
The Effects of Exfoliated Graphite Concentration on Sensing
Properties of Chitosan/Exfoliated Graphite Film Crosslinked with
Glutaraldehyde
I. Nainggolan
1
, P. Faradilla
1
, T. I. Nasution
2
, H. Agusnar
1
1
Chemistry Department, Faculty of Mathematics and Natural Science, Universitas Sumatera Utara, Medan 20155,
Sumatera Utara, Indonesia
2
Physics Department, Faculty of Mathematics and Natural Science, Universitas Sumatera Utara, Medan 20155, Sumatera
Utara, Indonesia
Keywords: Chitosan, Exfoliated Graphite, Glutaraldehyde, Films, Sensors.
Abstract: In this study, chitosan/exfoliated graphite (CEG) sensors were used for formaldehyde detection in various
concentration i.e. 1 ppm; 1,5 ppm; 2 ppm; 5 ppm; and 10 ppm. The sensors were fabricated using
electrodeposition method to form film sensors. The cross-linking agent is glutaraldehyde, it was used to
enhance the life time of CEG film sensors. The effect of exfoliated graphite concentration on sensing
properties of CEG film sensors has been proven by improvement on response and sensitivity of sensors when
the concentration of EG increased. Formaldehyde solution was dropped onto chitosan/EG film sensor and the
response of the chitosan/EG film sensors toward formaldehyde was recorded as output voltage. The
measurement result of maximum output voltage from chitosan/EG film sensors is greater than chitosan
sensors for 10 ppm formaldehyde. Increasing on concentration of formaldehyde made the output voltage
of the sensors increased.
1 INTRODUCTION
The control on utilization of formalin by goverment
is weak (Noordiana, 2011). Formalin is a forbidden
food as stated in The Food Regulation. According to
WHO standards in 2002, the maximum formalin
content contained in food was 1 mg/l equivalent to 1
ppm (WHO, 2002).
Chitosan is biopolymer which produced by treating
seafood waste and it functions as an attractive sensitive
material. Chitosan easily can be modified to be used as
an effective sensitive material due to modification of the
chitosan structure, excellent film-forming ability,
adhesive, high heat stability (Yang, 2013). The high
solubility in acidic media makes chitosan easily
deposited onto a substrate to form film (Sun, 2011). The
use of thin films of chitosan continues to expand in
various industries such as industrial biotechnology,
.
environmental, agricultural industries etc. (Majety,
2000). The advantages of non-porous film layers offer
high selectivity, permeability and mechanical strength
(Kanti, 2004).
Graphite is produced naturally or synthetically.
Graphite is a crystalline carbon which is highly
conductive (with an electrical conductivity of 10
4
S/cm). Graphite’s derivative called exfoliated
graphite or expanded graphite could be a filler for
producing conductive materials. The electrical
conductivity of exfoliated graphite is high. Exfoliated
graphite has a good affinity for organic compounds
(Debelak, 2007). Neat graphite (NG) can be
converted to intercalated or expandable graphite
through chemical oxidation in the presence of
concentrated acid such as H
2SO4 or HNO3. Expanded
graphite is then obtained by exfoliation and expansion
of graphite by heating in a furnace above 600
o
C
(Demitri, 2015). Surface area and bulk density
essentially affect the physicochemical and physical
properties of carbon materials. Exfoliated graphite
can be also synthesized from graphite oxide (OG)
(Buchsteiner, 2006).
Nainggolan, I., Faradhilla, P., Nasution, T. and Agusnar, H.
The Effects of Exfoliated Graphite Concentration on Sensing Properties of Chitosan/Exfoliated Graphite Film Crosslinked with Glutaraldehyde.
DOI: 10.5220/0010103810871090
In Proceedings of the International Conference of Science, Technology, Engineering, Environmental and Ramification Researches (ICOSTEERR 2018) - Research in Industry 4.0, pages
1087-1090
ISBN: 978-989-758-449-7
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
1087

Most of current techniques to detect fomaldehyde
are sophisticated equipment, expensive and time
consuming. So, it is highly desirable to develop a
sensitive and easy-to-use method for formaldehyde
detection.
2 MATERIALS AND METHOD
2.1 Materials and Instruments
Materials used in this research are chitosan powder
supplied by Sigma–Aldrich (medium molecular
weight), graphite, glutaraldehyde, CH
3COOH 2%,
H
2SO4(p), HNO3(p), CoCl2 0,01 M, KSCN 1 M and
HCl 2 N (Merck).
Oven, Furnace, hot plate, magnetic stirrer, printed
circuit board (PCB), centrifuge, an ultrasonic, a set of
FTIR Shimadzu IR prestige-21 and a set of XRD
Shimadzu XRD-6100 were used as tools to perform
the
material preparation and material characterization.
2.2 Preparation of Chitosan Solution
Chitosan powder was dissolved in acetic acid 2% then
stirred using a magnetic bar for 24 hours at room
temperature to prepare the chitosan gel.
2.3 Preparation of Exfoliated Graphite
Exfoliated graphite was prepared using graphite and
it was mixed with nitric and sulfuric acid for 24 hours.
Then, it became intercalated graphite. The mixture
was filtered and washed until the pH became neutral.
Exfoliated graphite was dried in an oven. Then the
intercalated graphite compound was subjected
thermal shock to temperatures of 900
o
C.
2.4 Preparation of Chitosan/Exfoliated
Graphite Solution
Exfoliated graphite 400 mg was added into chitosan
solution and stirred for 1 hour then sonicated. After
that, the solution was centrifuged 5000 rpm to collect
supernatant solution for chitosan/EG film sensors
fabrication.
2.5 Preparation of Chitosan/Exfoliated
Graphite (EG) Film Sensor
Chitosan/exfoliated graphite film sensors were made
by chitosan/EG solution using electrodeposition
method. The substrate of the sensor used is a printed
circuit board (PCB). The electrodeposition process is
illustrated as in Figure 1. The supplied voltage was
fixed at 2,5 volts. Then it left to dry for 5 minutes at
105
o
C in an vacuum oven. Chitosan/EG films were
cross-linked using glutaraldehyde 25%. CoCl
2 0,01
M was used as a template to protect amine groups.
KSCN 1 M is a solution to remove the template and
HCl 2 N was used to ensure the template is
completely removed. Formaldehyde solution was
varied into 1 ppm; 1,5 ppm; 2 ppm; 5 ppm; and 10
ppm. Formaldehyde solution was dropped onto
chitosan/EG film sensors and it was detected by
amperometric method. The output voltage was
displayed based on the characteristic of the film
sensor. The response of the sensor towards
formaldehyde was recorded as output voltage.
Figure 1. Electrodeposition Process of Chitosan/EG film
sensor.
3 RESULTS AND DISCUSSION
Chitosan would assemble onto the PCB surface when
it is positively charged in acidic conditions to form
chitosan sensor. Exfoliated graphite was found
improving the sensitivity of chitosan film sensor. The
addition of glutaraldehyde as crosslinking agent
improved the lifetime of chitosan/EG. The output of
chitosan/EG sensors was in the potential voltage.
Chitosan/EG film sensors showed good sensitivity
and long lifetime in measurement with various
concentration of formaldehyde. The measurements
were repeated three times. The output voltage of
chitosan/EG film sensors when detecting
formaldehyde are reported in Table 1.
Table 1 shows the output voltage of chitosan/EG
film sensors when the sensor’s surface was exposed
to formaldehyde with various concentration. The
output voltage of the sensor indicates the sensitivity
of chitosan/EG film sensor during detecting various
concentration of formaldehyde. The output voltage
values were within the range of 0,421 V to 0,589 V for
1-10 ppm of formaldehyde. The highest output voltage
(0,589 V) was observed when chitosan/EG film sensor
exposed to 10 ppm formaldehyde, while the lowest
ICOSTEERR 2018 - International Conference of Science, Technology, Engineering, Environmental and Ramification Researches
1088
output voltage (0,421 V) was observed at 1 ppm
formaldehyde. If it is compared to chitosan sensor which
gave output voltage 0.143 V when the sensor was
exposed to 1 ppm of formaldehyde. As shown by the
table, increasing concentration of formaldehyde shows
the increasing of output voltage.
Table 1: The output voltage of crosslinked chitosan/EG
film sensor towards formaldehyde.
Formaldeh
y
de
Avera
g
e of Output
Concentration (ppm) Volta
g
e (V)
1
0,421
1,5
0,434
2
0,447
5
0,504
10
0,589
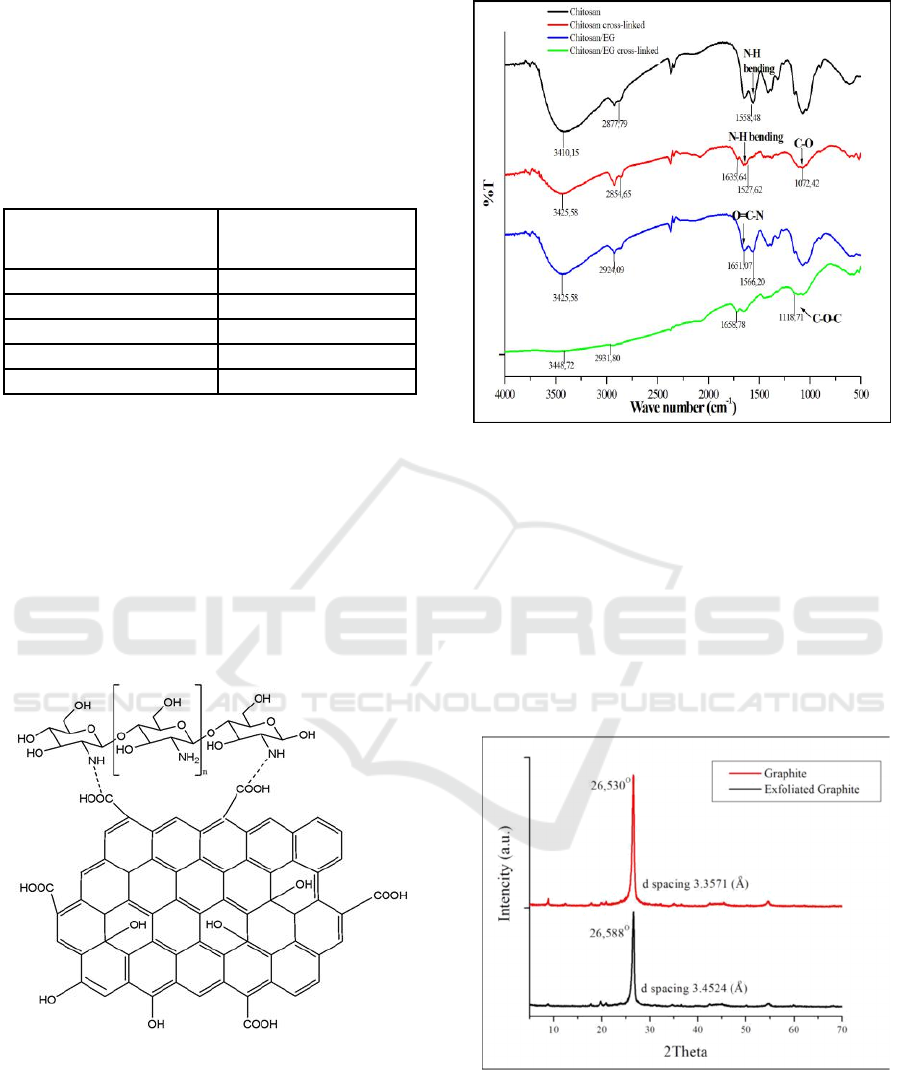
FTIR studies confirmed the successful of
crosslinking of chitosan and chitosan/EG with
glutaraldehyde as displayed in Figure 2. The peak at
1072,42 cm
-1
shows stretching vibration of –C-O-
from chitosan and glutaraldehyde. The absorption
band at the wave number 1651,07 cm
-1
is the O=C-N
group which proves that exfoliated graphite binds to
chitosan. Wave number of 1118.71 cm
-1
is a
stretching of C-O-C bridge which proves cross-
linkage between chitosan/EG and glutaraldehyde.
Figure 2: The FTIR spectrum of chitosan, chitosan/EG,
crosslinked chitosan and crosslinked chitosan/EG films.
The interaction between exfoliated graphite and
chitosan formed hydrogen bonding between
carboxylic acid of exfoliated graphite and amine
group of chitosan. The illustration of the reaction can
be seen in Figure 3.
Figure 3: Reaction between chitosan and exfoliated
graphite
The XRD patterns of graphite and exfoliated
graphite are represented in Figure 4. Based on XRD
analysis, the diffraction angles of the graphite forms
peak at 26,530
o
with d-spacing 3,3571 Å while the
diffraction angles of exfoliated graphite forms peak at
26,588
o
with d-spacing 3,4524 Å. It proved that there
was an expansion of the lattice of exfoliated graphite
thus exfoliated graphite was obtained in a greater d-
spacing value than graphite.
Figure 4: XRD of graphite and exfoliated graphite
4 CONCLUSIONS
The electrical testing results of chitosan/EG film
sensors showed that chitosan/EG film sensor was
The Effects of Exfoliated Graphite Concentration on Sensing Properties of Chitosan/Exfoliated Graphite Film Crosslinked with
Glutaraldehyde
1089

potentially capable to detect formaldehyde in various
concentration of formaldehyde. The sensitivity of
chitosan/EG film sensors has been proven by the
different output voltage values when the sensors
exposed to different concentration of formaldehyde.
Increasing concentration of formaldehyde indicated
the increasing on output voltage. The highest output
value (0,589 V) was recorded during detecting 10
ppm of formaldehyde, while the lowest value (0,4217
V) for 1 ppm of formaldehyde. The cross-linking
process of chitosan with glutaraldehyde has been
successfully done and it has been proved by FT-IR
spectrum. The crosslinking improved the lifetime f
the sensors.
ACKNOWLEDGEMENTS
This work was financially supported by TALENTA
USU research grant No. 2590/UN5.1.R/PPM/2018
REFERENCES
Noordiana, N., Fatimah, AB., Farhana, YCB. 2011. Int.
Food Res. J. 18: 125–136
World Health Organization (WHO)., 2002. Formaldehyde.
Concise International Chemical Assessment Document
40. Geneva; 48: 6-7
Yang, Y., Fang, G., Liu, G., Pan, M., Wang, X.,
Kong, L., 2013. Biosens. Bioelectron. 47: 475–
481.
Sun, K., Li, ZH., 2011. Express Polymer Letters 5(4): 342
- 361. Majety, NV., Kumar, R., 2000. Reactive &
Functional Polymers, 46 1-27
Kanti, P., Srigowri., Madhuri, J., Sridhar, S., 2004.
Separation and Purification Technology, 40 259-266
Debelak, B., Lafdi, K., 2007. Carbon 45: pp. 1727-1734
Demitri, C., Moscatello, A., Giuri, A., Rauci, MG.,
Corcione, CE., 2015. Polymers 7 2584–2594
Buchsteiner, A., Lerf,A., Pieper, J., 2006. J. Phys. Chem. B
110 22328-22338.
ICOSTEERR 2018 - International Conference of Science, Technology, Engineering, Environmental and Ramification Researches
1090
