
Electrolytic Wire as an Alternative Bio Interface:
A Case Study in Plant Tissue
Ernane José Xavier Costa, Luciana Vieira Piza and Ana Carolina de Sousa Silva
Computational and Applied Physics Lab – Basic Science Department – FZEA, University of São Paulo,
Rua Duque de Caxias Norte, Pirassununga, Brazil
Keywords: Bio-electrode, Bioelectricity, Aqueous Junction.
Abstract: One of challenge in physiological research is how to reconnect bioelectricity or turn on the transduction of
signals in biological systems such as nerves and other tissues after some injuries or degenerative process. The
electrical interactions in biological system can be understood by looking into the extracellular space between
cells. In such spaces, contain ions and several charged organic molecules. Despite the fact that the common
way to artificially link biological systems reported in the literature is by using metallic wires or bio-potentials
electrodes, this paper present the hypothesis that an electrolytic conductor is more efficient to transmit
information between biological systems when compared to the transmission carried out using electronic
conductors. To test this hypothesis an experiment was conducted using two leaves of ornamental plant (Agave
atenuata) connected by means of electronic and electrolytic wire and stimulated with electrical square waves
with 1V of amplitude at 20Hz. The quality of signal transmitted using electronic conductor was compared to
the signal transmitted using electrolytic conductor by measuring the distortion of the signal transmitted. The
results shown that the transmission of stimuli using electrolytic wire is less disturbed than by using electronic
wire.
1 INTRODUCTION
In this century, many technological approaches have
been tested to develop systems that link biological
system with electronic systems (Navarro et al., 2005)
or others biological interfaces (Agnew et al., 1989;
Hwang et al.,2016) to improve or to restore sensory
functions in several kinds of diseases. A number of
scientific approaches have been presented in order to
the establishment of state-of-art in the biological and
bionic interfaces (Lauer et al.,2000).
Each interaction among biological system involve
bioelectrical information generated, transmitted, and
broadcasted through organics pathways that link the
bio-processors, biosensors and bio-actuators
embedded throughout the organic systems (Enderle,
2011). The development of an interfacing method to
artificially place information into the biological
system, or to monitoring the information from it,
would improve the way one can make the biological
systems interacts each other and with the artificial
systems.
Bioelectrical signals like EEG, EKG and EMG are
detected by means of skin contact with electrodes
connected to signal amplifiers (Enderle, 2011). The
electrical contacts are performed by using
biocompatible conductive hydrogels applied between
skin and electrode. This process create an interface
that influences the quality of signal and depending of
this interface the signal quality is improved due the
presence of conductive hydrogel (Pedrosa et
al.,2017). The use of such electrolytic gels provides
an effective contact but the information acquired from
the biological system mediated by ionic interaction
must be transduced in to electronic information in the
metal electrode side (Clement et al.,2011). Despite
this electrode provide excellent signal quality there
are several difficulties related to it is use and to
overcome such difficulties, some alternative
electrodes that would be acceptable in physiological
research were tested, for example dry electrodes
(Mesiane et al.,2013). Even the most efficient
electrodes still present an interface between an
electronic conductor and the biological medium.
So far, an evaluation of electrodes technology
applied to link biological system is little understood,
this paper present an investigation on conductive
hydrogels capability to be used like an electrolyte
Costa, E., Piza, L. and Silva, A.
Electrolytic Wire as an Alternative Bio Interface: A Case Study in Plant Tissue.
DOI: 10.5220/0006549101330138
In Proceedings of the 11th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2018) - Volume 4: BIOSIGNALS, pages 133-138
ISBN: 978-989-758-279-0
Copyright © 2018 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
133

wire to conduct bioelectrical signal in plant tissue and
then we suggest that this kind of wire could be
considered as a future solution to bio-electronic
interface.
2 ELECTROLYTIC CONDUCTOR
The electrical interactions in biological system can be
understood by looking into the extracellular space
between cells. In such spaces, with no more than 150
Å wide, contain ions and several charged organic
molecules that are not only sensitive to electric fields
but also generate their own fields and these electrical
interactions flow to neighbouring disturbing it. For
the sake of simplicity, it is inferred that this space is
filled by an electrolytic solution.
Before the application of an external field in an
electrolytic solution, it is behaviour can be
understood by having a time-averaged electro
neutrality in the electrolyte i.e. the net charge in any
macroscopic volume of solution is zero because the
total charge due to the positive ions is equal to the
total charge due to the negative ions. If an electric
field arise in an electrolytic solution, the opposite
occur. The effect of this ionic drift on the state of
charge of an electrolytic solution is to send positive
ions near the positively charged area and the negative
ions near the negatively one producing a spatial
separation of charge. Because of this gross charge
separation, the electro neutrality tends to be disturbed.
Additionally, the separated charge tends generate its
own field, which would be contrary to the externally
applied field but equal in magnitude and then the
resultant field in the solution will vanish. This not
imply that an electrolytic solution would sustain only
a transient migration of ions because in practice, an
electrolytic solution can conduct electricity i.e., keep
a continuous flow of ions. It can be understand by
comparing electrolytic solution with a metallic
conductor (Horno et al.,1992).
There is a lattice of positive ions that hold their
equilibrium positions during the conduction process
in a metallic conductor and, there are free conduction
electrons, which transport charge. In the electrolytic
conductor, however, if an electrical contact to and
from the electrolyte is mediated by an interface
electricity in the interface side and ions carry the
charge in the electrolytic solution and if a change of
charge carrier at the interface exist then a steady flow
of charge in the entire circuit will be maintained. This
electron transfers phenomenal between ions and the
interface result in chemical changes.
The occurrence of a reaction at interface side is
equivalent to the removal of equal amounts of
positive and negative charge from the solution. In
other words, when electronic disturbance reactions
occur in the interface side, the ionic drift does not lead
to the charges separation and no opposite field is
created and then the flow of charge can continue i.e.,
the solution conducts, it is an electrolytic conductor.
In view of the above, the hypothesis that an
electrolytic conductor is more efficient to transmit
information between biological systems when
compared to the transmission carried out using
electronic conductors is postulated. To test this
hypothesis an experimental setup using plant tissue is
presented.
3 PLANT BIOELETRICITY
Literature studies shown that bioelectrical signals
play central role in both cell-cell and long-distance
communication in plants (Van Bel et al.,2014). There
are four types of bioelectrical signals generated by
plants: oscillatory potentials – OP, action potentials –
AP, variations potentials – VP and system potentials
–SP. Although AP, SP and VP is generated by
distinct molecular dynamic, OP arise from complex
mixing of bioelectric activities (AP, SP,VP) by means
of a complex web of systemic interaction at short a
long range level (Cabral et al.,2011; From et al.,
2013; Choi et al., 2016). Plant Bio-potentials has lot
of important information related with plant behavior
and then can be used for test several aspects of
bioelectricity.
4 MATERIAL AND METHODS
This experiment was carried out in Applied Physics
and Computational Laboratory (LAFAC) at the
Faculty of Animal Science and Food Engineering,
University of São Paulo (USP), Brazil.
Leaves of ornamental plants Agave atenuata
were collected in pairs and packaged in a beaker
containing water. One leave was designed to be
stimulate (Ls) and another leave (Lr) was designed to
receive the stimuli transmitted by Ls. Each
experimental section took place over 1 hour after
leaves preparation. The experiment were conducted
in the Faraday cage with controlled light incidence,
and the temperature and relative humidity in the cage
did not change significantly during the experience. To
monitor the bioelectricity transmission between Ls
BIOSIGNALS 2018 - 11th International Conference on Bio-inspired Systems and Signal Processing
134
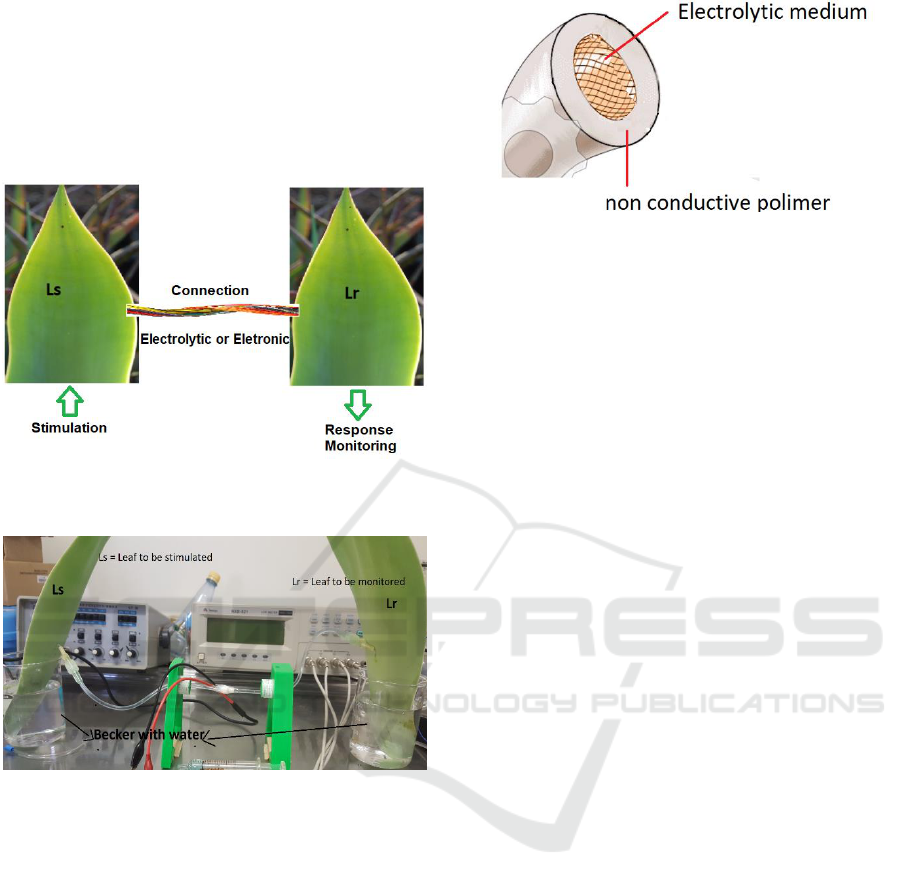
and Lr, they were connected each other in two
experimental arrangement. The first using normal
wire needle electrode connecting the leaves and the
second using electrolytic wire conductor. The
schematic diagram of experimental setup concept is
illustrated in diagram of Figure 1 and experimental
arrangement is illustrated in Figure 2.
Figure 1: Schematic diagr am of experimental setup
concept.
Figure 2: Experimental arrangement.
The electrical signals from the leave Lr were
monitored using two Ag/AgCl disc electrodes
connected to the leave surface, by means of a
conductive aqueous gel as described in Cabral et al.,
(2011). The electrodes were connected with screened
cables to a high-input impedance (≈109 MΩ)
electrometer that sent the data acquired to a computer
by using wireless technology in real-time at sample
rate frequency of 200 Hz.
The electrolytic wire was built by using a flexible
non-conducting polymeric tube with the extremity
needle-shaped to be connected in the leaf tissue and
filled with a hydrophilic polymer known as hydrogels
(Farina et al., 2004). Figure 3 illustrate the
electrolytic wire developed.
Figure 3: Electrolytic wire.
The Ls was stimulated using a square wave
electrical signal with 1 V of amplitude and 20 Hz in
frequency. Square wave was chosen because it is
commonly used in experiments with stimuli (Declan
et al.,2014) and 20 Hz was chosen due the fact that
plants have oscillatory characteristics in a frequency
range from 5 to 15 Hz (Cabral et al., 2011) so 20 Hz
is out of this range. Each experimental arrangement
was repeated six times. To compare the effect of the
type of wire used to transmit the signal from Ls to Lr
the signal acquired was analysed by measuring the
signals to noise and distortion ratio (SINAD)
(Karandjeff et al.,2011, Grigoriev and Kharin, 2011)
of signal acquired in relation of a signal acquired
locally in Ls. The SINAD was used due the
oscillatory characteristic of plant response to the
stimuli. So, the signal source is the Ls leave response
to square wave and the receiver is the Lr bioelectrical
signal acquired. The bioelectrical signal from the Ls
source is converted in to a form convenient for
transmission along the communications channel
represented by connection between the leaves. During
the conversion, the initial information is distorted due
to the fact that the conversion is non ideal.
The distortion measurement was made by using
the Matlab
®
signal processing toolbox and the
normalized SINAD results were presented.
The hydrophilic gel have demonstrated great
potential to be used in biological systems. The
hydrophilic gel polymer is biocompatible due its high
water content. Hydrophilic gel polymer have a high
affinity for water, nevertheless do not dissolve into it,
because of its chemical and physical property; water
molecules can only penetrate into the chains of the
polymer network, subsequently causing swelling and
formation of a hydrogel (Katter et al.,2017). For
convenience was used the hydrogel currently
available in local pharmaceutical market with
conductivity (σ) and constant phase element
parameters (A) measured in some papers in the
literature. The chemical compositions of hydrogel
used are listed as follows: water, disodium
ethylenediaminetetraacetic acid, lithium chloride,
Electrolytic Wire as an Alternative Bio Interface: A Case Study in Plant Tissue
135

propylene glycol, methylparaben and sodium
carbomer. Conductivity (σ) and constant phase
element (A) parameters of the hydrogel used has the
follow value: σ = 2.02 S m
−1
and A = 0.90 x 10
4
Ω/s
ν
(Freire et al., 2010).
5 RESULTS AND DISCUSSION
In Figure 4 the signal acquired in Lr without any
stimulation is shown.
Figure 4: Signal acquired from leaf Lr without any
stimulation and disconnected from Ls leaf.
Figure 5 shown the results of signal transmission
from Ls connected to Lr by mean of copper wire or
electronic conductor. Ls was stimulated by a square
wave with 1 Volts of amplitude and 10 Hz of
frequency.
Figure 5: Leaves connected by electronic conductor.
Experience with electric square wave stimuli.
Figure 6 shown the results of signal transmission
from Ls connected to Lr by mean of electrolytic
conductor.
Figure 6: Leaves connected by electrolytic conductor.
Experience with electric square wave stimuli.
The signal to noise and distortion ratio calculate
for each signal acquired is shown in Table 1.
Table 1: SINAD measurement of signal acquired after
electrical stimulation of the leaf Ls. Mean of six
experimental run.
Transmission
medium
SINAD of signal transmitted
measured in Lr (dB)
Electronic
14.45 ± 0.07
Electrolytic
15.12 ± 0.3
6 A PRELIMINAR MODEL
PROPOSITION
One of challenge in physiological research is how to
reconnect bioelectricity or turn on the transduction of
signals in biological systems such as nerves and other
tissues after some injuries or degenerative process
(Lauer et al.,2000). Some biological systems like in
mammals, looks like wired systems, bio-structures
like the brain send commands to others bio-structure
like muscles. However, the mechanism related to the
information transmission along such structures
remain misunderstood. In fact, the information is
related to the bioelectricity that pass among the
structures like nerves, but this bioelectricity do not
move in such structures as electrons move through a
metallic conductor, for example, the rate of passage
in bio structures is slower than that of electrons
through a wire.
Another fact is the relationship between the
bioelectricity and the electrolytic medium present in
the extracellular medium of the biological structures,
that is modelled via Nernst-type equation (Horno et
al.,1992). Thus, the theoretical approach to the
passage of bioelectricity through biological systems
is related to the electrolytic conduction.
Despite the fact that the common way to
artificially link biological systems reported in the
literature is by using metallic wires or bio potentials
electrodes (Navarro et al.,2005) the result in this
paper allow us to discuss that another way to do that
is using a electrolytic wire. The main argument to
support this idea is that if two electrolytic solution is
interfaced each other with different ions
concentration and different ions mobility then a
liquid-junction potential (Enderle, 2011) will arise
between them with the magnitude given by:
(1)
When
is the liquid-juction potential;
and
represent the mobility;
and
are the activities
of the ions of each side of liquid-junction; R is the
universal gas constant; T represent the system’s
50 100 150 200 250 300 350 400
-0.5
-0.4
-0.3
-0.2
-0.1
0
0.1
0.2
0.3
0.4
samples (1/s)
Amp. (mV)
BIOSIGNALS 2018 - 11th International Conference on Bio-inspired Systems and Signal Processing
136

temperature; z represent the system’s valence and F
the system’s Faraday constant.
Based in equation (1) the transduction of signal
from junction is not necessarily linear and therefore
can be modelled as illustrated in Figure 7.
Figure 7: A simple non-linear model proposition for
bioelectricity information transmission in an aqueous
interface.
In the model of Figure (7) the ions activities will
act like a negative feedback function F() and the
coefficients
and
represent the mobility
responsible by the nonlinear transduction in the
interface. The signal transduction between the
interfaces can be described as:
u(t) = v(t) – Fy(t) (2)
F represent the ions activities that act like an
unilateral transference function. In this model, the
distortion of the information caused by the interface
is dependent of how the coefficients
,
and the
ions activities are adjusted.
The results presented in this paper, suggest that de
SINAD is related to several property of the interface,
as described in the literature. When a liquid junction,
i.e, an electrolytic wire, makes the transmission, the
model proposed in Figure 7 can give a direction to the
explanation of why the SINAD of signal transmitted
by electrolytic wire is less than the signal transmitted
by electronic wire. Future works will conducted in
order to test if the model proposed will explain the
data obtained. Overall results in this study allow the
follow scientific question: is electrolytic transmission
the alternative way to send signal in biological
structures? To answer this question more experiments
must be done with different kinds of biological
structures but this start point open a door in the area
of bioelectricity transmission.
7 CONCLUSIONS
A new method for transmission of bioelectricity using
electrolytic wire was presented. The signal distortion
of signal transmitted by electrolytic wire was less
than the signal transmitted by an electronic wire. In
addition, a non-linear model to explain the effect of
aqueous junction was proposed and could explain the
results obtained. Overall results allow to conclude
that in plant tissues the transmission of bioelectricity
is less disturbed than by means of electronic
transmission. This research open a new door in the
area of bioelectricity transmission among biological
structures.
ACKNOWLEDGEMENTS
The author would like to thanks the National Agency
for Research Support CNPq (Proc Num, 311084).
REFERENCES
Agnew, W.F., McCreery, D.B., Yuen, T.G.H., Bullara,
L.A. 1989. Histologic and physiologic evaluation on
electrically stimulated peripheral nerve: considerations
for the selection of parameters. Ann Biomed Eng.
17:39–60.
Cabral, E. F., Pécora, P.C., Arce, A.I.C., Tech, A.R.B.,
Costa, E.J.X., 2011. The oscillatory bioelectrical signal
from plant explained by a simulated electrical model
and tested using Lempel-Ziv Complexity. Computers
and Electronics in Agriculture. 76: 1-5.
Choi, W., Hilleary, R., Swanson, J., Kim, S.H., Gilroy, S.,
2016. Rapid, long-distance electrical and calcium
signalling in plants. Annu. Rev. Plant. Biol. 67:287-
307.
Clement, R.G.E., Bugler, K.E., Oliver, C.W., 2011. Bionic
prosthetic hands: A review of present technology and
future aspirations. The Surgeon, vol.9, 336–340.
Enderle, J.D., Bronzino J., 2011. Introduction to
Biomedical Engineering. 3 rd Edition, ISBN:
9780123749796.
Farina, D., Merletti, R., Enoka, R.M., 2004. The extraction
of neural strategies from the surface EMG. Journal of
Applied Physiology. 96(4):1486-1495.
Freire, F C M., Becchi, M., Pontil, S., Miraldi, E., Strigazzi,
A., 2010. Impedance spectroscopy of conductive
commercial hydrogels for electromyography and
electroencephalography. Physiol. Meas. 31:S157–
S167.
Fromm, J., Hajirezaei, M., Becker, V.K., Lautner, S., 2013.
Electrical signalling along the phoem and its
physiological responses in the maize leaf. Front Plant
Sci. 4:1-7.
Grigoriev, V.A., Kharin, V.N., 2011. An estimate of the
error of measuring sensitivity by the SINAD method.
Measurement Techniques, v 54, n 5, p 544-553.
Horno, J., Castilla, J., Gonzalez-Fernandez., C. F. 1992. A
new approach to nonstationary ionic transport based on
Electrolytic Wire as an Alternative Bio Interface: A Case Study in Plant Tissue
137

the network simulation of time-dependent Nernst-
Planck equations. J. Phys. Chem., 96 (2), 854–858.
Hwang, D., Ihn, Y. S., Hwang, S., Oh, S., Kim, K., 2016. A
Preliminary Study on the Method for Stable and
Reliable Implantation of Neural Interfaces into
Peripheral Nervous System. 6 th IEEE RAS/EMBS
International Conference on Biomedical Robotics and
Biomechatronics (BioRob). 561-566.
Kather, M., Skischus, M., Kandt, P., Pich, A., Conrads, G.,
Neuss, S., 2017. Functional Isoeugenol-Modified
Nanogel Coatings for the Design of Biointerfaces
Angew. Chem. Int. Ed. Engl. 56(9):2497-2502.
Karandjeff, C., Hannaford, C., Llgglero, R., Max, S.,
Tilden, S.J., 2011. Measuring SNR, SINAD, and THD
quickly.. Evaluation Engineering, 50(10) , 18-21.
Lauer, R.T., Peckham, P.H., Kilgore, K.L., Heetderks,
W.J., 2000. Applications of cortical signals to
neuroprosthetic control: a critical review. IEEE Trans.
Rehabil. Eng. 8:205–208.
McKeefry, D., Kremers, J., Kommanapalli, D., Challa,
N.K., Murray, I.J., Maguire, J., Parry, N.R.A., 2014.
Incremental and decremental L- and M-cone-driven
ERG responses: I. Square-wave pulse stimulation. J.
Opt. Soc. Am. A 31, A159-A169.
Meziane, N., Webster, J. G., Attari, M. Nimunkar, A. J.,
2013. Dry electrodes for electrocardiography. Physiol.
Meas. 34, R47–R69.
Navarro, X., Krueger, T. B., Lago, N., Micera, S., Stieglitz,
T. and Dario, P., 2005. A critical review of interfaces
with the peripheral nervous system for the control of
neuroprostheses and hybrid bionic systems. Journal of
the Peripheral Nervous System, 10:229–258.
Pedrosa, P., Fiedler, P., Schinaia, L., Vasconcelos, B.,
Martins, A.C., Amaral, M.H., Comani, S., Haueisen, J.,
Fonseca C., 2017. Alginate-based hydrogels as an
alternative to electrolytic gels for rapid EEG monitoring
and easy cleaning procedures. Sensors and Actuators B
247: 273–283.
Van Bel, A.J.E., Hafke, J.B., (2013). Calcium along the
phloem pathway as a universal trigger and regulator of
systemic alarms and signals. In: Baluska F. (ed), Long-
distance Systemic Signalling and Communication in
Plants. Berlin: Springer. 363-392.
BIOSIGNALS 2018 - 11th International Conference on Bio-inspired Systems and Signal Processing
138
