are
involved in each criterion. Third, we related entities
to existing ontologies, most of which coming from the
OBO foundry and aligned onto the Basic Formal On-
tology (Bittner and Smith, 2004; Smith et al., 2005b).
When needed new ontology classes were specified.
Fourth, we specified and defined the axioms charac-
terizing these entities and relations between them. Fi-
nally, we made sure that all possible configurations
for each feature Fi can be modeled in a formal way.
In our work, we described the thirty VASARI fea-
tures but this paper focuses on seven of them, namely:
lesion location, lesion side, enhancement quality, pro-
portion nCET (non contrast enhanced tumor), cortical
involvement, extent of resection of enhancing tumor
and lesion size (see Table 1).
After a deep analysis of the meaning of the VA-
SARI features and the identification of the different
entities that they involve, we have proceeded with
their formal description. This step was not a trivial
task given that we faced several modeling problems
summarized in Table 2 and more detailed in (Am-
douni and Gibaud, 2016). Modeling problems con-
cern: negative findings (MP1) (Ceusters et al., 2006),
spatial knowledge (MP2) (Bennett et al., 2013) and
complex entities representation (MP3).
We have used the version 2 of the Basic Formal
Ontology (BFO) as a foundation for the VASARI on-
tology, thus facilitating the integration of specialized
ontologies that come from the Open Biological and
Biomedical Ontologies (OBO) foundry (Smith et al.,
2007). In particular, we reused the following onto-
logies: the Foundational Model of Anatomy (FMA)
(Smith et al., 2006), the Information Artifact Onto-
logy (IAO) (Ceusters, 2012), the Phenotypic Quality
Ontology (PATO) (Mungall et al., 2007), the Open
KEOD 2018 - 10th International Conference on Knowledge Engineering and Ontology Development
64
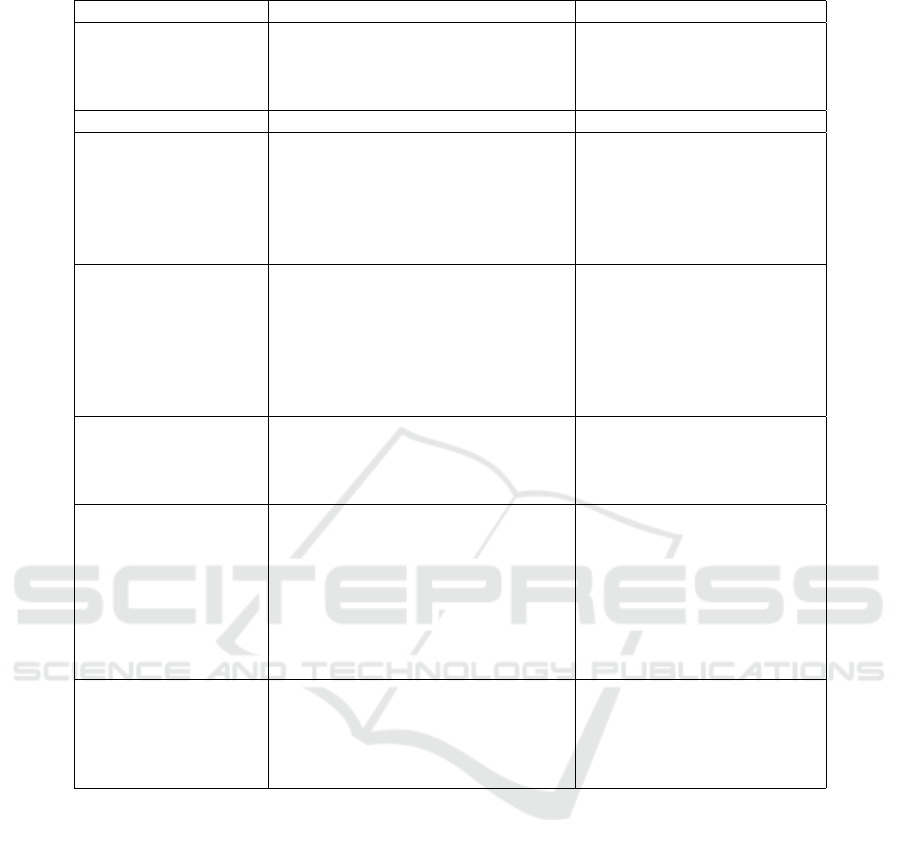
Table 1: Selected subset of the VASARI features defined in the VASARI terminology.
Feature Feature definition feature
F1.Lesion location Location of lesion geographic epi-
center; the largest component of the
tumor either contrast enhancing or
non contrast enhancing.
frontal, parietal, temporal,
occipital, corpus callosum,
thalamus
F2.Lesion side Side of lesion epicenter. right, central, bilateral
F4.Enhancement
quality
Qualitative degree of contrast en-
hancement is defined as having all
or portions of the tumor that demon-
strate significantly higher signal on
the post contrast T1W images com-
pared to pre contrast T1W images.
n/a, none, mild, marked
F6.Proportion nCET What proportion of the entire tumor
is non enhancing? Non enhancing
tumor is defined as regions of T2W
hyper intensity that are associated
with mass effect and architectural
distortion, including blurring of the
gray-white interface.
n/a, 0%, >5%, 6-33%, 34-
67%, 68-95%, >95%, 100%,
indeterminate.
F20.Cortical invol-
vement
Non-enhancing o enhancing tumor
extending to the cortical mantle, or
cortex is no longer distinguishable
relative to subjacent tumor.
no, yes
F26.Extent of re-
section of enhancing
tumor
Using the first postoperative scan
(contrast enhancing MR imaging)
assessed for tumor residual estima-
ting the proportion of enhancing
tumor. Total resection component
should be scored to 100%, subtotal
resection of enhancing tissue should
be scored accordingly.
n/a, 0%, >5%, 6-33%, 34-
67%, 68-95%, >95%, 100%,
indeterminate
F29.Lesion size Largest perpendicular (x-y) cross
section diameter of T2 signal ab-
normality (longest dimension X
perpendicular) measured on single
sectional image only.
unidimensional, largest dia-
meter in centimeters
Biomedical Investigations (OBI) (Brinkman et al.,
2010), the Ontology for General Medical Sciences
(OGMS) (Scheuermann et al., 2009), the Unit On-
tology (UO) (Gkoutos et al., 2012) and the Relation
ontology (RO) (Smith et al., 2005a); in our work we
have considered that RO relations are integrated under
the BFO 2 ontology. Note that ontologies acronyms
will be used in the rest of the paper.
2.2 Design of the Experimental Work
In our experimental work, we have developed a se-
mantic annotation software of VASARI data using the
VASARI ontology. This software enables the user
to transform the informal description of the 30 VA-
SARI features into a formal one. This software was
applied to a corpus of VASARI data called the REM-
BRANDT repository. The resulting semantic data set
was used to evaluate the added value of our work; es-
pecially, we performed some reasoning tasks by for-
mulating semantic queries as well as some consis-
tency tests to detect inconsistent assertions.
2.3 Presentation of the REMBRANDT
Repository
The REMBRANDT repository is freely accessible
on this link16. The ultimate objective of the REM-
BRANDT data set is to facilitate the discovery of
significant correlations between clinical and genomic
information in order to provide patients with more
personalized treatments in the clinical context. The
REMBRANDT data set contains 30 VASARI features
labeled by 3 radiologists that concern 34 patients with
GBM tumors. Features values are stored in an Excel
Semantic Representation of Neuroimaging Observations: Proof of Concept based on the VASARI Terminology
65
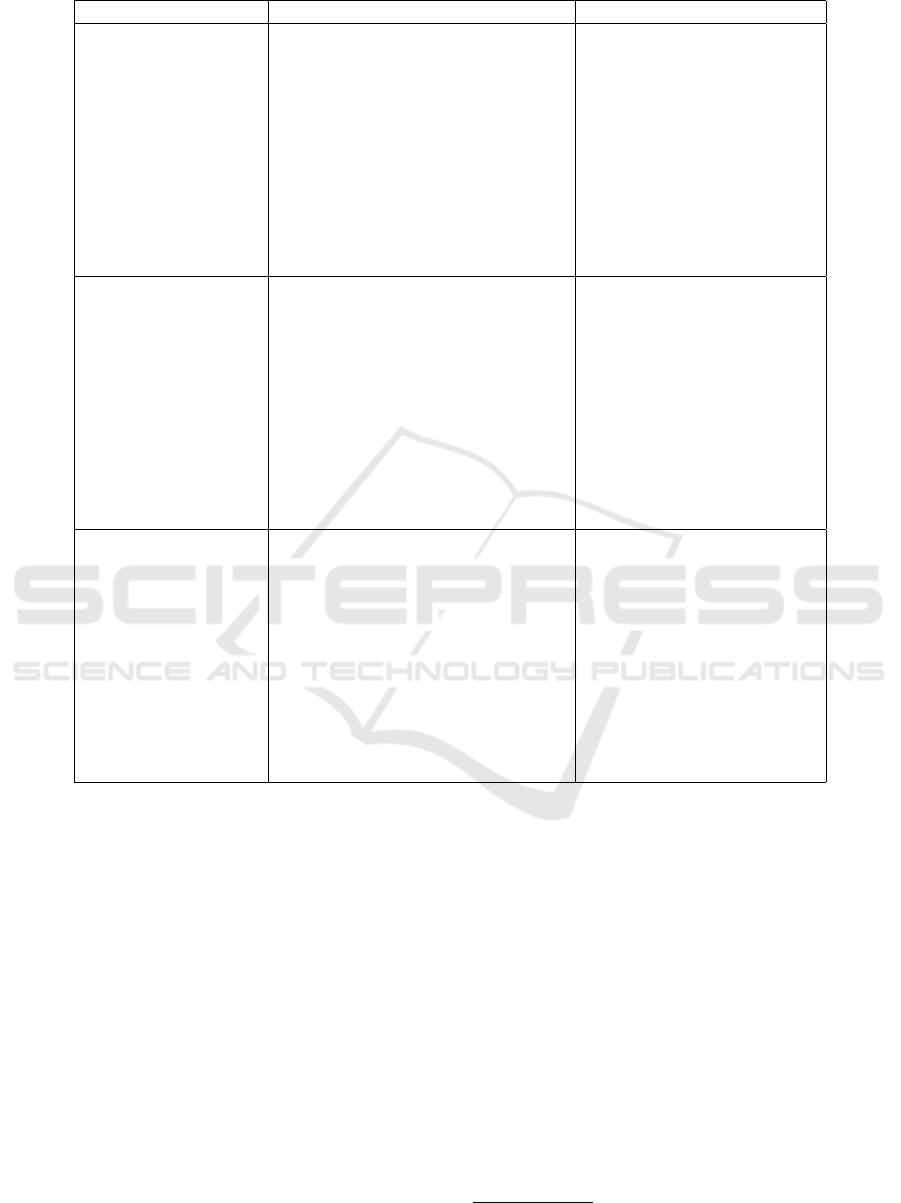
Table 2: Modeling problems.
Modeling problem VASARI expressions Use cases and examples
MP1: How to re-
present negative
neuroimaging obser-
vations that indicate
the non-existence of
a dependent conti-
nuant (category C1)
or an independent
continuant (category
C2): bfo:Quality or
bfo:Disposition?
Negative findings are expressed by
the use of negative qualifiers as
for example none or expressions as
indeterminate, not applicable, wit-
hout etc.
Non-existence of an entity
or a quality/disposition. Ex-
amples: C1:Johns cerebral
tumor is without an enhan-
cing region. C2:Johns cere-
bral tumor is not edematous.
Or Johns cerebral tumor is
not infiltrative.
MP2: How to ensure
a faithful represen-
tation of the studied
pathological struc-
ture and describe
how its components
are situated in space?
Containment is denoted by natural
language expressions like within,
portion of, comprise of whereas
overlapping is denoted by the term
invasion. The proximity of a given
entity to another one is expressed
with terms such as surrounding and
adjacency, and separation is deno-
ted by terms like not contiguous and
separated.
Spatial location of existing
entities, entities that are re-
lated to each other, enti-
ties that are separated from
each other, entities that are
adjacent to each other, etc.
Examples: Johns cerebral
tumor epicenter is in the pa-
rietal lobe. A cerebral tumor
has part a cerebral tumor
margin.
MP3: How to encode
complex entities as
for example deri-
ved measurements
(proportions of vo-
lume measurements,
length measure-
ments, etc.), and
associate them to
their corresponding
clinical findings?
Volume proportions: 0%, <5%,
6-33%, 34-67%, 68-95%, >95%,
100%. Or, two-dimensional length
(x,y) that represents cross-sectional
diameters; scores are between <0.5
cm and >8cm.
The representation of the ex-
tent of the resection of a gi-
ven cerebral tumor compo-
nent (nCET, necrotic, etc.).
Examples: 35% of Johns
cerebral tumor is enhanced,
Johns cerebral tumor of size
1cm2cm.
document where each spreadsheet contains evaluati-
ons asserted by a radiologist.
2.4 Implementation Details
We have designed the VASARI ontology in the Onto-
logy Web Language 2 (OWL2) format using the ver-
sion 5 of the Prot ´eg ´e tool (Tudorache et al., 2013).
To extract modules from the relevant OBO ontolo-
gies we used the Ontofox web interface (Smith et al.,
2007). Our semantic annotation tool was developed
with the JAVA language and created using the NetBe-
ans IDE 8.0.2 programming environment. To design
the semantic annotation software, we used the version
2.8.3 of the JENA35 semantic web programming fra-
mework and the version 2.3.2 of the JENA-Pellet re-
asoner engine (external engine of the JENA API) (Si-
rin et al., 2007) to perform automatic reasoning tasks.
To execute some SPARQL queries, we have used the
CORESE tool 3.2
2
. Note that instance data are seria-
lized in the RDF/XML format.
3 RESULTS
3.1 The VASARI Ontology
The VASARI ontology imports eight ontology modu-
les and contains around 570 OWL classes and 120
properties. Figure 1 shows that the four major se-
mantic aspects that constitute the VASARI domain,
namely: pathological structures, anatomical localiza-
tion, qualities and dispositions, and measurements. In
2
http://wimmics.inria.fr/corese
KEOD 2018 - 10th International Conference on Knowledge Engineering and Ontology Development
66

this section, classes are represented in Italic and rela-
tionships between them in Bold.
In our proposed ontology, a
vasari:CerebralTumor is a va-
sari:CerebralPathologicalStructure. Different
regions of the cerebral tumor are introduced with
the entity vasari:CerebralTumorComponent that is
defined as a vasari:CerebralPathologicalStructure
and bfo:continuant
part at some time some
vasari:CerebralTumor. In our semantic model
and as it is defined in the VASARI termino-
logy, we considered that a vasari:CerebralTumor
can be composed of four basic components that
characterize the brain tissue abnormality: va-
sari:EnhancingCerebralTumorComponent, va-
sari:NonEnhancingCerebralTumorComponent,
vasari:NecroticCerebralTumorComponent and
vasari:CerebralEdemaComponent.
The following paragraphs describe how the speci-
fic domains requirements mentioned in the Material
and Methods Section were addressed in the VASARI
ontology.
Modeling Problem 1: In order to respect the basic
principles of realist ontology, no instance should be
created when no concrete entity exist in reality. The
solution that we proposed uses an equivalentclass ax-
iom (condition if and only if involving some negative
somevaluesfrom assertion). Thus, classes are defined
as follows:
• Case category C1: A
vasari:CerebralTumorComponentNotLocatedIn-
BrainCortex [definition] is a va-
sari:CerebralTumorComponent and
not (bfo:located in at some time
some vasari:CerebralCortex), an
vasari:EnhancingCerebralTumorWithoutNonEn-
hancingCerebralTumorComponent [definition]
is a vasari:EnhancingCerebralTumor and not
(bfo:has continuant part at some time some
vasari:NonEnhancingCerebralTumorCompone-
nt).
• Case category C2: A va-
sari:NonCysticCerebralTumorComponent [de-
finition] is a vasari:CerebralTumorComponent
and not (bfo:has quality at some time
some pato:Cystic), a va-
sari:NonEnhancingCerebralTumorComponent
[definition] is a va-
sari:CerebralTumorComponent and not
(bfo:has disposition at some time some
vasari:DispositionToBeEnhancing).
Modeling Problem 2: Three main spatial relations
are modeled:
• Containment relation: We employed the spa-
tial relation bfo:located in at some time
and the foundational relation
bfo:part of continuant at some time.
We suppose that C1 and C2 are classes
of continuants. As asserted in BFO, C1
bfo:part of continuant at some time C2 means
that for every particular c1, if c1 instance of C1
then there is some c2 such that c2 instance of C2
and c1 bfo:part of continuant at some time
c2, C1 bfo:located in at some time C2 as-
serts that for every c1 if c1 instance of
C1, then there is some c2 instance of
C2 and c1 bfo:located in at some time
c2. In our ontology, we used the re-
lation bfo:located in at some time, for
example to associate a particular va-
sari:CerebralTumorEpicenter to its specific
vasari:LobeOfCerebralHemisphere and the rela-
tion bfo:has continuant part at some time to
define that the vasari:EdematousCerebralTumor
[definition] is a vasari:CerebralTumor and
(bfo:has continuant part at some time some
vasari:CerebralEdemaComponent).
• Overlapping vs adjacency relation: We employed
the spatial relation ro:adjacent to to express that
two continuants do not share a common spatial re-
gion and we defined the relation vasari:overlaps
to represent the case of overlapping. As des-
cribed in Table 1, F20 evaluates the location
of the cerebral tumor regarding the cerebral
cortex. To describe these different situations
and ensure a correct classification of the cerebral
tumor, we have defined the following classes:
vasari:CerebralTumorInvadingBrainCortex
[definition] vasari:CerebralTumor and
(vasari:overlaps some vasari:CerebralCortex),
vasari:CerebralTumorAdjacentToBrainCortex
[definition] vasari:CerebralTumor and
(ro:adjacent to some vasari:CerebralCortex).
• Separation relation: We used the relational
quality vasari:ContiguousWithCerebralTumor
with the logical negation operator (not) to
qualify and identify cerebral component that
are separated from the cerebral tumor, as
for example: vasari:SatelliteLesion [defini-
tion] vasari:CerebralPathologicalStructure
and (not (bfo:has quality at some time some
vasari:ContiguousWithCerebralTumor)) and
(bfo:has disposition at some time some va-
sari:DispositionToBeEnhancing).
• Separation relation: We used the relational
quality vasari:ContiguousWithCerebralTumor
with the logical negation operator (not) to
Semantic Representation of Neuroimaging Observations: Proof of Concept based on the VASARI Terminology
67
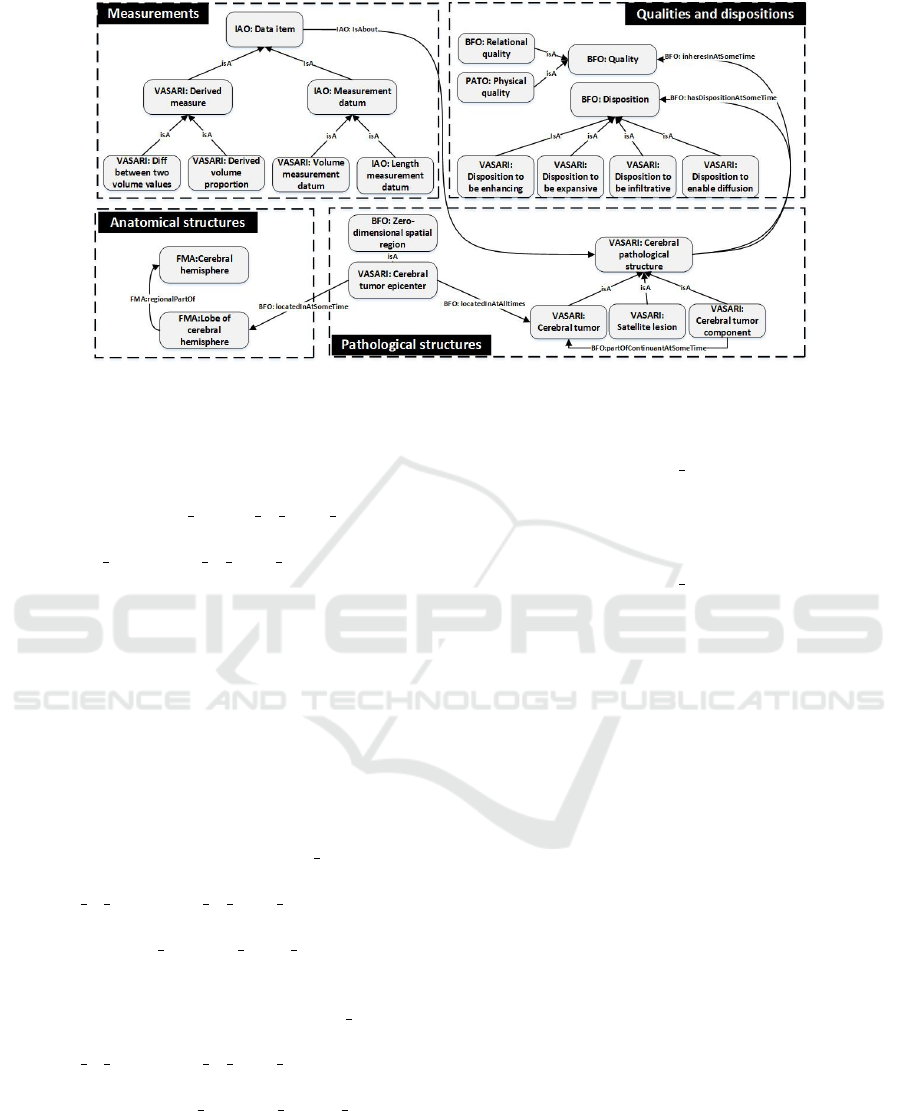
Figure 1: The basic pattern of the main classes in the VASARI ontology.
qualify and identify cerebral component that
are separated from the cerebral tumor, as
for example: vasari:SatelliteLesion [defini-
tion] vasari:CerebralPathologicalStructure
and (not (bfo:has quality at some time some
vasari:ContiguousWithCerebralTumor)) and
(bfo:has disposition at some time some va-
sari:DispositionToBeEnhancing).
Modeling Problem 3: The representation of the
extent of the resection of an enhancing cerebral tumor
component appears to be simple data to represent,
but in reality it involves hidden information that
are not explicit in the definition of the feature.
To model this kind of feature, we consider that
the vasari:EnhancingCerebralTumorComponent
will not preserve its identity after and before the
surgery. Thus, we identified two distinct entities:
vasari:EnhancingCerebralTumorComponentBefore-
Surgery [definition] is
a va-
sari:EnhancingCerebralTumorComponent and
(bfo:part of continuant at some time some
vasari:EnhancingCerebralTumorComponent)
and (bfo:is specified input of some
vasari:ResectionOfEnhancingCerebralTumorComp-
onent). vasari:EnhancingCerebralTumorCompone-
ntAfterSurgery [definition] is a va-
sari:EnhancingCerebralTumorComponent and
(bfo:part of continuant at some time some
vasari:EnhancingCerebralTumorComponentBefore-
Surgery) and (bfo:is specified output of some
vasari:ResectionOfEnhancingCerebralTumorComp-
onent).
We considered that the measured qua-
lity, i.e. the volume, is the same, but
the measured volume values are distinct:
vasari:volumeMeasurementDatumOfEnhancingCer-
ebralTumorAfterSurgery [defini-
tion] vasari:VolumeMeasurementDatum
and (bfo:is
about some
vasari:EnhancingCerebralTumorComponentAfterSu-
rgery), vasari:volumeMeasurementDatumOfEnhanc-
ingCerebralTumorBeforeSurgery [defi-
nition] vasari:volumeMeasurementDatum
and (bfo:is about some
vasari:enhancingCerebralTumorComponentBeforeS-
urgery). The interpretation of extreme values of F26
will be as follows:
• %0 means that the enhancing tumor component
is totally preserved and that the measured volume
value before the surgery is the measured volume
value after the surgery.
• %100 means that the enhancing cerebral
tumor component is totally resected, thus the
cerebral tumor component is classified as a
vasari:NonEnhancingCerebralTumorComponen-
tAfterSurgery.
3.2 Semantic Annotation Software of
VASARI Data
The annotation software begins by reading as an
input the set of imaging features values of the
REMBRANDT repository and the VASARI ontology
schema. Then, to semantically annotate the data the
software realizes four main tasks. First, it instantia-
tes the VASARI ontology based on the VASARI la-
beled values. Second, it describes imaging features
by creating RDF triples that establish semantic links
between instances. Third, it adds these triples as sta-
tements in an RDF graph. Fourth, it serializes data in
the RDF/XML grammar and records the RDF graph
in memory or in a JENA triple database (TDB). Note
KEOD 2018 - 10th International Conference on Knowledge Engineering and Ontology Development
68

that the software stores separately the schema and the
instance data (denoted by the terms Tbox and Abox
in the following paragraph). It returns the generated
RDF graph of the whole REMBRANDT dataset in
1.06 s ( 0.47s per radiologist).
3.3 Semantic Exploitation of the
VASARI Annotation Data
The developed software bases its reasoning on an in-
ferred model generated with a reasoner. The infor-
mation stored in the inferred graph is contained in a
knowledge base (KB) that can be exploited in two
ways: 1) accessed via SPARQL queries to retrieve
data based on their semantics 2) checked via consis-
tency tests.
Querying a knowledge base: Let us consi-
der this example to illustrate inference capabilities
and demonstrate how the semantic format enables
the exploitation of the anatomical knowledge co-
ming from the FMA ontology. We suppose that
a given KB is composed of an Abox that con-
tains the following assertions: (A1) cte instance of
CerebralTumorEpicenter, (A2) tl instance of Tem-
poralLobe, (A3) rch instance of RightCerebralHe-
misphere, (A4) cte bfo:located in at some time tl,
(A5) tl regional part of rch. A Tbox that contains:
(T1) RightTemporalLobe [equivalentClass] Tempo-
ralLobe and regional part of some RightCerebralHe-
misphere, (T2) regional part of [equivalentProperty]
part of continuant at some time.
Based on the assertions of the Abox and on
the (T1) axiom we can deduce that: (A6) tl in-
stance of RightTemporalLobe (see Figure 3). Using
(T2), (T3) and (A6), we can infer: (A7) cte
bfo:located in at some time rtl (see figure 2).
Figure 3 presents an example of SPARQL
query that retrieves the location of cerebral tumors
(?lobe, ?cerebral hemisphere) and their correspon-
ding length measurements (?long axis value, ?per-
pendicular long axis value). The lower part of the
figure presents the results returned by the CORESE
semantic query tool.
Validation of a knowledge base: The pellet JENA
reasoner enables the detection of conflicts in the kno-
wledge content; thus we have exploited this capa-
city to perform a global check across the KB and
looked for inconsistencies between the radiologists
assertions. For example lets suppose that (A1) ct in-
stance of CerebralTumor and that one radiologist said
that (A2) ct is a HemorrhagicCerebralTumor howe-
ver, another radiologist said that (A3) ct is a NonHe-
morrhagicCerebralTumor. Reasoning on this know-
ledge base is impossible given because the two clas-
ses HemorrhagicCerebralTumor and NonHemorrha-
gicCerebralTumor are defined as disjoint classes in
the Tbox; this means that they cannot share the same
set of instances (i.e., ct). As a consequence, a classifi-
cation error should occur when the reasoner performs
inference tasks.
The result of a consistency checking is provi-
ded via the object ValidityReport of the JENA API.
This data structure encapsulates all detected incon-
sistent axioms and assertions. To generate explana-
tions about inconsistencies, we have used the met-
hod explainconsistency(). The output generated af-
ter the execution of this method consists on the lis-
ting of the set of all involved axioms; Figure 4 de-
picts an example of inconsistency that is caused by
the assignment of two different length measures to the
same cerebral tumor. This multiple attribution viola-
tes the owl:functionalProperty axiom of the property
iao:has measurement value that is declared as functi-
onal.
4 DISCUSSION
In our work, we followed the realism-based appro-
ach to describe the neuroimaging reality on the side
of the patient which appeared to us the most rele-
vant methodology in the context of the biomedical re-
search; the realism-based approach is being adopted
by a growing community of researchers in the medi-
cal context. Actually, medical terminologies such as
the DICOM SR and the AIM model do not refer to
concrete existing phenomena on the side of the pa-
tient, but they only code medical statements in a for-
mal way. The adoption of the realism-based approach
enabled us to provide a faithful representation of ima-
ging features by considering both the universals level
(e.g. cerebral tumor concept) and instances of univer-
sals level (e.g. Davids cerebral tumor). To follow this
modeling perspective, we have used two foundational
and realism-based ontologies; namely the BFO on-
tology to describe existing entities and relationships
between them. The use of BFO ontology has facilita-
ted the integration of heterogeneous knowledge from
different ontologies that are specialized in anatomy,
quality phenotypes, measurements, etc.
The developed ontology answers to some challen-
ging points that are highlighted in many papers (Ceus-
ters et al., 2006; Cimino, 2006; Levy et al., 2012)
mainly: 1) the formalization of the description of neu-
roimaging information via the use of specialized onto-
logies; we can cite the example of the FMA ontology
that allowed the description of some clinical state-
ments or the BFO and RO ontologies that helped us in
Semantic Representation of Neuroimaging Observations: Proof of Concept based on the VASARI Terminology
69
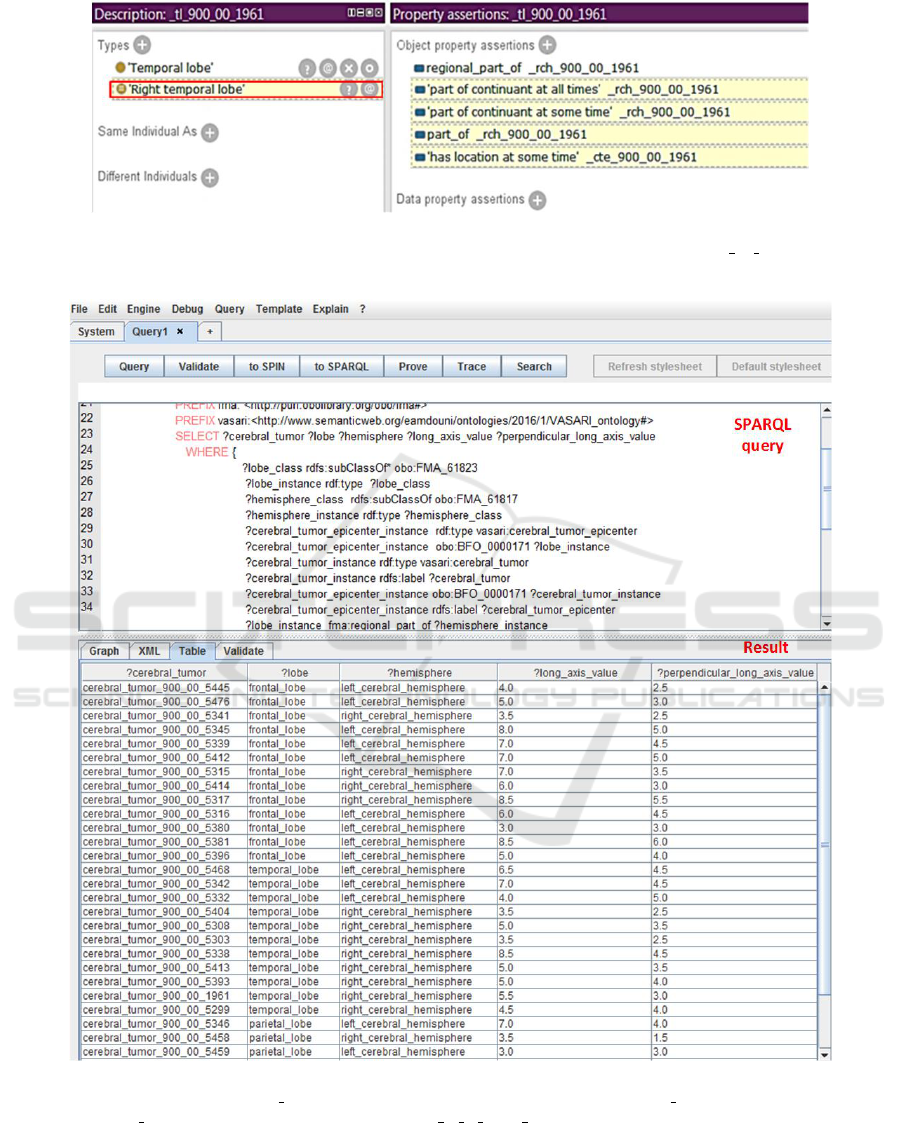
Figure 2: Illustrative example of semantic exploitation of anatomical knowledge from the FMA ontology (protg tool); instan-
ces names are automatically generated with our developed annotation tool, the ID of the patient 900 00 1961 is included to
refer to existing entities on the side of the patient.
Figure 3: Execution result to a SPARQL query on observations of the radiologist1 in the REMBRANDT repository (demon-
stration with the CORESE tool). FMA 61823 denotes the LobeOfCerebralHemisphere, FMA 61817 denotes the CerebralHe-
misphere and BFO 0000171 denotes the relation bfo:located in at some time.
the description of foundational (i.e., is a, part of) and
spatial relations (i.e., location, adjacent to) between
pathological structures, 2) the representation of nega-
tive neuroimaging observations via the use of OWL
axioms and 3) the representation of complex entities
was made more explicit by referring to the involved
concrete entities; but we have faced some difficulties
to represent mathematical expressions with the IAO
KEOD 2018 - 10th International Conference on Knowledge Engineering and Ontology Development
70
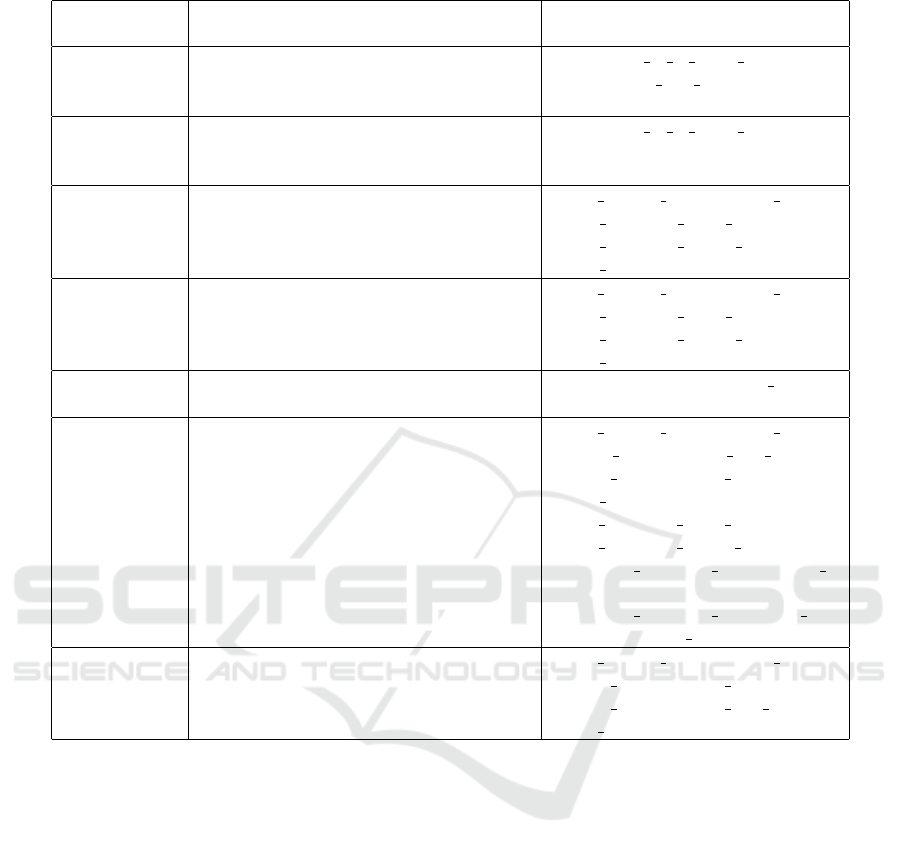
Table 3: Alignment of ontologies to represent VASARI features.
VASARI fea-
ture
Main involved classes Main involved relations
F1:lesion lo-
cation
vasari:CerebralTumorEpicenter,
fma:LobeOfCerebralHemisphere,
fma:Brainstem, fma:Cerebellum
bfo:located in at some time
fma:regional part of
F2:lesion side vasari:CerebralTumorEpicenter,
fma:CerebralHemisphere,
fma:MedianSagittalPlane
bfo:located in at some time
F4:enhancem-
ent quality
pato:Volume,
vasari:CerebralTumorComponent,
vasari:VolumeMeasurementDatum,
obi:ValueSpecification
iao:is quality measurement of,
bfo:is specified input of,
bfo:is specified output of,
bfo:is about
F5: propor-
tion nCET
pato:Volume,
vasari:CerebralTumorComponent,
vasari:VolumeMeasurementDatum,
obi:ValueSpecification
iao:is quality measurement of,
bfo:is specified input of,
bfo:is specified output of,
bfo:is about
F20:cortical
involvement
vasari:CerebralTumor,
fma:CerebralCortex
vasari:overlaps ro:adjacent to
F26:extent
of resection
of enhancing
tumor
pato:Volume,
vasari:VolumeMeasurementDatum,
uo:VolumeUnit,
vasari:RatioValueSpecification,
vasari:ProportionOfEnhancingAndRemo-
vedTumor,
vasari:EnhancingCerebralTumor-
Component,
vasari:ResectionOfEnhancingCerebralTu-
morComponent
iao:is quality measurement of,
bfo:has measurement unit label,
iao:has measurement value,
bfo:is about,
obi:is specified input of,
obi:is specified output of,
vasari:has specified denominator -
value,
vasari:has specified nominator -
value, ro:derives from
F29 and F30:
lesion size
obi:LengthMeasurementDatum,
uo:LengthUnit, pato:Quality
iao:is quality meausrement of,
iao:has measurement value,
iao:has measurement unit label,
iao:is about
ontology, thus we think that it will be interesting to
extend it to cover this kind of information that is nee-
ded in the definition of biomedical experiments.
It is important to note that our approach is not li-
mited to the translation of VASARI features resulting
from the subjective assessment of MRI images by hu-
man neuro-radiologists. In contrast, it would express
its full value if it were implemented as a complement
to an automated or semi-automated image analysis sy-
stem (Velazquez et al., 2015). For example systems
that are described in these papers (Rubin et al., 2014b;
Porz et al., 2014) enable to segment the various parts
of the tumor, and to automatically determine their
anatomical environment (e.g. what anatomical struc-
tures they are contained in, or they overlap or they are
adjacent to). Such mereotopological properties could
be directly translated in semantic form using the rela-
tionship discussed above. Similarly, the volume me-
asurements and the derived proportions could be ge-
nerated automatically and with a better accuracy than
through the current subjective assessments. VASARI
features could be derived from the detailed image-
based observations and measurements rather precede
them, and our model provides the conceptual basis to
make such enrichment of image processing systems.
The Radiology Reading Room of the Future (Gil-
lies et al., 2015) will entail a reading room where in
practicing radiologists interact with picture archiving
and communication system software to identify, seg-
ment, and extract features from regions of interest. If
prior studies obtained in the same patient are availa-
ble, the previous regions of interest will be automa-
tically identified by the reading software. As part of
the reading, the extracted size, shape, location, and
textural features will be automatically uploaded to a
shared database and algorithmically compared with
prior images to enable more precise diagnoses.
The first limit of our proposal is that it is imple-
mented in OWL and thus it does not generate tempo-
ralized instances (Smith et al., 2006). We think that
Semantic Representation of Neuroimaging Observations: Proof of Concept based on the VASARI Terminology
71

Figure 4: Example of a validation output: detection of a logical contradiction, IAO 000004 denotes the relation
iao:has measurement value and paldmd 900 00 1961 refers to the measurement value of the perpendicular longest axis
of the patient 900 00 1961.
taking into consideration the temporal aspect in the
representation of neuroimaging features is needed es-
pecially in longitudinal imaging studies to, for exam-
ple, evaluate cancer treatment response. In this con-
text, we recommend to select a logic-based language
that is capable to represent n-ary relationships. The
second limit is intrinsic to the problem of logical con-
tradiction that is due to the fact that radiologists des-
cribe what they observe based on their thoughts and
experiences. As a consequence they may describe
differently the reality and produce different clinical
records about the identified entities. To resolve the
disagreement in interpretation many medical systems
require preserving in data entry a single correct value
for the evaluated feature to facilitate the aggregation
of data. According to Rector et al., electronic medi-
cal records should allow the presence of conflicting
statements, multiple measurements, etc. to faithfully
reflect the reality of clinical practice. Thus, they pro-
pose three types of foundational information models
to describe (a) the medical records, (b) the state of
the patient and (c) clinical care (Rector et al., 1991)
and they consider that a meta-language should be used
to separate between what can be said and what actu-
ally occurs, and avoid the problem of inconsistency.
Smith et al. have mentioned that even the adoption of
a meta-language cannot remove errors because medi-
cal dialogues are also subject to error (Smith et al.,
2006). In our work, we have stored (a) observations
in separate data sets and we have not included (b and
c) the meta-observation level.
The experimental work regarding the VASARI on-
tology shows that the semantic representation of neu-
roimaging features can enhance search operations;
e.g. the exploitation of the VASARI ontology with the
DL reasoner can resort the list of cerebral tumor epi-
centers that are located in the right temporal lobe by
retrieving cerebral tumor epicenters that are located in
the temporal lobe and the right hemisphere. Certainly,
such classification task cannot be obtained with non
semantic representations such as DICOM SR and the
AIM model. We believe that our work can be reu-
sed in other image-based reasoning contexts as for
example, RECIST criteria that base the tumor clas-
sification task (i.e. measurable and non-measurable
lesions) on the knowledge of the location of the le-
sion and the calculation of its length. Added to the
reasoning task, the consistency checking functiona-
lity offered by OWL reasoners can detect inconsistent
statements that can be caused by inappropriate or er-
roneous diagnosis or treatment, in the clinical context,
until now DICOM SR and the AIM model do not offer
this semantic capability.
5 CONCLUSION
We believe that neuroimaging data should be held in
a structured format that makes their meanings explicit
to the systems and thus facilitate their comprehension
as well as management. Semantic data about imaging
features (measurement values, qualities, lesion com-
ponents, lesion localization, etc.) are important (1)
KEOD 2018 - 10th International Conference on Knowledge Engineering and Ontology Development
72

to support the clinical research on the development of
new imaging biomarkers by combining clinical data
with information coming from different medical dom-
ains (2) to improve the quality of the clinical healt-
hcare that tend to provide personalized treatments to
patients via the use of clinical guidelines that are ba-
sed on evaluation criteria. In this paper, we employed
the VASARI terminology as a proof of concept for the
demonstration of the feasibility and the importance of
making RDF and OWL data available to describe ce-
rebral tumors observations and determining the key
concepts and relationships that are central in their eva-
luation. Our work can be easily expanded to answer
to other use cases; thanks to the modular aspect of
the ontology and to the OWL language that is self-
descriptive (concepts are textually and formally des-
cribed in the ontology to guide users) and extendable.
REFERENCES
Amdouni, E. and Gibaud, B. (2016). Concept-based ver-
sus realism-based approach to represent neuroima-
ging observations. In Keod: Proceedings of The
8th International Joint Conference On Knowledge
Discovery, Knowledge Engineering and Knowledge
Management-Vol. 2, pages 179–185.
Bennett, B., Chaudhri, V., and Dinesh, N. (2013). A voca-
bulary of topological and containment relations for a
practical biological ontology. In International Confe-
rence on Spatial Information Theory, pages 418–437.
Springer.
Bittner, T. and Smith, B. (2004). Normalizing medical on-
tologies using basic formal ontology.
Brinkman, R., Courtot, M., Derom, D., Fostel, J., He, Y.,
Lord, P., Malone, J., Parkinson, H., Peters, B., Rocca-
Serra, P., et al. (2010). Modeling biomedical experi-
mental processes with OBI. J. Biomedical Semantics,
1(S-1):S7.
Ceusters, W. (2012). An information artifact ontology per-
spective on data collections and associated represen-
tational artifacts. In MIE, pages 68–72.
Ceusters, W., Elkin, P., and Smith, B. (2006). Referent
tracking: The problem of negative findings. Studies
in health technology and informatics, 124:741.
Ceusters, W. and Smith, B. (2005). Tracking referents in
electronic health records. Studies in health technology
and informatics, 116:71.
Channin, D., Mongkolwat, P., Kleper, V., Sepukar, K.,
and Rubin, D. (2010). The caBIG annotation and
image markup project. Journal of digital imaging,
23(2):217–225.
Cimino, J. (2006). In defense of the desiderata. Journal of
biomedical informatics, 39(3):299–306.
Clunie, D. (2000). DICOM structured reporting. PixelMed
Publishing.
Clunie, D. (2007). DICOM structured reporting and cancer
clinical trials results. Cancer informatics, 4:33.
Eisenhauer, E., Therasse, P., B. J., Schwartz, L., Sargent,
D., Ford, R., Dancey, J., Arbuck, S., Gwyther, S., M.,
M., et al. (2009). New response evaluation criteria in
solid tumours: revised recist guideline (version 1.1).
European journal of cancer, 45(2):228–247.
ESR (2011). Medical imaging in personalised medicine:
a white paper of the research committee of the euro-
pean society of radiology (esr). Insights into imaging,
2(6):621–630.
Gillies, R., Kinahan, P., and Hricak, H. (2015). Radiomics:
images are more than pictures, they are data. Radio-
logy, 278(2):563–577.
Gkoutos, G., Schofield, P., and Hoehndorf, R. (2012). The
units ontology: a tool for integrating units of measu-
rement in science. Database, 2012:bas033.
Grams, A., Gempt, J., Ringel, F., Soehngen, E., Astner, S.,
Schlegel, J., Meyer, B., Zimmer, C., and Forschler,
A. (2014). Multimodal imaging to delineate tumor
heterogeneity in cerebral gliomas. Open Journal of
Radiology, 4(02):182.
Gruber, T. (1993). A translation approach to portable onto-
logy specifications. Knowledge acquisition, 5(2):199–
220.
Gutman, D., Cooper, L., Hwang, S., Holder, C., Gao, J.,
Aurora, T., Dunn Jr, W., Scarpace, L., Mikkelsen,
T., Jain, R., et al. (2013). Mr imaging predictors
of molecular profile and survival: multi-institutional
study of the tcga glioblastoma data set. Radiology,
267(2):560–569.
Kahn J.R., C., Langlotz, C., Channin, D., and Rubin, D.
(2011). Informatics in radiology: an information mo-
del of the dicom standard. Radiographics, 31(1):295–
304.
Levy, M., Freymann, J., Kirby, J., Fedorov, A., Fennessy,
F., Eschrich, S., Berglund, A., Fenstermacher, D., Tan,
Y., G. X., et al. (2012). Informatics methods to enable
sharing of quantitative imaging research data. Magne-
tic resonance imaging, 30(9):1249–1256.
Levy, M., OConnor, M., and Rubin, D. (2009). Seman-
tic reasoning with image annotations for tumor asses-
sment. In AMIA Annual Symposium Proceedings, vo-
lume 2009, page 359. American Medical Informatics
Association.
Madhavan, S., Zenklusen, J., Kotliarov, Y., Sahni, H., Fine,
H., and Buetow, K. (2009). Rembrandt: helping per-
sonalized medicine become a reality through integra-
tive translational research. Molecular cancer rese-
arch, 7(2):157–167.
M
¨
oller, M., Regel, S., and Sintek, M. (2009). Radsem: Se-
mantic annotation and retrieval for medical images.
In European Semantic Web Conference, pages 21–35.
Springer.
Mungall, C., Gkoutos, G., Washington, N., and Lewis, S.
(2007). Representing phenotypes in OWL. In Pro-
ceedings of the OWLED 2007 Workshop on OWL: Ex-
perience and Directions. Innsbruck, Austria Edited by
Golbreich C, Kalyanpur A, Parsia B.
Oberkampf, H., Zillner, S., Bauer, B., and Hammon, M.
(2012). Interpreting patient data using medical back-
ground knowledge. ICBO, 897:3.
Semantic Representation of Neuroimaging Observations: Proof of Concept based on the VASARI Terminology
73

Porz, N., Bauer, S., Pica, A., Schucht, P., Beck, J., Verma,
R., Slotboom, J., Reyes, M., and Wiest, R. (2014).
Multi-modal glioblastoma segmentation: man versus
machine. PloS one, 9(5):e96873.
Rector, A.L., N. W. K. S. et al. (1991). Foundations
for an electronic medical record. Methods Inf Med,
30(3):179–186.
Rubin, D., Mongkolwat, P., and Channin, D. (2009). A se-
mantic image annotation model to enable integrative
translational research. Summit on translational bioin-
formatics, 2009:106.
Rubin, D., Willrett, D., O’Connor, M., Hage, C., Kurtz, C.,
and Moreira, D. (2014a). Automated tracking of quan-
titative assessments of tumor burden in clinical trials.
Translational oncology, 7(1):23–35.
Rubin, D., Willrett, D., O’Connor, M., Hage, C., Kurtz,
C., and Moreira, D. (2014b). Automated tracking of
quantitative assessments of tumor burden in clinical
trials. Translational oncology, 7(1):23.
Scheuermann, R., Ceusters, W., and Smith, B. (2009).
Toward an ontological treatment of disease and di-
agnosis. Summit on translational bioinformatics,
2009:116.
Seifert, S., Kelm, M., Moeller, M., Mukherjee, S., Caval-
laro, A., Huber, M., and Comaniciu, D. (2010). Se-
mantic annotation of medical images. In SPIE medical
imaging, pages 762808–762808. International Society
for Optics and Photonics.
Sirin, E., Parsia, B., Grau, B., Kalyanpur, A., and Katz, Y.
(2007). Pellet: A practical owl-dl reasoner. Web Se-
mantics: science, services and agents on the World
Wide Web, 5(2):51–53.
Smith, B. (2006). From concepts to clinical reality: an es-
say on the benchmarking of biomedical terminologies.
Journal of biomedical informatics, 39(3):288–298.
Smith, B., Ashburner, M., Rosse, C., Bard, J., Bug, W.,
Ceusters, W., Goldberg, L., Eilbeck, K., Ireland, A.,
Mungall, C., et al. (2007). The OBO Foundry: coor-
dinated evolution of ontologies to support biomedical
data integration. Nature biotechnology, 25(11):1251–
1255.
Smith, B. and Ceusters, W. (2010). Ontological realism:
A methodology for coordinated evolution of scientific
ontologies. Applied ontology, 5(3-4):139–188.
Smith, B., Ceusters, W., Klagges, B., K
¨
ohler, J., Kumar, A.,
Lomax, J., Mungall, C., Neuhaus, F., Rector, A., and
Rosse, C. (2005a). Relations in biomedical ontolo-
gies. Genome biology, 6(5):R46.
Smith, B., Kumar, A., and Bittner, T. (2005b). Basic formal
ontology for bioinformatics. Journal of Information
Systems, pages 1–16.
Smith, B., Kusnierczyk, W., Schober, D., and Ceusters, W.
(2006). Towards a reference terminology for ontology
research and development in the biomedical domain.
In KR-MED, volume 2006, pages 57–66.
Tudorache, T., Nyulas, C., Noy, N., and Musen, M. (2013).
Webprot
´
eg
´
e: A collaborative ontology editor and kno-
wledge acquisition tool for the web. Semantic web,
4(1):89–99.
Van Soest, J., Lustberg, T., Grittner, D., Marshall, M.,
Persoon, L., Nijsten, B., Feltens, P., and Dekker, A.
(2014). Towards a semantic pacs: using semantic web
technology to represent imaging data. Studies in he-
alth technology and informatics, 205:166.
Velazquez, E., Meier, R., Dunn Jr, W., Alexander, B., Wiest,
R., Bauer, S., Gutman, D., Reyes, M., and Aerts, H.
(2015). Fully automatic gbm segmentation in the tcga-
gbm dataset: Prognosis and correlation with vasari fe-
atures. Scientific reports, 5.
KEOD 2018 - 10th International Conference on Knowledge Engineering and Ontology Development
74
