
Model of Formation Graphene from Graphite with Ammonia
H. Sitohang
1
, N. Pasaribu
1
, R. Siburian
1*
C. Simanjuntak
1
1
Department of Chemistry, Faculty of Mathematics and Natural Sciences, Universitas Sumatera Utara, Medan
Keywords: Graphene, Graphite, FTIR, Hummer’s Method.
Abstract: The reaction mechanism of commercial graphite using ammonia reducing agents has been carried out based
on Hummer's modification method. The purpose of this study was to determine the reaction model of graphene
formation and characterization using an infrared spectrophotometer (FTIR). The results showed that the
characterization using infrared spectrophotometer (FTIR) analysis on graphene oxide showed a ring
consisting of three one oxygen atom or called an epoxy group (C-O-C) at a number wave
1396 cm
-1
which
reacted with ammonia thus replaced by the availability of CN groups in wave numbers
1033 cm
-1
on
graphene. This happens due to the deoxygenation process. These data proved that graphene can be synthesized
from graphite using ammonia reducing agents.
1 INTRODUCTION
Graphene is believed to be a promising material in the
future (1). Graphene is a two-dimensional carbon
from the structure of graphite. Graphene has various
extraordinary properties, such as mechanical
resistance, strength and elasticity, thermal and
electrical conductivity and high surface area (2,3).
That is, graphene can replace other materials and
applications. By laboratory, graphene can be
produced from a simple, high-quality and inexpensive
method. Graphene characterization produced
approaches the theoretical parameters namely
electron mobility (2.5 × 105 cm2 V-1s-1) (4),
Young's modulus 1 TPa and 130 GPa intrinsic
strength (5,6), high thermal conductivity (> 3000 W
mK-1) (7), optical absorption πα = 2.3% (α =
structural stability) (8), can react with various gases
(9), ability high density of electric current (1000 times
that of Cu) (10) and has a functional chemistry (11).
Graphene is also called a magical material (11) and
can be deposited on special substrates such as
hexagonal boron nitride (4, 13). Thus, graphena will
be of particular concern to be applied to the industry.
Thus, the production of graphene on a large scale
becomes the target of scientists.
2 MATERIALS AND METHODS
2.1 Materials
The materials used are: Graphite; strong acids,
oxidizing agents and ammonia reducing agents.
2.2 Synthesis of Graphene Oxide
A total of 0.2 g of graphite powder were fed into a
250 mL erlenmeyer, then 0.2 g of NaNO3 and 15 mL
of 96% H2SO4 were added. The solution is stirred for
2 hours. Furthermore, the Erlenmeyer containing the
mixture was placed in an ice container and added
gradually 1 gram of KMnO4 then stirred for 24 hours.
After stirring for 24 hours, 20 mL 5% H2SO4 and 1
mL H2O2 30% were added to the solution and stirred
for 1 hour. The solution is confused with a speed
centrifuge of 6500 RPM for 20 minutes to separate
the filtrate and supernatant. Then into the solution
added 25 ml distilled water and messed up using a
centrifuge with a speed of 6500 RPM (Rotor
PerMinute) for 20 minutes. The solution was
transferred to a beaker glass and added with 100 mL
distilled water and then ultrasonicated for 5 hours,
then allowed to cool and produced graphene oxide.
1036
Sihotang, H., Pasaribu, N., Siburian, R. and Simanjuntak, C.
Model of Formation Graphene from Graphite with Ammonia Reduktor.
DOI: 10.5220/0010096210361038
In Proceedings of the International Conference of Science, Technology, Engineering, Environmental and Ramification Researches (ICOSTEERR 2018) - Research in Industry 4.0, pages
1036-1038
ISBN: 978-989-758-449-7
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
2.3 Synthesis of Graphene
The second graphene oxide solution was added with
10 ml of ammonia then stirred for 72 hours. The
solution was filtered and dried at 80ºC for 24 hours
and characterized by using XRD, FTIR, and SEM-
EDX.
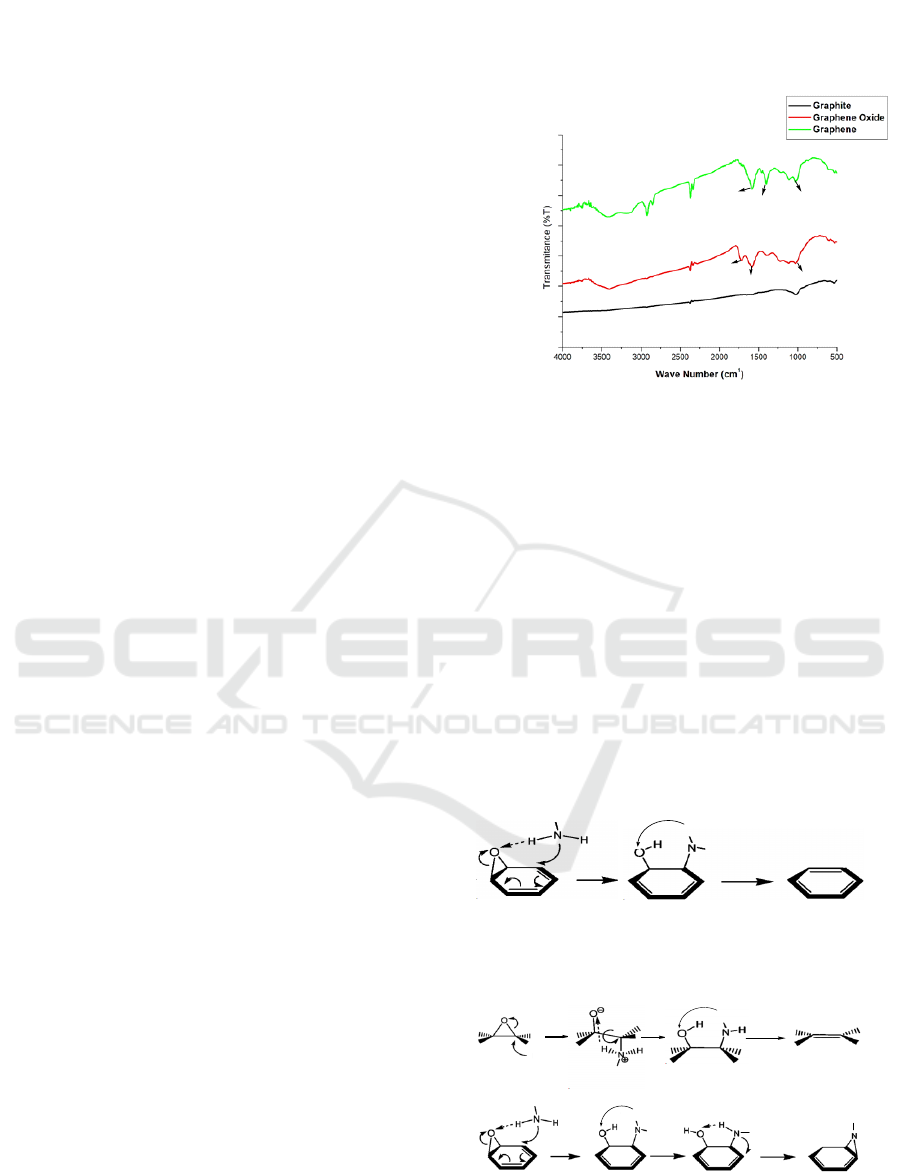
3 RESULTS
Characterization using FTIR was carried out to
determine changes in functional groups before and
after the oxidation process in graphite, the synthesis
of graphite oxide, graphene oxide and graphene and
to determine the interaction between graphene and
ammonia reducing agents. FT-IR analysis using
Shimadzu IR Prestige-21 tool was carried out in the
wave number range of 500-4500 cm
-1
. All samples
were analyzed in the form of smooth curls. In Figure
1 shows that the peak wavelength FT-IR has occurred
in graphite, graphene oxide and graphene.
In the Graphite FT-IR spectrum, the wave number
1581cm
-1
shows the bond between the aromatic group
C = C, and the presence of a support group in another
wave number that appears at peak 1026 cm
-1
which is
the bond between C-C. Based on the data in Figure1
it can be seen that graphite only has the structure C =
C (π bond) and C-C.
In the FT-IR spectrum of Graphene Oxide has
formed bonds between oxygen and hydrogen (OH)
groups occurring at wave numbers 3402 cm1, a weak
absorption spectrum occurring at wave numbers
1705.07 cm-1 indicates the bond between C = O of
the carboxylic acid group ( COOH), the spectrum of
the wave number is formed by the aromatic group C
= C in the wave number 1581 cm
-1
and there is also a
weak spectrum at wave number 1396 cm
-1
indicating
the bond between C-OH or CO (epoxy) bond at 900-
1300 cm
-1
.
In the Graphene FT-IT spectrum shows the
appearance of absorption peaks in the wave number
1033 cm
-1
which indicates the bond between C-N.
The wavelength range~ 900 cm
-1
is the range of wave
numbers for epoxy groups (C-O) (Li, et al, 2008).
Based on these data it can be seen that there has been
a de-epoxidation process. However, the stretch
vibration of O-H at 3410 cm
-1
and the stretching
vibration of C-O at 1404 cm
-1
are still observed due to
the presence of hydroxyl groups and carboxylic
groups even after being reduced by ammonia.
Synthesis of graphene oxide from graphite has
occurred the process of peeling graphite oxide into
graphene oxide by ultrasonication of graphite oxide.
The mechanism of the oxidation reaction can be
stated in equations 1.1 (a) and 1.1 (b).
C=C
(1581 cm
1
)
C-C
(1026 cm
1
)
O-H
(3402 cm
1
)
C=O
(1705 cm
1
)
C=C
(1581 cm
1
)
C-O epoksi
(1118 cm
1
)
O-H
(3410 cm
1
)
C=C
(1581 cm
1
)
C-N
(1404 cm
1
)
C-O
(1033 cm
1
)
Figure 1: FTIR data of Graphite, Graphene Oxide and
Graphene.
This transformation process can only occur under
strong acid conditions, so the presence of sulfuric acid
as well as solvent from graphite also plays a role in
further oxidation processes.
𝐾𝑀𝑛𝑂
4
+ 3H
2
SO
4
→
𝐾
+
+
𝑀𝑛𝑂
3
+
+ 3HSO
4
+
1.1(a)
𝑀𝑛𝑂
3
+
+ Mn
𝑂
4
-
→ M
2
O
7
1.1(b)
(Rizkietal, 2014).
Mechanism of reaction between epoxy and ammonia:
Phase 1:
H
H
H
-H
2
O
-NH
Phase 2:
NH
3
H
H
-H
2
O
-NH
Mn
H
H
H
H
H
-H
2
O
-NH
Model of Formation Graphene from Graphite with Ammonia Reduktor
1037

Phase 3 :
HH
H
-H
2
O
-NH
4 CONCLUSION
Synthesis of graphene oxide with the addition of
ammonia pereductor to produce graphene from
commercial graphite has been successfully carried
out with the modified Hummer's method. The success
of this synthesis is evidenced by the presence of FTIR
data.
Graphene formation reaction model of graphite
and graphene oxide reduction has been obtained
according to FTIR data.
ACKNOWLEDGEMENT
We would like to thankful for “TALENTA”,
Universitas Sumatera Utara, No. 2590/UN5.1
R/PPM/2017, 16 March 2018 who funding supported
on our research.
REFERENCES
Novoselov, K.S., Fal’ko, V.I., Colombo, L., Gellert, P.R.,
Schwab, M.G. and Kim, K. 2012. A road map for
graphene. Nature. 490: 192– 200.
Geim, A. K., and Novoselov, K. S. 2007. The rise of
graphene. Nature Mater. 6: 183–191.
Geim, A. K. 2009. Graphene: status and prospects. Science.
324: 1530–1534.
Mayorov, A.S.et al. 2011.Micrometer-scale ball is tic
transport in encapsulated graphene at room temperature.
Nano Lett. 11: 2396–2399.
Lee, C., Wei, X.D., Kysar, J.W.and Hone, J.2008.
Measurement of the elastic properties and intrinsic
strength of monolayer graphene. Science. 321: 385–
388.
Liu, F., Ming, P. M. and Li, J. 2007. Ab initio calculation
of ideal strength and phonon in stability of grapheme
under tension. Phys. Rev. B. 76: 064120.
Balandin, A.A. 2011. Thermal properties of grapheme and
nanostructured carbon materials. Nature Mater. 10:
569–581.
Nair, R.R.et al. 2010. Fluoro graphene: a two-dimensional
counter part of Teflon. Small. 6:2877–2884.
Bunch, J.S.et al. 2008. Impermeable atomic membranes
from grapheme sheets. Nano Lett. 8:2458–2462.
Moser, J., Barreiro, A. and Bachtold, A. 2007. Current-
induced cleaning of graphene. Appl. Phys. Lett. 91:
163513.
Elias, D.C.et al. 2009. Control of graphene’s properties by
reversible hydrogenation: evidence for graphene.
Science.323: 610–613.
Novoselov, K.S. et al. 2004. Electric field effect in
atomically thin carbon films. Science.306:666–669.
Dean, C.R. et al. 2010. Boron nitride substrates for high-
quality grapheme electronics. Nature Nanotechnology.
5, 722–726.
ICOSTEERR 2018 - International Conference of Science, Technology, Engineering, Environmental and Ramification Researches
1038
