
Cell Cycle Analysis of Plectranthus amboinicus, (Lour.) Spreng.
Leaves Ethanol Extract Nanoparticles on T47D Cell Lines
Poppy Anjelisa
1
, Zaitun Hasibuan
2
and Sumaiyah
3
1
Nanomedicine Center of Innovation,Universitas Sumatera Utara,Medan, Indonesia
2
Department of Pharmacology, Faculty of Pharmacy, Universitas Sumatera Utara,Medan, Indonesia
3
Department of Pharmaceutical Technology, Faculty of Pharmacy, Universitas Sumatera Utara,Medan, Indonesia
Keywords: Cell Cycle, Plectranthus Amboinicus, Nanoparticle, T47D Cell Line.
Abstract: The development of molecular targeted therapy in cancer is necessary to reduce the occurrence of cell
resistance and toxicity towards normal cell. The aim of this study is to evaluate the effect of Plectranthus
amboinicus (Lour.) Spreng. leaves ethanol extract nanoparticle on T47D cell cycle. The dried leaves powder
of Plectranthus amboinicus (Lour.) Spreng. was extracted with ethanol by maceration method. Ionic
gelation method was implemented for the preparation of Plectranthus amboinicus leaves ethanolic extract
nanoparticles. The cell cycle of T47D treated with ethanol extract nanoparticle of Plectranthus amboinicus
(Lour.) Spreng. leaves were analyzed using FACScan flow cytometer. Treatment of PAEEN with IC
50
concentration, ½ IC
50
(44.582 µg/mL, and ¼ IC
50
(22.291 µg/mL) caused cell accumulation at G
0
– G
1
phase. At S phase, the percentage of accumulation at ¼ IC
50
(22.291 µg/mL) higher than control. The study
showed that cells underwent apoptosis indicated by occurrence of inhibition of cell cycle on G0-G1 phase
and S phase.
1 INTRODUCTION
Several advantages have been achieved for breast
cancer treatment, including combination treatment
of chemotherapy, antibody therapy and endocrine
therapy. However, the resistance of cancer cells
being one of a major problem in breast cancer
treatment (Lifiani, 2018).
The development of molecular targeted therapy
in cancer is necessary to reduce the occurrence of
cell resistance and toxicity towards normal cell.
Therapeutic targets may involve many proteins and
mechanisms, including the inhibition of protein in
the signaling process which regulates the growth and
the development of cancer cells and of proteins
which cause the resistance of cancer treatment
(Hasibuan, 2016).
The previous studies have demonstrated the
activity of Plectranthus amboinicus (Lour.) Spreng.
leaves extracts on cancer cell could be due to the
inhibition of cell cycle. The study showed that
ethylacetate extracts of Plectranthus amboinicus
(Lour.) Spreng. leaves changed the accumulation of
cell cycle phase from G0-G1 phase (54.61%) to sub
G1 phase (69.73%) (Hasibuan, 2014). In this study,
we aimed to investigate the effect of Plectranthus
amboinicus (Lour.) Spreng. leaves ethanol extract
nanoparticle on T47D cell cycle.
2 METHODS
The extraction was conducted by maceration
method. Dried leaves powder of Plectranthus
amboinicus (Lour.) Spreng. was extracted with
ethanol for 3 days at room temperature. The extract
then concentrated using rotary evaporator, and was
dried by freeze-dryer.
2.1 The Preparation of Nanoparticles
of Plectranthus amboinicus (Lour.)
Spreng. Leaves Ethanolic Extract
(PAEEN)
Ionic gelation method was implemented for the
preparation of Plectranthus amboinicus leaves
ethanolic extract nanoparticles. 0.3% PAEEN
(Plectranthus amboinicus (Lour.) Spreng. leaves
ethanolic extract nanoparticles) was diluted in 1.5%
Anjelisa, P., Hasibuan, Z. and Sumaiyah, .
Cell Cycle Analysis of Plectranthus amboinicus, (Lour.) Spreng. Leaves Ethanol Extract Nanoparticles on T47D Cell Lines.
DOI: 10.5220/0010072904590461
In Proceedings of the International Conference of Science, Technology, Engineering, Environmental and Ramification Researches (ICOSTEERR 2018) - Research in Industry 4.0, pages
459-461
ISBN: 978-989-758-449-7
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
459
acetic acid. The sodium tripolyphosphate (1mg/ml)
was added to the extract with dropwise under
magnetic stirring for an hour. The mixture result of
PAEEN nanoparticles was separated by centrifuge at
the speed of 15000 rpm for 20 minutes. The pellet
was collected and used for characterization (Raj,
2015).
1 gram of the ethanolic extract of Plectranthus
amboinicus leaves was diluted into 35 mL ethanol
p.a, added by 15 mL distilled water, chitosan in 100
mL glacial acetic acid 1% and 350 mL NaTPP
solution. Stirred by using magnetic stirrer for ± 2
hours. Then the colloid of nanoparticle chitosan-
NaTPP of Plectranthus amboinicus leaves ethanolic
extract were separated by centrifugation. The result
was put in to the freezer (± -4
o
C) for ± 2 days. Then,
it was moved to refrigerator (± 3°C) to dry. The
resulting nanoparticles were characterized using
PSA (Particle Size Analyzer). The formed solids are
characterized using TEM (Transmission Electron
Microscope) to determine the morphological form in
its solid form.
2.2 Cell Line and Culture Condition
T47D cell lines were obtained from Parasitology
Laboratory, Faculty of Medicine, Gadjah Mada
University, Indonesia. The cell line was maintained
in RPMI 1,640 suplemented with 10% Foetal
Bovine Serum v/v (Gibco). Cells were cultured in
the presence of 1% penicillin-streptomycine
(Gibco), and 0.5% fungizone (Gibco) and incubated
at 37
o
C in humidified atmosphere containing 5%
CO
2
.
2.3 Cell Cycle Analysis
The cell cycle analysis was carried out according to
our previous study (Hasibuan, 2014). T47D cells
(5×10
5
cells/well) were treated with 89.166 µg/mL
(IC
50
concentration), 44.582 µg/mL (½ IC
50
), and
22.291 µg/mL (¼ IC
50
) PAEEN for 24 hrs. Cells
then washed, harvest and fixed with 70% ice-cold
ethanol. Cells were washed 3 times with ice-cold
PBS, resuspended and centrifuged at 3000 rpm for 3
minutes. Cells were treated with RNAse 100 μg/mL
containing PI 40 μg/mL incubated at 37°C for 30
minutes. Cell cycle distribution was then were
analyzed using FACScan flow cytometer. Data were
calculated using ModFit Lt. 3.0.s; (Satria, 2017).
3 RESULTS AND DISCUSSIONS
The cell cycle involves mainly four steps which lead
to cell growth and cell division in order to produce 2
daughter cells. The phases are G1, S, G2 and M
(Dalimunthe, 2017). Recent study has showed that
IC
50
of PAEEN was 89.166 µg/mL which is
potential as anticancer. Next, we explore the
mechanism of PAEEN by analyzing cell cycle
distribution using propidium iodide staining and
flowcytometry. Propidium iodide is a fluorogenic
dye wich binds to DNA, allowing the DNA content
of the stained cells to be anayzed by flow cytometry.
Cells can be classified according to phases of the
cell cycle ((G0/G1, S, and G2/M) based on DNA
content (Xuereb and Blundell, 2008). Treatment of
PAEEN with IC
50
concentration, ½ IC
50
(44.582
µg/mL, and ¼ IC
50
(22.291 µg/mL) caused cell
accumulation at G
0
– G
1
phase. At S phase, the
percentage of accumulation at ¼ IC
50
(22.291
µg/mL) higher than control which mean that
PAEEN could inhibit on S phase as well. During the
G1/S phase checkpoint, DNA damage is sensed and
the cell cycle is paused until the DNA is thoroughly
repaired. This ensure that the S phase is embarked
only when the DNA damage accumulated
throughout the entire cycle has been eliminated. If
the damage is so severe, apoptosis can be induced.
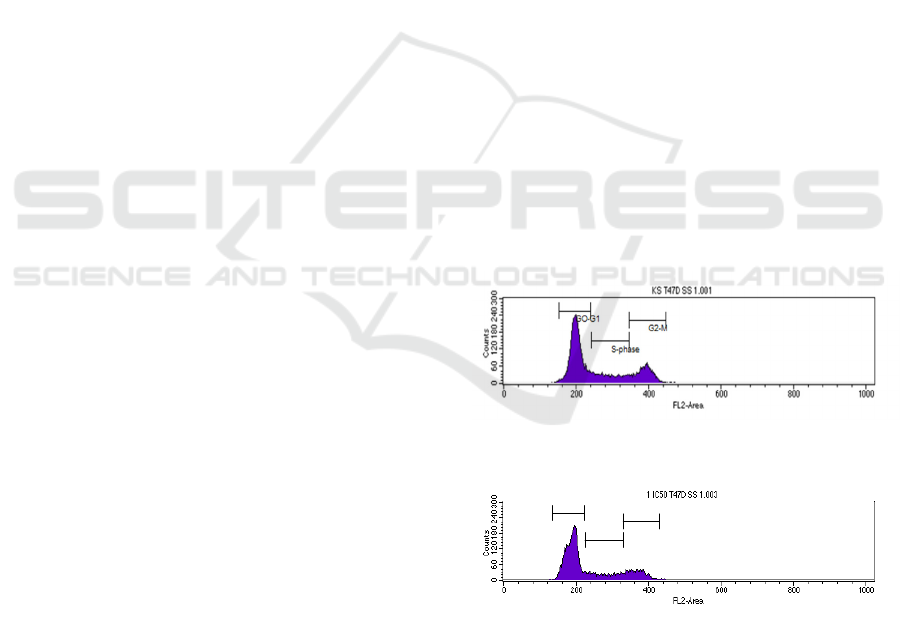
The cell cycle accumulations were showed on Figure
1-4 as follow:
Figure 1: Control cells T47D.
Figure 2: cell cycle inhibition by Plectranthus amboinicus
ethnolic extract nanoparticles with IC
50
.
GO-G1
S-phase
G2-M
ICOSTEERR 2018 - International Conference of Science, Technology, Engineering, Environmental and Ramification Researches
460
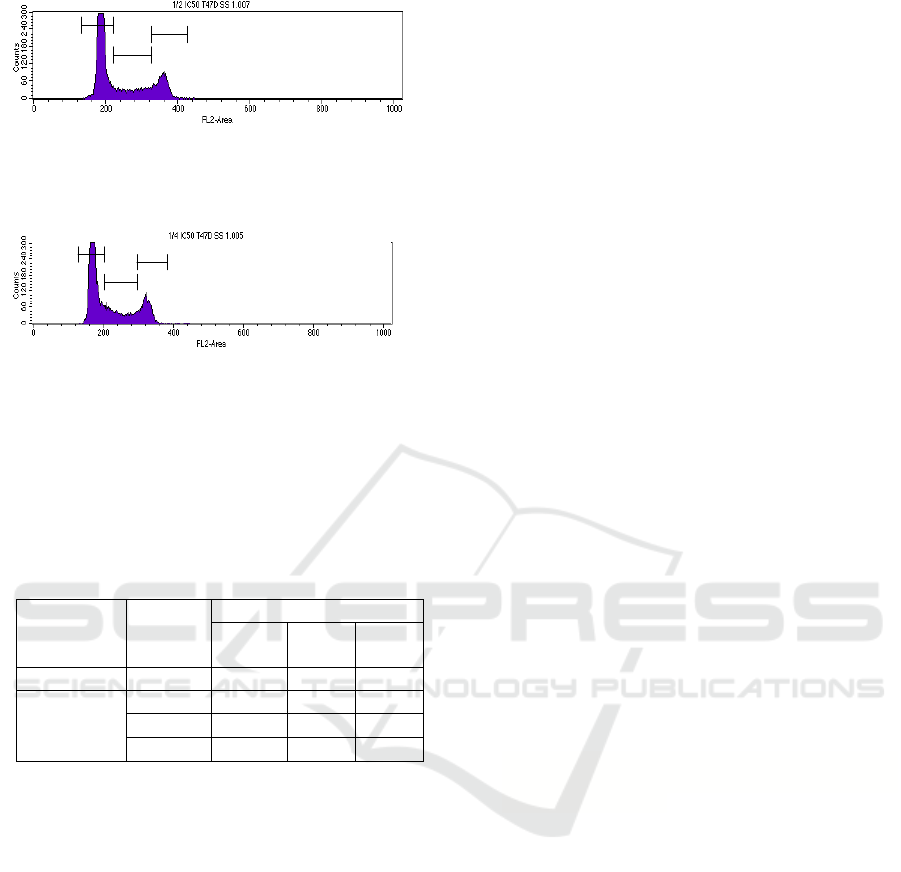
Figure 3: cell cycle inhibition by Plectranthus amboinicus
ethnolic extract nanoparticles with ½ IC
50
.
Figure 4: cell cycle inhibition by Plectranthus amboinicus
ethnolic extract nanoparticles with ¼ IC
50
.
The percentage of cell accumulation on every
phase showed that there is a cell cycle arrest on G0-
G1 phase because the percentage was higher than
control as shown on table 1.
Table 1: The percentage of cell accumulation.
We have previously reported that PAEEN can
prevent proliferation and induce apoptosis on T47D
breast cancer cell lines. On this study, we showed
that cells underwent apoptosis indicated by
occurrence of inhibition of cell cycle on G0-G1
phase and S phase. Cell cycle control is deregulated
in cancer cells in spite of defects in their genome,
they can easily pass cell cycle protein such as cyclin
D1 and drive the cycle to proliferation without any
interruption (Xuereb and Blundell, 2008).
4 CONCLUSIONS
PAEEN could inhibit the cell cycle T47D cell lines
at G0-G1 phase and S phase. The data provide the
promising candidate for further studies.
ACKNOWLEDGEMENTS
This research is funded by Universitas Sumatera
Utara, Indonesia. The support is under the “Hibah
Talenta” Research Grant 2018 No.
233/UN5.2.3.1/PPM/KP-TALENTA USU/2018.
REFERENCES
Lifiani, R., Harahap, U., Hasibuan, PAZ., Satria, D. 2018.
Anticancer Effect of African Leaves (Vernonia
Amygdalina, Del.) to T47D Cell Resistant. Asian J of
Pharm and Clin Research. Volume 11 issue 1. P:4-7.
Hasibuan, PAZ., Harahap, U., Sitorus, P., Satria, D. 2016.
Ethylacetate Extract of Zanthoxylum acanthopodium
DC. Fruit Againts Doxorubicin-Resistanced T47D
Cells. Der Pharma Chemica. 8(20): 172-174
Hasibuan, Rosidah, Satria, D. 2014. Cell Cycle Inhibition
from Ethylacetate Extract of Plectranthus amboinicus,
(Lour) Spreng. Leaves on HeLa Cell Lines.
Proceeding. Medan International Conference on
Advanced Pharmaceutical Sciences.
Raj, L.F.A.A., Jonisha, R.,Revathi, B., Jayalakhsmy, E.
2015. Preparation and Characterization of BSA and
Chitosan Nanoparticle for Sustainable Delivery
System for Quercetin. J of Applied Pharmaceutical
Sciences. Vol 5 (07). P: 01-05
Hasibuan, PAZ, Chrestella, J, Satria, D. 2015.
Combination Effect of Ethylacetate Extracts of
Plectranthus amboinicus, (Lour.) Spreng. With
Doxorubicin Againts T47D Breast Cancer Cells. Int J
of Pharmacy and Pharmaceutical Sciences.volume 7
issue 10. P: 156-159
Satria D, Furqan M, Hadisahputra S, Rosidah.. 2015.
Combinational Effects of Ethylacetate Extract of
Picria Fel-Terrae Lour And Doxorubicin On T47D
Breast Cancer Cells. Int J Pharmacy & Pharm
Sci;7:73-6.
Satria D, Silalahi J, Haro G, Ilyas S, Hsb PA. 2017.
Antioxidant and Antiproliferative Activities of an
Ethylacetate Fraction of Picria fel-terrae, Lour. Herbs.
Asian Pac J Cancer Prev;18(2):399-403.
Dalimunthe, A. Hasibuan, PAZ., Satria, D. 2017. Cell
Cycle Arrest Activity of Litsea cubeba Lour.:
Heartwood and Fruit Extracts Againts T47D Breast
Cancer Cells. Asian J Pharm Clin Res. Vol 10, issue
11. Page: 404-406
Xuereb, J. and Blundell, R. 2008. The Role of Cell Cycle
Regulation in Cancer. Res J of Biological Sci 3(2):
251-257
GO-G1
S-phase
G2-M
GO-G1
S-phase
G2-M
Treatments
concentr
ations
Phase (%)
G
0
–
G
1
S G
2
-
M
Control 59.07 18.93 21.36
PAEEN IC
50
63.92 17.30 18.32
½ IC
50
66.89 16.19 16.74
¼ IC
50
60.94 20.46 19.05
Cell Cycle Analysis of Plectranthus amboinicus, (Lour.) Spreng. Leaves Ethanol Extract Nanoparticles on T47D Cell Lines
461
