
The Activity of Virgin Coconut Oil to Increase Proliferation and
COX-2 Expression towards NIH 3T3 Cell Line
Jansen Silalahi
1*
, Dian Ika Perbina Meliala
1
, Yuandani
1
, Linda Margata
1
and Denny Satria
1
1
Faculty of Pharmacy, Universitas Sumatera Utara, Medan, Indonesia
Keywords: VCO, Acid Value, %FFA, NIH 3T3, Proliferation Cell, Percent of Wound Closed, Expression of COX-2.
Abstract: This research aims to investigate the effect of VCO (Virgin Coconut Oil) to increase the activity of NIH
3T3 cell proliferation and COX-2 expression. The sample used were VCO, and acid value was determined.
Proliferation was appraised using the MTT method. Furthermore, wound healing activity assays were
established with a microscopic system, and expression of COX-2 was determined using RT-PCR. Acid
value and % FFA of VCO are 1.07±0.01 and 0.51±0.02. VCO 62.5µg/mL with viability cells were
107.758±0.45%; 104.45±0.48% and 104.45±0.48% after 24h, 48h, and 72 h incubation, cell migration in the
wound healing assay after 24 h and 48 h incubation (49.11±0.09% and 74.82±0.22%), and increase in
expression of COX-2 (Control=1; and VCO=1.21). The results explain that VCO supply potent proliferation
output. This study is planned to appraise of wound closure activity of VCO on the scratched monolayer of
NIH 3T3 cell line.
1 INTRODUCTION
Wound healing is a complicated process involving
many cells consisting of four phases namely the
phases of hemostasis, inflammation, proliferation,
and remodeling (Stamm, et al., 2016). The
hemostasis phase is the beginning of the wound
healing process by involving platelets (Rodrigues, et
al., 2016). During the inflammatory phase,
fibroblasts function as cytokine secretions, and
growth factors to activate the body's defense system
(Ridiandries, et al., 2018). During the proliferation
and remodeling phases, fibroblasts are important for
granulating and reorganizing tissues of the
extracellular matrix (Ariffin and Hasham, 2016).
Wound healing is associated with bacterial
contamination in the wound area (Ariffin and
Hasham, 2016).
The ultimate aim of wound healing
is to restore the functional properties of the leather
and prevent infection (Ariffin and Hasham, 2016).
The COX enzyme consists of 2 isoenzymes such
as COX-1, COX-2 and COX-3 (COX-1 variants)
(Chandrasekharan, et al., 2002). COX-2 plays a role
in the process of angiogenesis The expression of
COX-2 affects the process of migration,
angiogenesis, and proliferation of fibroblasts
(Futagami, et al., 2002).The process of angiogenesis,
proliferation, and migration of fibroblasts is very
important in wound healing. The expression of
COX-2 affects the process of proliferation,
angiogenesis, and migration of fibroblasts
(Futagami, et al., 2002).
This research aims to evaluate the effect of VCO
(Virgin Coconut Oil) in increasing the activity of
cell proliferation, COX-2 expression, and wound
healing migration NIH 3T3 cells.
2 MATERIALS AND METHODS
2.1 Materials
VCO (Palem Mustika®, Indonesia) and all
chemicals and reagents that were used in this study
were of analytical grade. NIH 3T3 cells were gotten
from Institute of Pharmacy, Gajah Mada University.
NIH 3T3 cells were modified in Dulbecco’s
modified Eagle’s medium added with 10% Fetal
bovine serum and saved 37
0
C with a CO
2
provide of
5%.
778
Silalahi, J., Meliala, D., Yuandani, ., Margata, L. and Satria, D.
The Activity of Virgin Coconut Oil to Increase Proliferation and COX-2 Expression towards NIH 3T3 Cell Line.
DOI: 10.5220/0010088907780781
In Proceedings of the International Conference of Science, Technology, Engineering, Environmental and Ramification Researches (ICOSTEERR 2018) - Research in Industry 4.0, pages
778-781
ISBN: 978-989-758-449-7
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
2.2 Methods
2.2.1 Acid Value Determination
Five (5) g VCO was weighed and procedure titration
carried out, then the acid value and free fatty acid
(FFA) percentage of HVCO was calculated as
previously described
(Margata, et al., 2018; Silalahi,
et al., 2016).
2.2.2 Proliferative Activity
HVCO (1000 μg/mL to 15.625 μg/mL in co-solvent
DMSO (Sigma) was submitted for proliferative test.
In that way, NIH3T3 cell line (58.5 x10
4
cells/mL)
was grown in DMEM complete medium. After 24;
48 and 72 h treatment, MTT assay was performed
and cell viability was counted to determine the
proliferative activity (Harahap, et al., 2018).
2.2.3 Wound Healing Migration Assay
The migration assay was carried out with NIH3T3
cells were seeded at 5x104cells/well in 24-well
plates and saved for 24 h at 37
o
C. Cultured cells
were washed up with PBS and added culture media
which containing 0.5% FBS and saved for 24 h.
Scratch was done in the bottom center of the well
within cell layer using yellow tip. Cell residues in
the plate were washed up with PBS and treated with
HVCO and incubated for 48 h at 37
o
C and
documented under the inverted microscope against
cell migration rapidity after 0, 24, and 48 h. The
space from scratch treatment between control and
treatment cultur cell was measured using Image J
software and defined as cell migration area
(Freiesleben, et al., 2017; Harahap, et al., 2018).
2.2.4 Expression of COX-2
NIH3T3 cells (5x10
4
cells/well) were planted into
6-well plate and saved for 24 h and RNA extraction
followed Harahap, et al., 2018. The supernatant was
divided and used for RNA extraction (Genaid, USA)
and RNA concentration was determined by
spectrophotometric method (Nanodrop) and stored at
-80
o
C until used. Complementary DNA (cDNA) was
synthesized from 3.0 μg total RNA using RT-PCR
kit (Toyobo, Japan) to make final volume of 20 μL
using random primers based on the manufacturer’s
instructions. RT-PCR was carried out in AB 7500
Fast (ABI, USA). The reaction mixture consisted of
GoTaq Green (12.5 μL) (Promega), 1.0 μL of cDNA
1 μL forward primers, 1 μL reverse primers, and 9.5
μL ddH
2
O to make a total volume of 25 μL. β-actin
was used as internal reference control. The PCR
primers were used for β-actin (F: 5’-gtc gta cca ctg
gca ttg t-3’; R: 5’-cag ctg tgg tga agc t-3’), Cox-2 (F:
5’-cca gca ctt cac gca tca gt-3’; R: 5’-acg ctg tct agc
cag agt ttc ag-3’). The PCR condition were
comprised of first incubation at 95
o
C for 2 minutes,
95
o
C for 30 sec, annealing at 55
o
C 30 sec, extension
at 72
0
for 1 minute, and 35 cycles. The PCR
products were detected by electrophoresis in 2%
agarose gels, and added gel red 10 μL. Then, they
were visualized with gel doc (Li, et al., 2017;
Harahap, et al., 2018).
2.2.5 Statistic Analysis
The results were served as means ± SD. The
statistical analysis was carried out by using SPSS
edition 21.
3 RESULT
3.1 Acid Value and %FFA of VCO
Acid value is 1.07±0.01 mg NaOH/g oil and free
fatty acids (FFA) is 0.51±0.02%.
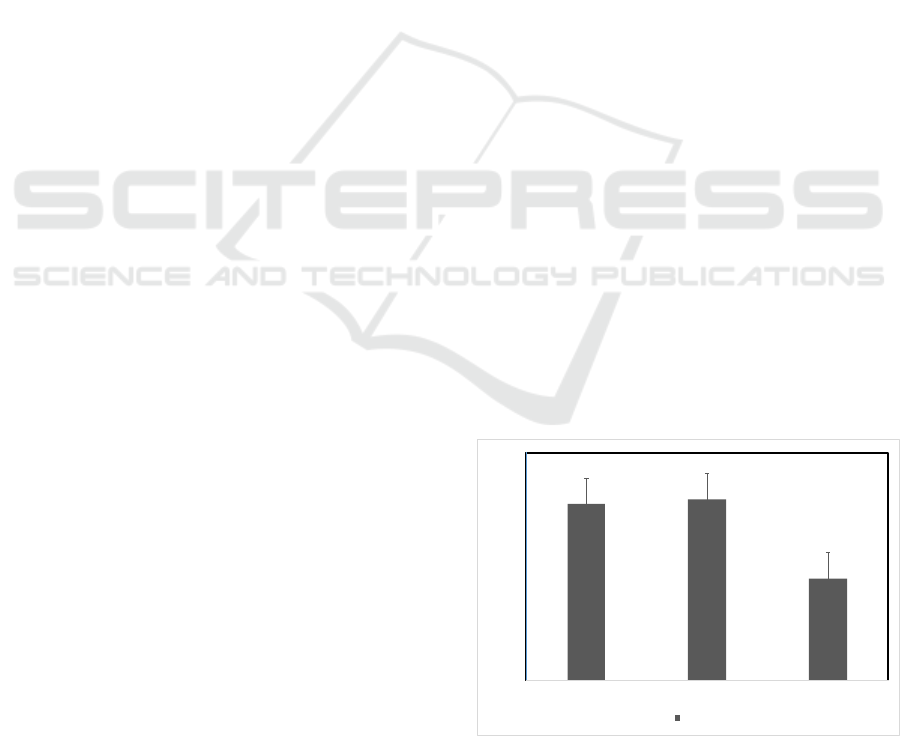
3.2 Proliferative Activity
To appraise the effect of VCO to increase the
quantity of cells by stimulating cell proliferation.
The percentage of viable cells after treatment and
incubation for 24h, 48h, and 72h (107.76±0.45;
107.94±0.45; 104.45±0.48) showed the stimulation
effect of VCO towards proliferation of NIH3T3
cells. The effect of VCO is given in Figure 1.
100
101
102
103
104
105
106
107
108
109
110
24h 48h 72h
ViableCells
Hours
Figure 1: Percentage of Viable Cells of NIH3T3 Cells
were Treated by VCO 62.5 µg/mL for 24; 48 and 72 h and
Measured Viable Cells.
The Activity of Virgin Coconut Oil to Increase Proliferation and COX-2 Expression towards NIH 3T3 Cell Line
779
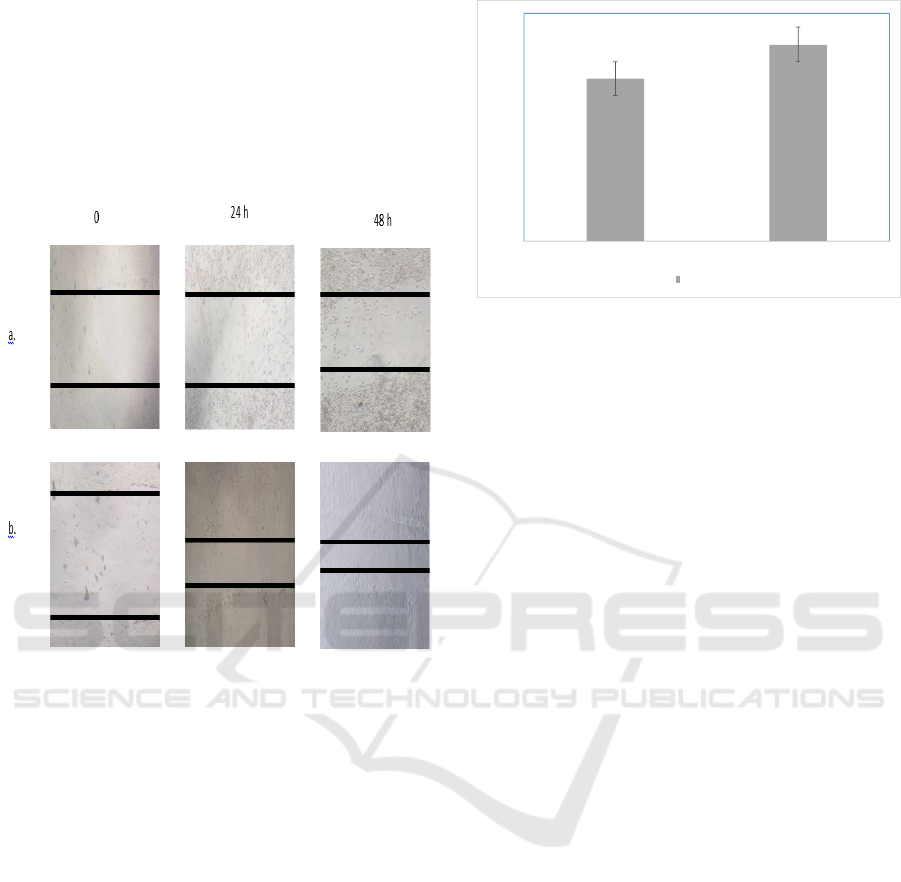
3.3 Wound Healing Migration
The scratch wound healing assay was done to
evaluate the influence of VCO on NIH3T3
migration. The wound healing migration of VCO is
given in Figure 2. A little wound repair was
observed in wells with VCO at 62.5 μg/mL after 24
and 48h incubation with 49.11 ± 0.09% and 74.82 ±
0.22% respectively closure area.
Figure 2: Wound Healing Migration Assay. NIH3T3 cells
were treated by VCO for 0; 24 and 48h and measured the
closure area. (a) control cells; (b) VCO 62.5 μg/mL.
3.4 COX-2 Expression
Two steps RT-PCR were used to evaluate COX-2
expression in NIH3T3 cells after the treatment with
VCO. VCO showed a significant up-regulatory
effect on the expression of COX-2. The COX-2
expression is given in Figure 3.
4 DISCUSSION
Acid value is defined as mg NaOH used to
neutralize FFA contained in 1 g of fats or oils to
indicate the amount of FFA in one gram fats or oils
(Silalahi, et al., 2016).
Lauric acid is antibacterial and anti-
inflammatory agent that able to overcome skin
problems (Ariffin and Hasham, 2016). Lauric acid
can decrease the time for complete epithelialization,
because lauric acid can increase proliferation cells
and migration cells (Ariffin and Hasham, 2016).
0
0.2
0.4
0.6
0.8
1
1.2
1.4
Contro lCells VCO
COX‐2Expression
COX ‐2
Figure 3: COX-2 Expression (a) control cells; (b)VCO
62.5 µg/mL.
Thourghout wound healing process, cells at the
wound side migrate, and proliferate, caused to re-
epithelialization of the wound side. Migration of
NIH 3T3 fibroblasts was evaluate with wound
healing assay. Lauric acid can increase
proliferation cells and migration cells (Ariffin and
Hasham, 2016). Cell migration activity in the VCO
group is faster than control group. Lauric acid can
increase proliferation cells and migration cells.
Lauric acid is found in VCO that stimulate cells to
migrate. So the percentage of VCO group cell
migration is faster than control group.
Cyclooxygenase-2 (COX-2) is an inducible
enzyme which plays a critical role in multiple
pathophysiological processes including
atherosclerosis, inflammation, tumorigenesis, tissue
injury, and angiogenesis (Futagami, et al., 2002).
COX-2 protein and mRNA were expressed primarily
in the head and basal layers of the epidermal wound
edges, which are arranged of proliferative and
migratory cells (Futagami, et al., 2002). In the
Ebeling, et al study, COX-2 is one of the wound
healing parameters. Pentacyclic triterpene and
botulin are active compounds of birch bark extract.
They influence the inflammatory phase of wound
healing by upregulating chemokines,
proinflammatory cytokines, and cyclooxygenase-2
(COX-2) in human primary keratinocytes. COX-2
and IL-6 that their mRNA enlargement is due to a
mRNA stabilizing effect, a process in which p38
MAPK and HuR (human antigen R) are primarily
involved (Ebeling, et al., 2014). In this study, Lauric
acid increase of expression COX-2 which mediated
angiogenesis and migration NIH 3T3 cell Two steps
RT-PCR were used to evaluated COX-2 expression
in NIH3T3 cells after the treatment with VCO.
ICOSTEERR 2018 - International Conference of Science, Technology, Engineering, Environmental and Ramification Researches
780

5 CONCLUSION
VCO can increase proliferation cells, COX-2
expression, and accelerate wound closure. So that
VCO has wound healing activities.
ACKNOWLEDGEMENTS
This work was supported by University of Sumatera
Utara through “Hibah Penelitian Guru Besar”
funding 2018.
REFERENCES
Ariffin NHM, Hasham R. Potential Dermatological
Application on Asian Plants. Biotechnology and
Bioprocess Engineering 2016;21:337-54.
DOI:10.1007/s12257-015-0750-
Chandrasekharan NV, Dai H, Roos KLT, Evanson NK,
Tomsik J, Elton TS, et al. COX-3, A Cyclooxygenase-
1 Variant Inhibited by Acetaminophen and other
Analgesic/Antipyretic Drugs: Cloning, Structure, and
Expression. PNAS 2002;99(21):13926-31.
Ebeling S, Naumann K , Pollok S , Wardecki T, Vidal S ,
Nascimento JM, et al. From a Traditional Medicinal
Plant to a Rational Drug: Understanding the Clinically
Proven Wound Healing Efficacy of Birch Bark
Extract. Plos One 2014;9(1): e86147.
DOI:10.1371/journal.pone.0086147
Freiesleben SH, Soelberg J, Nyberg NT, Jäger AK.
Determination of the Wound Healing Potentials of
Medicinal Plants Historically Used in Ghana.
Evidence-Based Complementary and Alternative
Medicine 2017;1-6.
http://dx.doi.org/10.1155/2017/9480791
Futagami A, Ishizaki M, Fukuda Y, Kawana S, Yamanaka
N. Wound Healing Involves Induction of
Cyclooxygenase-2 Expression in Rat Skin. Laboratory
Investigation 2002;82(11):1503-13.
DOI:10.1097/01.LAB.0000035024.75914.39
Li X, Kong L, Liao S, Lu J, Ma L, Long X. The
Expression and Significance of Feces
Cyclooxygensae-2 mRNA in Colorectal Cancer and
Colorectal Adenomas. Saudi Journal of
Gastroenterology 2017;23(1):28-34.
DOI: 10.4103/1319-3767.199112
Margata L, Silalahi J, Harahap U, Satria D. The Effect of
Dietary Oils and Hydrolyzed Coconut Oil on Minerals
Absorption in Rats. Asian Journal of Pharmaceutical
and Clinical Research 2018;11(1):185-90.
DOI: 10.22159/ajpcr.2017.v11i1.20687
Harahap U, Hasibuan PAZ, Sitorus P, Arfian N, Satria D.
Antimigration Activity of an Ethylacetate Fraction of
Zanthoxylum acanthopodium DC. Fruits in 4T1 Breast
Cancer Cells. Asian Pacific Journal of Cancer
Prevention 2018;19:565-9.
DOI:10.22034/APJCP.2018.19.2.565
Ridiandries A, Bursill C, Tan J. Broad-Spectrum
Inhibition of the CC-Chemokine Class Improves
Wound Healing and Wound Angiogenesis.
International Journal of Molecular Sciences
2017;18:155.
DOI:10.3390/ijms18010155
www.pnas.org/cgi/doi/10.1073/pnas.162468699
Rodrigues HG, Vinolo MAR, Sato FT, Magdalon J, Kuhl
CMC, et al. Oral Administration of Linoleic Acid
Induces New Vessel Formation and Improves Skin
Wound Healing in Diabetic Rats. Plos One
2016;11(10):1-19. DOI:10.1371/journal.pone.0165115
Stamm A, Reimers K, Strauß S, Vogt P, Scheper T,
Pepelanova I. In vitro wound healing assays – state of
the art. BioNanoMat 2016;17(1-2):79–87. DOI
10.1515/bnm-2016-0002
The Activity of Virgin Coconut Oil to Increase Proliferation and COX-2 Expression towards NIH 3T3 Cell Line
781
