
Dynamic Detection of Cytomegalovirus in Breastmilk
Towards a Device for Self Monitoring Risks of Postnatal Infection
S. Py
1
, A. Guitton
2
, F. Lardet-Vieudrin
2
, N. Marthouret
2
, L. Pazart
1
, A. Coaquette
3
, W. Boireau
2
,
G. Thiriez
4
, G. Herbein
3, 5
and B. Wacogne
1, 2
1
INSERM-CIC 1431, Besançon University Hospital, Besançon, France
2
FEMTO-ST Institute, University of Bourgogne Franche-Comté, CNRS, Besançon, France
3
Laboratory of Virology, Besançon University Hospital, Besançon, France
4
Department of Neonatal Medicine, Besançon University Hospital, Besançon, France
5
UPRES EA4266, SFR FED 4234, Pathogens and Inflammation Laboratory, Department of Virology,
University of Bourgogne Franche-Comté, Besançon, France
Keywords: Cytomegalovirus, Screening, Biochips, Preterm Infants, Breastfeeding, Self-Testing.
Abstract: Human cytomegalovirus (HCMV) infection is a major cause of morbidity worldwide especially in newborn
infants. While congenital HCMV infection affects 2-5% of preterm newborns, the risk of postnatal infection
particularly through breast milk is higher in this population (prevalence about 20%) since more than one
mother on two is affected. Congenital and postnatal infection can lead to important clinical complications
such as deafness, learning disabilities, and mental retardation during childhood. Neonatologists are squeezed
in their clinical practice: either breastfeeding is favored without any milk treatment going on exposure of
preterm infants to a potential infection, or milk is systematically treated by freezing or pasteurization but
with deprivation of non-at-risk infants from the benefits of fresh milk. In this position paper, we propose a
possible solution to differentiate milk with risk of HCMV contamination from milk without any risk. This
would allow subsequent adaptation of the milk feeding strategy. Also, because the HCMV contamination
peak appears 4 to 8 weeks after birth, the work we present here should lead to a device meant to be used
both at hospital and at home in a self-testing manner.
1 INTRODUCTION
Human cytomegalovirus (HCMV), rarely dangerous
for immune-competent person, is a real threat for
immune-depressed people (organ transplanted or
pregnant women). HCMV is the most frequent
etiologic agent of congenital and postnatal infection
of newborns and can have a significant impact on
the neurosensory development of newborns and
especially preterm infants (Hayashi et al. 2011).
Recent studies showed that postnatal HCMV
infection in preterm infants can lead to serious
clinical consequences and can lead to death in rare
cases (Lanzieri et al. 2013; Hamele et al. 2010;
Hamprecht et al. 2008). Although the long-term
follow-up of the neurosensory development of
congenitally infected preterm infants is well
documented, very few studies concern postnatal
infected preterm infants. However, actual data
suggest a negative influence on long-term cognitive
development (Kurath et al. 2010; Bevot et al. 2012;
Goelz et al. 2013).
Breastfeeding is now clearly recognized as being
superior to artificial feeding. However, breastfeeding
plays a major role in the epidemiology of
transmission and postnatal HCMV infection. It is
now well established that HCMV is excreted in milk
from about 80% of seropositive lactating mothers
(Kurath et al. 2010). Excretion can start since the
first post-partum week and reaches a maximum
value 4 to 8 weeks after birth. Mother-to-child
transmission generally occurs during this period
(Hamprecht et al. 2003, 2008). About 20% of
breastfed children are HCMV positive and the
200
Py, S., Guitton, A., Lardet-Vieudrin, F., Marthouret, N., Pazart, L., Coaquette, A., Boireau, W., Thiriez, G., Herbein, G. and Wacogne, B.
Dynamic Detection of Cytomegalovirus in Breastmilk - Towards a Device for Self Monitoring Risks of Postnatal Infection.
DOI: 10.5220/0006634602000205
In Proceedings of the 11th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2018) - Volume 1: BIODEVICES, pages 200-205
ISBN: 978-989-758-277-6
Copyright © 2018 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

contamination risk increases with lactating duration.
For preterm infants, the weak transmission of
mother antibodies and the non-mature immune
system increases the risk of symptomatic HCMV
infection.
Today, almost no national recommendations on
the manipulation of the breast milk of HCMV
positive mothers are proposed. Methods exist to treat
breast milk. Systematic pasteurization is not done
because it alters the immune components of milk
(Chang et al. 2013). Freezing at -20 °C does not
completely destroy the virus (Yoo et al. 2015).
Neonatologists are then squeezed in their clinical
practice between the potential risk to transmit
infection when breast milk is not treated and the risk
to favor complications if the milk is treated. The
ideal solution would be to differentiate “at risk” and
“non at risk” situations in order to treat only the “at
risk” cases. This would be extremely more
satisfactory than a systematic attitude.
The techniques currently available to detect
HCMV are Polymerase Chain Reaction (PCR) and
cell culture but are not adapted to rapid and early
HCMV detection in breast milk (Hamprecht et al.
2008). Indeed, PCR is expensive and time
consuming and cell culture gives a result only
several days after sampling. Enzyme-linked
immunosorbent assay (ELISA) can hardly be used
because in this static fluidic configuration, nutritive
components of the milk shield the antibodies used
for detection.
In order to help clinicians in their practice, our
project aims at developing a simple, fast and low-
cost system namely a rapid diagnostic test (RDT)
which could be used at the hospital or at home in a
self-evaluation manner.
The biological and technological hypotheses
developed in this position paper concern the fact that
in a fluid flow configuration, HCMV remains
available for bio-recognition by specific antibodies.
Using specifically marked detection antibodies, an
optical measurement is possible. Prior to the
development of a RDT, a technico-clinical study is
ongoing to develop an integrated immuno-combined
device able to detect HCMV in native breastmilk.
The goal is to determine the most suitable antibodies
association to be used. In section 2, we show that a
fluid flow configuration is much more efficient than
ELISA-like methods although the laboratory model
we used suffered from instabilities. In section 3, we
present the very first results obtained with a stable
and reliable integrated device. In part 4, and in line
with the scope of a position paper, we present
scientific and socio-economic impacts the foreseen
RDT (still to be fabricated) could address.
2 PRINCIPLE AND
PRELIMINARY EXPERIMENTS
2.1 Biochip Principle
The technique we propose to detect HCMV is based
on antigen/antibody recognition. Biochips were
designed and homemade. The biosensor consists of a
polystyrene biochip coated by human polyclonal
anti-HCMV antibodies as shown in figure 1. HCMV
potentially present in the breast milk sample is
captured by these antibodies. HCMV detection uses
a specific secondary antibody coupled to horseradish
peroxidase (HRP) enzyme which subsequently
recognizes the captured virus. After addition of a
substrate of this enzyme, a colorimetric reaction
occurs and allows transforming the substrate to a
blue product. Then, the optical reading relies on an
absorbance measurement at about 640 nm or 450 nm
if the reaction is stopped with sulfuric acid.
Figure 1: Principle of the HCMV biosensor.
2.2 Preliminary Tests
The first HCMV detection tests were conducted with
an initial version of a homemade laboratory model
in parallel with ELISA experiments. The goal was to
compare dynamic (laboratory model) and static
(ELISA) conditions in terms of virus detection. The
laboratory model consists of a fluidic system
containing a biosensor inserted into a cartridge.
Syringes contain reagents which are driven on
biosensor surface. The system allows controlling the
fluid flows and interaction durations. The biosensor
is sandwiched between a light-emitting diode (LED)
emitting in the red wavelength region and a
photodiode as shown in figure 2(a). Initially, the
device was designed for 4 simultaneous tests.
Preliminary positive results were obtained with
the device from artificially contaminated breast milk
samples. Indeed, virus concentrations as low as 6
µg/mL can be detected using this simple opto-fluidic
model with a higher absorbance value than the one
Dynamic Detection of Cytomegalovirus in Breastmilk - Towards a Device for Self Monitoring Risks of Postnatal Infection
201
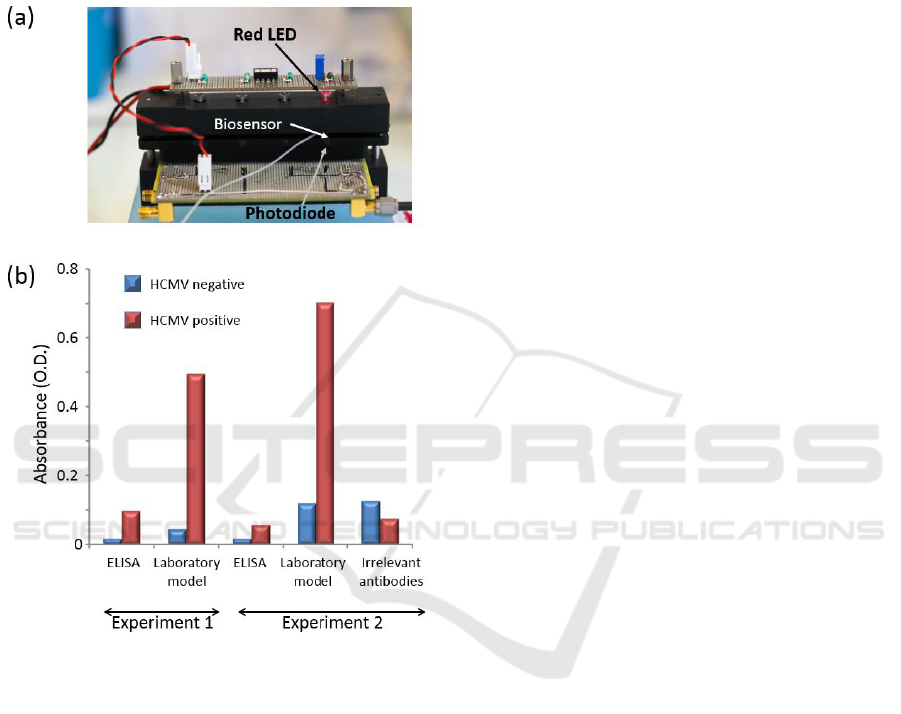
obtained with ELISA technique (figure 2(b)).
Furthermore, it can be seen that absorbance levels
achieved in ELISA are of the same order of
magnitude as those obtained using irrelevant
antibodies. This highlights the fact that a static
configuration is not suitable for virus detection in
native breastmilk.
Figure 2: HCMV (~6 µg/mL) detection in artificially
contaminated breastmilk in static conditions (ELISA) and
dynamic conditions (laboratory model). Two different
experiments performed with two distinct batches of
capture antibodies are presented.
In view of the first results obtained using
breastmilk, dynamic conditions proved to be
extremely efficient for HCMV detection. Indeed,
optical densities as high as 0.7 are achieved in
dynamic conditions against 0.1 maximum in static
condition. As previously mentioned, the high
proportion of lipids in this biological fluid makes
capture and detection of viruses more difficult due to
a shield effect of the components. These results
should be validated using naturally HCMV
contaminated breastmilk sample and with a much
larger number of samples. Indeed, for this position
paper, only a few experiments have been conducted.
We still don't have quantitative estimation of the
reliability and repeatability.
The laboratory model presented in figure 2
proved to be inappropriate for a large scale study.
Indeed, we experienced number of experimental
problems. The mechanical stability was not high
enough to ensure reproducible measurements. The
gap between the LED and the photodiode is open to
air and variations in the ambient light jeopardize
measurements. Optical densities presented in figure
2(b) were measured after the experiment was
finished by means of an optical spectrometer.
Therefore, no continuous absorption measurements
were possible.
In fact, the first laboratory model was a copy of
the model used to experiment on red cell immuno-
capture (Charrière et al. 2012). Biological reactions
involved in these experiments lasts for a few
minutes only. Optical absorption of red cells is
orders of magnitude higher than the absorption
measured in the current experiments. Also,
experimentation times were a few minutes for red
cells and more than 30 minutes for HCMV.
Therefore, variations of the optical power emitted by
the LED become an issue. Stabilization of the
emitted power is then required in our experiments.
This is why we designed a new, compact and opto-
mechanically stable prototype which allows
continuously recording optical absorption during the
experiment.
3 A STABILIZED AND
INTEGRATED PROTOTYPE
3.1 Description of the New Prototype
A Computer Aided Design of the new prototype is
presented in figure 3. A reaction chamber defined in
a sheet of polydimethylsiloxane is sandwiched
between two biosensors which are used to enhance
the sensor’s sensitivity. Sample and reagents are
introduced in and evacuated from the reaction
chamber through commercially available
microfluidic connectors. Fluid flows are controlled
using motorized syringes. Fluidic sealing is ensured
using two micro O-rings. Embedded spacers are
used to ensure that the thickness of the reaction
chamber (few tens of µm) remains constant between
experiments (new biosensors must be used for each
experiment). Also, embedded stops are used to
ensure a vertical repositioning between experiments.
Stops and spacers are not visible in the figure.
BIODEVICES 2018 - 11th International Conference on Biomedical Electronics and Devices
202
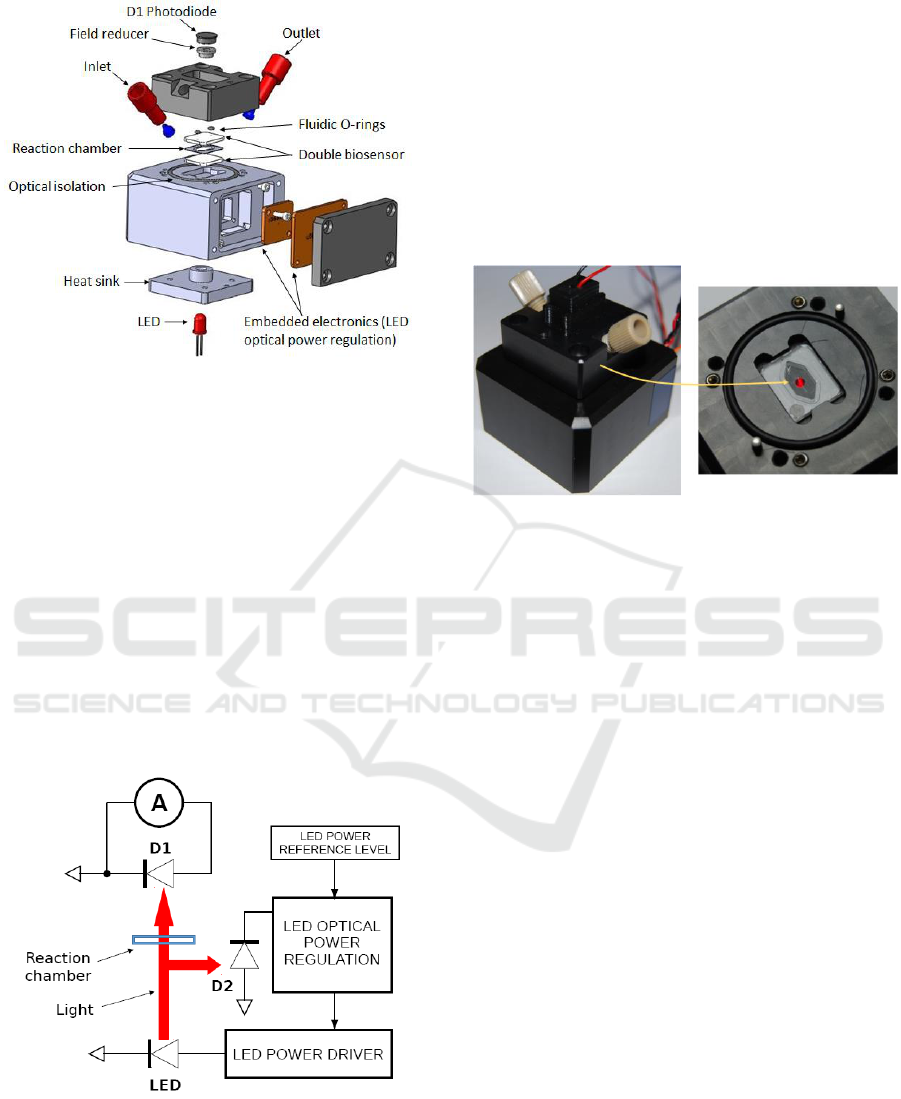
Figure 3: CAD view of the new opto-fluidic prototype.
The optical absorption measurement is provided
by the couple LED plus D1 photodiode. The LED is
embedded in a heat sink in order to reduce self-
heating due to long operations (more than 30 min).
Indeed, LED heating results in a continuous
decrease of its emitted power. Although the heat
sink cannot completely stabilized the LED
temperature, an optically stable operation is ensured
with an embedded electronic optical power
regulator. The beam sensing D2 photodiode is
inserted into a spacer. A 2 mm diameter hole is
drilled in the spacer. It allows collecting only light
which has propagated in straight line from the LED.
Isolation from ambient light is obtained using a large
diameter O-ring placed in a circular groove.
Figure 4: Schematic representation of the driving and
detection electronics.
Driving and detection electronics uses surface
mount component on printed circuit boards (PCBs)
directly integrated in the device. Internal wiring is
not shown in the figure. The D1 photodiode is
integrated in the top part of the prototype while the
optical power regulator and PCBs are integrated in
the bottom part. The general electronic circuit is
schematically described in figure 4. D1 is the
photodiode which produces a current proportional to
the absorption signal while D2 is the photodiode
dedicated to the LED power regulation. Figure 5
shows the actual prototype with a closer view to the
reaction chamber. Stops and light shield are also
visible.
Figure 5: Pictures of the prototype.
3.2 Sample Preparation
For this set of experiments, breastmilk samples were
not available. Experiments were then conducted
using a simplified biological model. Biochips and
samples were prepared as follows. Three biochips
were coated by human polyclonal anti-HCMV
antibodies at a concentration of 20 ng/µL in
carbonate/bicarbonate buffer overnight at 4°C. The
next day, a rinsing of chips with phosphate buffered
saline (PBS) 1X followed by a saturation step of the
surface with Bovine Serum Albumin 10% during 1 h
at room temperature (RT) was performed. A mixture
of commercial HCMV antigen (pure or diluted at
1/25) and an anti-HCMV antibody conjugated to
HRP diluted at 1/5000 (ETI-CYTOK-M reverse plus
Diasorin kit) was injected on two of the chips and
incubated 1 h at RT. A negative control was realized
by incubation of one chip with the antibody alone
diluted in PBS. Here, we call CMVneg the negative
control solution, CMV1/25 the test solution with the
antigen diluted at 1/25 and CMVpure the test
solution with no dilution of the antigen. Three
washing of 1 mL were realised with a wash solution
composed of PBS-Tween (ETI-CYTOK-M reverse
plus, Diasorin) and the HRP substrate (hydrogen
peroxide and tetramethylbenzidine) was incubated
during about 50 min at RT in the prototype.
Absorption measurements were collected during this
time of incubation.
Dynamic Detection of Cytomegalovirus in Breastmilk - Towards a Device for Self Monitoring Risks of Postnatal Infection
203
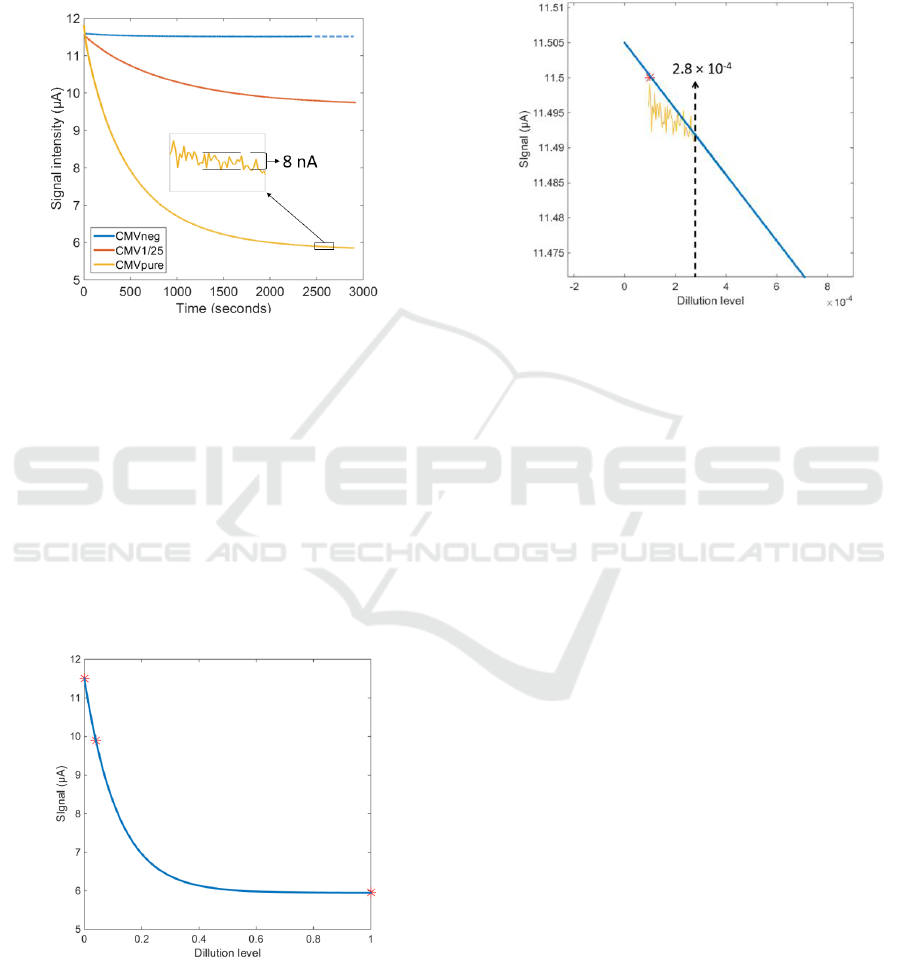
3.3 Experimental Results Obtained
with the New Prototype
Figure 6 shows the photodiode signal recorded over
the whole experiment for the CMVneg, CMV1/25
and CMVpure samples.
Figure 6: Absorption recorded during the whole
experiment session.
It can be observed that the electronic circuits
perfectly regulate the LED emitted power. The noise
level is extremely small as illustrated in the insert of
figure 6. The noise peak to peak level is of the order
of 8 nA which correspond to a root mean square
value less than 2nA. Also, the exponential decay of
the signal is clearly observed.
Figure 7 shows the signal recorded at the end of
the experiments as a function of the dilution levels:
1/1 CMVpure, 1/25 for CMV1/25 and 1/∞ for
CMVneg.
Figure 7: Final signal as a function of the dilution levels.
The blue curve in the figure is an exponential
fitting of the data. Of course, this is a very rough
approximation because of the very few experimental
data. However, it is expected that the viral charge in
the actual samples will be quite low. This means that
only the left part of the curve should be considered
as representative of real situations. Zooming this
region of the figure and reporting the noise level
allows roughly estimating the detection limit in
terms of dilution level (figure 8).
Figure 8: A tentative estimation of the detection limit.
The value of 2.810
-4
represents a sample where
the antigen dilution is 1/3500. However, this is
nothing but a rough estimation obtained using a
simplified biological model. We are now starting a
clinical trial in order to assess the accuracy and the
potential of the opto-fluidic technique we propose. If
successful, a step ahead will have been passed in the
direction of a home-use RDT. The impacts of such a
device are summarized in the next section.
4 SOCIO-ECONOMICAL
IMPACTS
As mentioned above and in the scope of a position
paper, we present the scientific and socio-economic
impacts such a device potentially.
HCMV is an opportunistic virus that infects a
large proportion of the population worldwide and
results in an asymptomatic latent infection in healthy
subjects. The disease burden is both medical and
economic. HCMV infection can lead to severe
diseases in the absence of an effective immune
response. HCMV is also the leading cause of
neonatal viral infection and can have a significant
impact on the neurosensory development of
newborns and especially preterm infants. HCMV
infection may result from maternal-fetal
transmission during pregnancy (2-5% of very
premature infants) or postnatal transmission (about
20% of children). Currently, viral status of breast
BIODEVICES 2018 - 11th International Conference on Biomedical Electronics and Devices
204

milk is not explored in practice and, depending on
the health centers, milk is systematically inactivated
or breastfeeding is continued with raw milk without
any caution. Finally, although the cost of HCMV
infection in the hospital community has not yet been
clearly established, it appears that HCMV infections
cost hundreds of thousands of euros each year to the
French health system in terms of medical and
surgical expenses, especially in taking care of long-
termed disabled children and adults infected early in
life or during pregnancy.
Therefore, an easy-to-use secured RDT to detect
HCMV infection in breastmilk from lactating
women of preterm infants is urgently needed. An
answer to this need is the subject of this
communication. About 8000 very preterm infants
could benefit from this test each year in France and
13 million worldwide. Considering that the test
should be repeated several times for a same couple
mother/baby pair in the early months of
breastfeeding, the market worth to be taken into
account. Indeed, the test will be practiced both at
hospital and at home since the peak of viral
excretion in breast milk occurs generally after
hospitalization of the child. In addition to detection
by caregivers in departments of neonatal medicine,
self-diagnosis of mothers will be possible given ease
of use and reading of this type of test.
5 CONCLUSIONS
Although the risk of HCMV congenital infection is
relatively low, the risk of postnatal contamination, in
particular via breast milk, can be dramatic for
preterm infants. Currently, the question is: should
we favor a better development and take the risk of
using contaminated breast milk, or should we use
treated milk, even when the HCMV infection is low
enough to be considered safe?
To address this problem, and in the current
context of breastfeeding promotion, we propose to
develop a HCMV biosensor based on sandwich
ELISA principle in a dynamic flow configuration
(lateral flow immunochromatography). This position
paper presents studies that have just started, but we
think it is possible to set-up an easy to use and rapid
"point-of-care" device to detect HCMV in
breastmilk. Therefore, a third answer can be
proposed to the above mentioned question. The idea
is to screen HCMV on a routine basis and to define a
personalized feeding strategy for “at risk”
population only. Without such a rapid HCMV test,
this third solution may never exist.
ACKNOWLEDGMENTS
This work is funded by the “APICHU-RBFC call” in
2015.
The authors would like to thank FEMTO-
engineering for the design and manufacture of the
prototype.
REFERENCES
Bevot, A, et al., 2012. Long-Term Outcome in Preterm
Children with Human Cytomegalovirus Infection
Transmitted via Breast Milk. Acta Paediatrica 101
(4): e167-172.
Chang, J.C., et al., 2013. Influence of Prolonged Storage
Process, Pasteurization, and Heat Treatment on
Biologically-Active Human Milk Proteins. Pediatrics
and Neonatology 54 (6): 360‑66.
Charrière, K., et al., 2012. SmarTTransfuser: A biochip
system for the final ABO compatibility test.
BIODEVICES 2012 - Proceedings of the International
Conference on Biomedical Electronics and Devices,
janvier, 257‑62. SCITEPRESS.
Goelz, R., et al., 2013. Long-Term Cognitive and
Neurological Outcome of Preterm Infants with
Postnatally Acquired CMV Infection through Breast
Milk. Archives of Disease in Childhood. Fetal and
Neonatal Edition 98 (5): F430-433.
Hamele, M., et al., 2010. Severe Morbidity and Mortality
with Breast Milk Associated Cytomegalovirus
Infection. The Pediatric Infectious Disease Journal 29
(1): 84‑86.
Hamprecht, K., et al., 2008. Cytomegalovirus transmission
to preterm infants during lactation. Journal of Clinical
Virology 41 (3): 198‑205.
Hamprecht, K., et al., 2003. Rapid detection and
quantification of cell free cytomegalovirus by a high-
speed centrifugation-based microculture assay:
comparison to longitudinally analyzed viral DNA load
and pp67 late transcript during lactation. Journal of
Clinical Virology 28 (3): 303‑16.
Hayashi, S., et al., 2011. Transmission of cytomegalovirus
via breast milk in extremely premature infants. J
Perinatol 31 (6): 440‑45.
Kurath, S., et al., 2010. Transmission of Cytomegalovirus
via Breast Milk to the Prematurely Born Infant: A
Systematic Review. Clinical Microbiology and
Infection 16 (8): 1172‑78.
Lanzieri, T., et al., 2013. Breast Milk-Acquired
Cytomegalovirus Infection and Disease in VLBW and
Premature Infants. Pediatrics 131 (6): e1937-1945.
Yoo, H.S., et al., 2015. Prevention of Cytomegalovirus
Transmission via Breast Milk in Extremely Low Birth
Weight Infants. Yonsei Medical Journal 56 (4):
998‑1006.
Dynamic Detection of Cytomegalovirus in Breastmilk - Towards a Device for Self Monitoring Risks of Postnatal Infection
205
