
Using Near Infrared Spectroscopy to Index Temporal Changes in Affect
in Realistic Human-robot Interactions
Megan Strait and Matthias Scheutz
Tufts University, 161 College Avenue, Medford, MA 02155, U.S.A.
Keywords:
Brain Computer Interfaces, Functional Near Infrared Spectroscopy, Human Robot Interaction, Affect
Detection, Signal Processing.
Abstract:
Recent work in HRI found that prefrontal hemodynamic activity correlated with participants’ aversions to
certain robots. Using a combination of brain-based objective measures and survey-based subjective measures,
it was shown that increasing the presence (co-located vs. remote interaction) and human-likeness of the
robot engaged greater neural activity in the prefrontal cortex and severely decreased preferences for future
interactions. The results of this study suggest that brain-based measures may be able to capture participants’
affective responses (aversion vs. affinity), and in a variety of interaction settings. However, the brain-based
evidence of this work is limited to temporally-brief (6-second) post-interaction samples. Hence, it remains
unknown whether such measures can capture affective responses over the course of the interactions (rather
than post-hoc). Here we extend the previous analysis to look at changes in brain activity over the time course
of more realistic human-robot interactions. In particular, we replicate the previous findings, and moreover
find qualitative evidence suggesting the measurability of fluctuations in affect over the course of the full
interactions.
1 INTRODUCTION
With recent advances in brain-imaging technology,
inexpensive sensors are becoming increasingly acces-
sible to researchers and consumers alike. Moreover,
the production of sensors that are also small and wire-
less (in addition to being affordable) is promising for
HRI, as that allows for the wearing of these sensors
while performing a large set of activities without be-
ing intrusive. Examples of such devices include the
Emotiv Epoch
1
and NeuroSky MindWave
2
, which
are two EEG headsets that measure electrical activ-
ity in the brain linked to states of excitement, atten-
tion, anxiety or cognitive load. Socially and affect-
aware robots that can capture and respond to some
of these states from a human have been found to be
more effective in engaging people, e.g., (Szafir and
Mutlu, 2012). For these reasons, research on neuro-
physiological signals has been attracting the attention
of researchers in the Human-Robot Interaction (HRI)
community over recent years (Rosenthal von der Put-
ten et al., 2013b).
1
http://emotiv.com/epoc/features.php
2
http://www.neurosky.com/Products/MindWave.aspx
Neural data, in particular, is extremely relevant
for HRI research in two main directions. First, it
can complement traditional survey methods such as
questionnaires and thus yield further understanding
of users genuine responses toward robots during the
interaction, e.g., (Rosenthal von der Putten et al.,
2013b; Strait et al., 2013a). Another potentially
promising facet for neurological signal processing is
affect detection in realtime, e.g., (Heger et al., 2013;
Zander, 2009), so that the robot can react accordingly.
Within the HRI community, there is a small, but
growing body of research employing brain-computer
interfaces (BCIs) as a modality for both understand-
ing and augmenting a person’s experience (Canning
and Scheutz, 2013; Zander, 2009; Zander and Kothe,
2011). Brain-based adaptivity of robotic agents has
shown to yield performance and learning enhance-
ments (Solovey et al., 2012; Szafir and Mutlu, 2012).
BCIs have also been used to further understand the
user’s perceptions of a robot, e.g., (Broadbent et al.,
2013; Kawaguchi et al., 2012; Rosenthal von der Put-
ten et al., 2013a; Strait et al., 2013a; Strait et al.,
2014). In particular, a recent mixed-methods study
employing a combination of brain-based and subjec-
tive measures reflected participants’ affinity towards
385
Strait M. and Scheutz M..
Using Near Infrared Spectroscopy to Index Temporal Changes in Affect in Realistic Human-robot Interactions.
DOI: 10.5220/0004902203850392
In Proceedings of the International Conference on Physiological Computing Systems (OASIS-2014), pages 385-392
ISBN: 978-989-758-006-2
Copyright
c
2014 SCITEPRESS (Science and Technology Publications, Lda.)

a robot (Strait et al., 2014; Strait and Scheutz, 2014).
This study used a brain-imaging technique – func-
tional near infrared spectroscopy (NIRS) – similar in
basis to fMRI, but less restrictive (e.g., participants
need not be confined to a tube).
However, the brain measurements were limited
to brief (6-second), post-hoc re-exposure (viewing
a series of images of the robots). Thus it re-
mains unknown whether NIRS-based BCIs can cap-
ture changes in participants’ affect over the course of
the human-robot interactions. Here we further probe
the dataset collected in (Strait et al., 2014) to quali-
tatively evaluate changes in brain activity over inter-
actions lasting two minutes in duration. While there
are still a number of limitations of neurophsyiology
and NIRS that concern the usage of BCIs in realistic
HRI interaction environments (Canning and Scheutz,
2013; Hoshi, 2011; Strait et al., 2013b), this inves-
tigation begins to bridge the gap between unrealistic
and realistic interaction settings (e.g., viewing images
of robots for several seconds versus unrestricted, live
interactions).
2 RELATED WORK
Within the HRI community, there is a small, but grow-
ing body of research employing brain-computer inter-
faces as a modality for both understanding and aug-
menting a person’s experience (Canning and Scheutz,
2013; Zander, 2009; Zander and Kothe, 2011). Brain-
based adaptivity of robotic agents based on students’
level of attention has been repeatedly shown to pro-
duce learning enhancements (Andujar et al., 2013;
Szafir and Mutlu, 2012; Szafir and Mutlu, 2013).
BCIs have also been used to further understand the
user’s perceptions of a robot, e.g., (Broadbent et al.,
2013; Kawaguchi et al., 2012; Rosenthal von der Put-
ten et al., 2013a).
In particular, brain-based measures have been
used for better understanding users’ perceptions of
robots. For example, fMRI has been used to inves-
tigate emotional responses towards robots (Rosenthal
von der Putten et al., 2013b) and towards a humanoid
robot displaying affective gestures (Chaminade et al.,
2010). Cooperation, rapport, and moral decision-
making have been investigated using NIRS-based sys-
tems (Kawaguchi et al., 2012; Shibata, 2012; Strait
et al., 2013a). A number of BCIs have been also
been employed for affect detection, e.g., (Heger et al.,
2013), by capitalizing on signal artifacts arising from
facial expressions (Heger et al., 2011) or by target-
ing the ventromedial prefrontal cortex (Strait et al.,
2013a).
While numerous exemplars of EEG-based BCI
systems exist for augmenting human-robot interac-
tions, we focus here on NIRS in particular for the
greater spatial resolution (which facilitates the tar-
geting of ventromedial prefrontal cortical activity
reflective of emotion regulatory processes). NIRS
(functional Near Infrared Spectroscopy, also called
‘fNIRS’) is a neuroimaging technique similar to func-
tional Magnetic Resonance Imaging (fMRI) that mea-
sures changes in blood flow corresponding to neural
activity (Canning and Scheutz, 2013). From usability
testing to animal-assisted therapy (e.g., (Kawaguchi
et al., 2012; Shibata, 2012)), NIRS has served for over
two decades as a quantitative metric in evaluating user
workload, as an alternative interaction modality in as-
sistive technologies, and more recently, as a passive
input technique to adapt computer interfaces based on
a user’s affective state (Zander and Kothe, 2011).
In comparison to other methods such as fMRI
and EEG, NIRS-based systems have been reported
as better suited for realistic settings, with the pri-
mary strenth of being more robust to user move-
ment (Cui et al., 2010a; Cui et al., 2011; Solovey
et al., 2009). Recent work in HCI has further en-
dorsed NIRS-based BCI as suitable for passive in-
put to adaptive user interfaces based on improve-
ments observed in behavioral indices of user perfor-
mance (Solovey et al., 2011; Solovey et al., 2012).
Moreover, although NIRS is vastly slower in tem-
poral resolution compared to EEG, recent work has
demonstrated reliable classification accuracies with
under two-seconds delay from a response onset (Cui
et al., 2010b). Thus it is potentially promising for
use as input to robotic agents for adapting social be-
havior appropriate to user’s perceptions or affective
state. However, few NIRS-based studies have investi-
gated sequential or prolonged tasks (e.g., (Hoshi and
Tamura, 1997)), rather NIRS is predominately em-
ployed in event-related experimental designs, limited
to stimulus periods of sub-60s (Canning and Scheutz,
2013; Strait et al., 2013b; Hoshi, 2011). Thus it has
yet to be shown whether NIRS is applicable to more
realistic human-robot interaction settings.
3 MATERIALS AND METHODS
In a previous mixed-design human-robot interaction
experiment, manipulating the type (MDS vs. PR2) of
robot helper and modality of interaction (3rd-person
remote vs. 1st-person remote vs. 1st-person co-
located; see Figure 1), participants’ prefrontal corti-
cal activity was recorded while they completed a set
of two drawing tasks with each robot (Strait et al.,
PhyCS2014-InternationalConferenceonPhysiologicalComputingSystems
386

Figure 1: Subject perspectives for each of the three interaction modalities. Left – the 3rd-person perspective interaction (3R)
condition with the PR2 as the robot helper. Right and center – the 1st-person perspective protocols, with the MDS robot helper
remotely interacting (1R; center) and co-located with the participant (1C; right).
2014).
Manipulation of the robot helper was intended
to vary the degree of the perceived human-likeness
of robot (human-like versus mechanical). Thus the
multi-dextrous social (MDS) robot was used for its
relatively human-like appearance in contrast to the
more typical appearance of Willow Garage’s PR2
robot. The three interaction conditions, on the other
hand, were intended to modulate the directness (ob-
servatory versus participatory) and proximity (remote
versus co-located) of the interaction.
To determine the effects of the aforementioned
manipulations on participants’ perceptions of the two
robot helpers (as indexed by prefrontal hemodynamic
activity), Strait et al. additionally presented partic-
ipants with a set of still images of each robot fol-
lowing completion of the four drawing tasks. This
post-task exposure using still images allowed for the
controlling of potential confounds including: artifacts
from participant movement, unrelated hemodynamic
changes (e.g., changes due to the drawing task), and
unrelated physiological artifacts (e.g., low frequency
signal drift).
A preliminary evaluation of the manipulation ef-
fects on the controlled post-exposure brain activity
showed the MDS elicited significantly greater activ-
ity than the PR2 in the 1C condition. This effect was
reversed in the 3R condition, which showed the PR2
elicited significantly greater activity. The effects in
the first-person, remote (1R) interaction condition fell
squarely between the two other interaction conditions,
with no significant differences in response to either
robot.
Here we extend the previous analysis, using the
aforementioned NIRS dataset collected during the
drawing tasks in (Strait et al., 2014) to investigate the
changes in brain activity over the course of the full-
length human-robot interactions.
3.1 Dataset
We utilized the NIRS dataset collected in the afore-
mentioned IRB-approved study (Strait et al., 2014).
In that study, 45 participants were instructed on four
distinct tasks by two robot helpers (see Figure 1).
Hardware and software issues of the NIRS equipment
lead to failure to record or fully record seven partici-
pants’ interactions, resulting in a NIRS dataset of 38
participants. Subject demographics showed a 55/45
female-to-male ratio (21 female/17 male participants)
and average age of 21.4 years (SD = 4.1).
3.1.1 Experimental Manipulations
Two primary manipulations were studied. To eval-
uate the effects of human-likeness, the Xitome De-
sign’s Mobile Dexterous Social (MDS) robot and Wil-
low Garage’s PR2 were used as the two robot in-
structors. They were chosen for their stereotypical
robotic (PR2) and human-like (MDS) appearances
and to avoid potential effects of height and girth of
the helper (which are approximately equal between
the two robots).
To measure the effects of interaction modality
(comprised of participant perspective – first-person
vs. third-person – and the robot’s presence – co-
located vs. remote), three interaction conditions were
created: (a) 3rd-person, remote (3R); (b) 1st-person,
remote (1R); and (c) 1st-person, co-located (1C) – see
Figure 1.
3.1.2 Data Acquisition
NIRS recordings of participants’ bilateral anterior
prefrontal cortex were taken while participants inter-
acted with each of the two robots using a two-channel
NIRS oximeter. This placement of the NIRS sensors
corresponds to areas linked, in particular, to emotion
UsingNearInfraredSpectroscopytoIndexTemporalChangesinAffectinRealisticHuman-robotInteractions
387

regulation (Chaminade et al., 2010; Ochsner et al.,
2012; Rosenthal von der Putten et al., 2013a; Strait
et al., 2013a; Urry et al., 2006).
3.2 Signal Processing
Although the individual tasks were not fixed in du-
ration, here we limit our analyses to the first two
minutes of interaction for consistency for between-
subjects comparisons. Thus, upon extracting the first
two recorded minutes of each interaction, the NIRS
dataset was then preprocessed in a similar manner as
in (Strait et al., 2014): (1) conversion of raw light at-
tenuation to changes in hemoglobin concentrations,
(2) linear detrending to remove signal drift, (3) fil-
tering of cardiac artifacts using a Savitzky-Golay low
pass filter with degree 1 and cut-off frequency of
0.5Hz, and (4) correlation-based signal improvement
((Cui et al., 2010a)) to correct non-systemic artifacts
(e.g., motion). Following, for each robot, we aver-
aged across participants by condition, using one chan-
nel which corresponded to participants’ oxygenated
hemoglobin concentration changes in the left PFC. As
there were two interaction tasks with each robot, this
yielded one average timeseries per robot (2), per in-
teraction modality (3), per task (2) for a total of 12
two-minute signals.
3.3 Statistical Inference
We first naively compared each pairing of signals
(e.g., MDS vs. PR2 in 1C, first task) using repeated
paired t-tests (Bonferroni corrected, α = .004). As
expected (given the signals were two minutes in dura-
tion), all pairings were statistically significantly dif-
ferent. As the tasks were free-form and not time-
constrained, and moreover, as the placement of the
NIRS probes were qualitative (aligned with the center
of the forehead, atop the brow) – quantitative compar-
isons across participants would be confounded with
differences in alignment of the interactions with the
robots and alignment of the precise cortical area be-
ing sample. Thus we proceeded with a qualitative dis-
cussion of their differences and the resulting implica-
tions for using NIRS to measure affective responses
in more realistic settings.
4 DISCUSSION
In this investigation, we extended previous work to
consider the following questions regarding the use of
NIRS for evaluating human-robot interactions: (1) are
there observable effects of interaction modality and
human-likeness over more realistic task durations?,
(2) what, if any, are the effects of prolonged exposure
or repeat interactions with a robot?, and (3) are these
effects observable at the level of an individual? We
first discuss the effects of interaction settings (modal-
ity and human-likeness of the robot agent), which
mirror those observed in (Strait et al., 2014). We then
examine the differences in activation between the first
and second interactions performed with each robot,
to discuss effects of repeated exposure. Lastly, we
consider the findings from (1) and (2) from a within-
subjects perspective.
4.1 Effect of Interaction Modality and
Robot Human-likeness
Consistent with the findings of (Strait et al., 2014;
Strait and Scheutz, 2014), a correlation was ob-
served between human-likeness and prefrontal hemo-
dynamic activity (see Figure 2). Specifically, in
the first-person, co-located interaction condition, the
MDS robot elicited a significant increase in activ-
ity compared to the PR2. Whereas, in the third-
person, remote interaction setting, the MDS elicited
a significantly greater decrease in activity than the
PR2. Furthermore, the first-person, remote settings
showed comparatively no change (with relatively mi-
nor changes oscillating around zero). Interestingly,
however, these results were only consistent with the
previous when limited to the first 15-20 seconds of
interaction.
In combination with the subjective responses re-
ported in (Strait et al., 2014), the significant differ-
ences in neural activity to the two robots according
to the interaction condition further underscores an ef-
fect of human-likeness and the corresponding percep-
tion of eeriness. In the 1PC condition, participants
showed markedly greater activity in response to the
MDS robot as compared to their response to the PR2.
As the prefrontal cortex has been shown to be active
in response to robots with high subjective ratings of
eeriness (Strait and Scheutz, 2014), this suggests a
strong emotional response was evoked in participants
directly interacting with the very human-like MDS.
Considering the subjective preferences reported in
(Strait et al., 2014) also showed strong preferences for
the PR2 versus the MDS (67% 1C participants versus
47% of 1R and 3R participants), this activity seems to
be reflective of aversion to the more human-like robot
when it is co-located with the participant. This effect
is seems initially contrary to the findings of (Broad-
bent et al., 2013; Lisetti, 2011) which suggest that
a robot with a more human-like face is found to be
more likeable. However, participants in Broadbent
PhyCS2014-InternationalConferenceonPhysiologicalComputingSystems
388
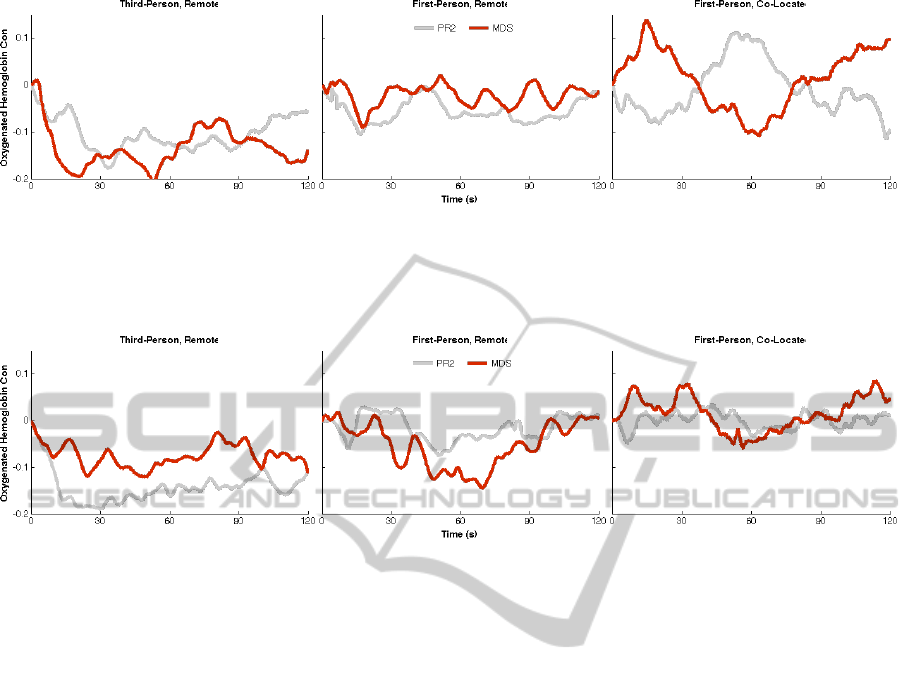
Figure 2: Average PFC activity during the first task with each robot (red depicts the interaction with the MDS, and gray,
the PR2), by interaction condition. Left – the 3R interaction condition shows a decrease (initially greater in response to the
MDS than PR2) in oxygenated hemoglobin across robots. Middle – similarly, the 1R interaction condition shows only slight
changes over the two minutes of interaction for both robots. Right – the 1C condition shows an initial increase in HbO in
response to the MDS and decrease in response to the PR2.
Figure 3: Average PFC activity over the course of the second interaction with each robot, by interaction condition. Left –
the 3R interaction condition shows a decrease (greater in response to the PR2 than MDS) in oxy-hemoglobin across robots.
Middle and right – the first-person interaction conditions show no major changes in hemoglobin in response to either robot.
et al. and Lisetti interacted with computer-generated
avatars or a robot with a display screen face, rather
than an embodied agent. Given similar settings (the
1R condition: interaction with the robots via Skype),
participants seemed emotionally unaffected, perhaps
due to relative reduction in robot presence.
Overall, these results replicate previous findings
which suggest the correspondance of prefrontal ac-
tivity with aversion (Strait et al., 2014; Strait and
Scheutz, 2014) – in that it seems the eeriness of the
MDS may elicit emotion regulation mechanisms in
first-person interaction to reduce the unnerving ef-
fects of human-likeness and the corresponding eeri-
ness. Whereas in a removed context such as that of
observing video of the two – much like viewing a
movie – the fear or anxiety elicited by the MDS’ eerie
appearance may have been reduced or non-present.
However, they also suggest that participants’ affective
responses may change over the course of the interac-
tion (e.g., the decrease in hemodynamic activity from
15s to 60s in the 1C interaction condition with the
MDS; Figure 2, right). Due to this observation of sig-
nificant change in the 1C condition, we next consid-
ered how the prefrontal activity during participants’
second interaction (i.e., second 2 minute task) with
each robot compared to their first.
4.2 Effect of Repeat Interactions
The repeat interactions (the second task performed
with each robot) or prolonged exposure to each robot
showed significantly reduced responses (e.g., the
magnitude of activity elicited in response to the MDS
was decreased) in some settings and significantly en-
hanced in others (see Figure 3).
Figure 3 shows the prefrontal activity during the
second interaction with each robot. In the third-
person interaction condition, this repeat interaction
shows the reverse trends from the first interaction:
originally the MDS elicited greater negative change
in comparison to the PR2 for 3R subjects. However,
in the second interaction, participants show a more
negative change in response to the PR2 (and the mag-
nitude of the response to the MDS is reduced). In ad-
dition, participants in the 1R condition now showed a
slight and slow decrease in HbO over the first 60+ sec-
onds of the interaction. Activity elicited by the PR2
in the 1R condition still remained around zero. Partic-
ipants in the 1C condition interestingly now showed a
response similar to participants in the 1R condition.
Here in the second task, we observe only a slight in-
crease in HbO in response to the MDS followed by
a slighter and slower decrease over the first 60 sec-
onds. Whereas previously, the 1R condition showed
UsingNearInfraredSpectroscopytoIndexTemporalChangesinAffectinRealisticHuman-robotInteractions
389
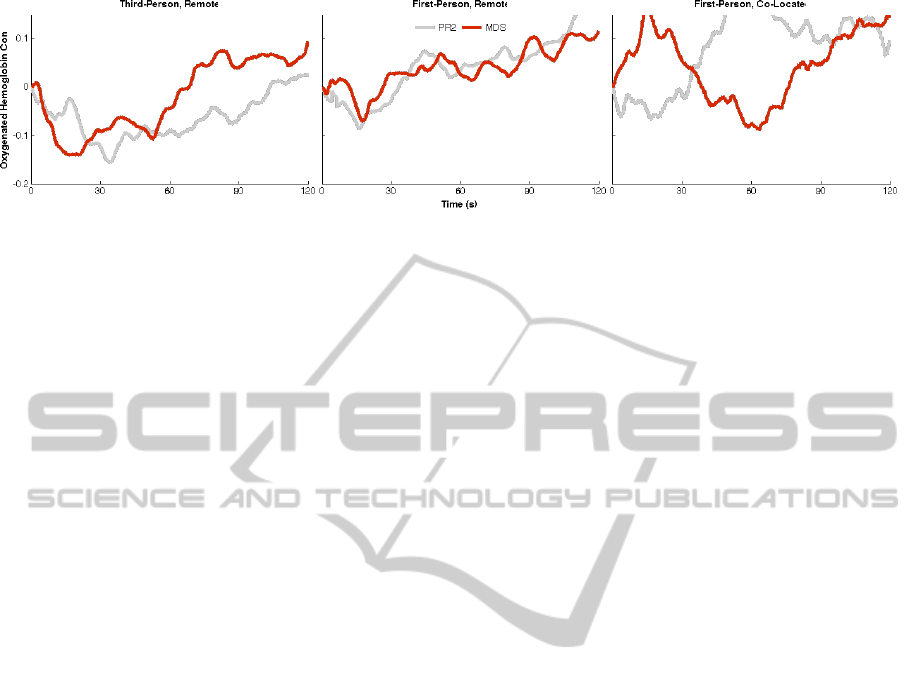
Figure 4: Non-detrended average PFC activity over the course of the first with each robot. Qualitatively the trends (decrease
in hemoglobin in response to the MDS in 3R versus increase in hemoglobin in response to the MDS in 1C) are apparent.
relatively no response and the 1C condition showed a
severe increase in response to the MDS. Moreover, 1C
participants showed a strong increase in HbO to the
PR2 in the middle 60s of the first interaction, which
is entirely absent from the second interaction.
While the changes occurring within the timeframe
of the tasks (e.g., PR2 activity peak in HbO at 60s in
the 1C condition) may be a function of the interac-
tion (e.g., the PR2’s cooling fans suddenly turn on
in the middle of the task), they nevertheless may be
reflective of temporally-brief aversions of the partici-
pants to the robots. If the activity, for instance, in the
2nd task in the 1R condition is reflective of a growing
aversion to the MDS or if the decrease in magnitude
of brain activity in the 2nd task in the 1C condition is
reflective of a decrease in aversion, this information
becomes particularly relevant to how a robot might
adjust it’s behavior. However, to deploy NIRS as a
mechanism for adapting robot behavior, it is also nec-
essary that these effects be observable at the individ-
ual level and not solely as an aggregate trend. More-
over, to influence a robot’s behavior in realtime, the
delay in signal processing becomes an important con-
sideration. Thus we next considered the persistence
of the observed effects in two facets: (1) whether the
effects are visible at the level of a single participant
and (2) whether the effects are qualitatively observ-
able in the absence of signal detrending.
4.3 Persistence of Effects
Since detrending, by definition, removes low-
frequency signal drift, it necessarily requires a tempo-
ral delay on the order of the lowest-frequency signal
artifact. While some work has shown using an expo-
nential moving average with a 20s processing delay is
sufficient to remove such artifacts (Cui et al., 2010a;
Cui et al., 2011), this window still may exceed the
duration of changes in affect. Hence, we first consid-
ered the effect of reducing the data preprocessing by
eliminating signal detrending (see Figure 4). Quali-
tatively, the magnitude of the effects (e.g., severe in-
crease in 1C response to MDS) are still observable.
However, smaller effect sizes may be obscured by the
low-frequency trends. For example, the 3R signal in
response to the MDS is highly similar to that of the
1C condition from 60s to then end of the task. How-
ever, in the detrended signals, the two are much more
disimilar. Depending on the robot’s adaptive behav-
iors, misclassification of the 3R signal as indicative
of aversion may or may not be important. Thus fu-
ture work to develop computational methods for de-
tection of such effects would need to address whether
a change in signal was a result of signal drift or of a
change in affect.
In addition, looking at the range (as opposed to
global average) of brain activity shows high variation
across participants and across conditions (see Fig-
ure 5). While the variation in hemodynamic activity
across subjects qualitatively follows the trends cap-
tured by the global averages, a number of subjects
(3 in each 1C and 3R conditions) in each interaction
condition show relatively no significant changes in
HbO throughout the task durations. One interpreta-
tion may be that such subjects did not have any affec-
tive response to the interactions. Further investigation
with a larger population size or additional measures
may help disentangle these responses. Conversely,
there are a number of participants who show large
signal spikes, suggestive that large motion artifacts
were not adequately filtered. Perhaps the latter can be
addressed through various approaches to motion fil-
tering; however, state-of-the-art NIRS signal process-
ing still suggests manual inspection or motion restric-
tions to adequately filter (Canning and Scheutz, 2013;
Solovey et al., 2009; Strait et al., 2013b). While these
analyses are qualitative in nature, they suggest that
further investigation is necessary of the persistence of
effects of robot appearance and interaction modality,
and whether they are observable at a more micro level.
PhyCS2014-InternationalConferenceonPhysiologicalComputingSystems
390
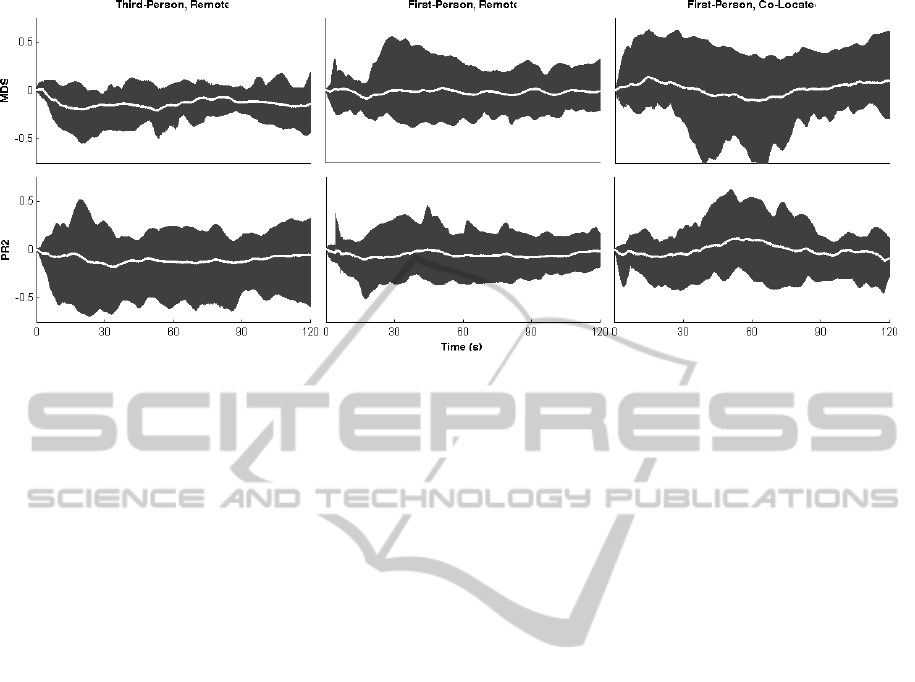
Figure 5: Range of hemodynamic activity in response to each robot across subjects. The average activity is shown in white,
and the range (minimum HbO concentration to max. HbO concentration) for subjects in the given condition is shown in gray.
5 CONCLUSIONS
The aim of this study was to investigate the use
of NIRS-based brain imaging for measuring human-
robot interactions in more realistic settings than pre-
viously. Specifically, we qualitatively analyzed pre-
frontal cortical activity over the course of two-minute
(semi) free-form interactions. Moreover, we evalu-
ated the changes in activity over repeat interactions
and whether the effects observed were large enough
to persist at an individual subject-level.
The findings are consistent with prior results, sug-
gesting, in combination with subjective measures of
preference, that PFC hemodynamics reflect a person’s
aversion to a robot and are moderated by the settings
of the interaction and the human-likeness of the robot
interlocutor. But our results also suggest that partic-
ipants’ responses fluctuate over the course of a task,
and may diminish with prolonged exposure to or in-
teraction with a given robot.
However, given the high variability and noise in
the NIRS data, it is unclear whether these effects
are reliably observable at an individual level. Thus
the use of NIRS as a feasible realtime measurement
for adapting robot behavior based on subject aversion
may be too premature to attempt without more con-
trolled investigations to better understand the individ-
ual variations in signal. Moreover, whether PFC ac-
tivity is representative of a negatively-valenced affec-
tive response requires further investigation (either in
combination with fMRI or with additional physiolog-
ical measures) in order to disconfirm the presence of
any confounds (e.g., signal artifacts or task-unrelated
activity).
Despite current limitations to the use and deploy-
ment of NIRS in realistic, realtime settings; this eval-
uation provides an important bridge between high-
controlled experiments showing observable effects
over brief exposure (e.g., viewing images for sev-
eral seconds) to actual, prolonged interactions. Future
work will thus continue to investigate this relation-
ship between signal changes and the extent to which
variations of physical appearance of the robot have an
influence on the perception of the interaction.
REFERENCES
Andujar, M., Ekandem, J. I., Gilbert, J. E., and Morreale, P.
(2013). Evaluating engagement physiologically and
knowledge retention subjectively through two differ-
ent learning techniques. In HCI International.
Broadbent, E., Kuman, V., Li, X., Sollers, J., Stafford,
R. Q., MacDonald, B. A., and Wegner, D. M. (2013).
Robots with display screens: a robot with a more hu-
manlike face display is perceived to have more mind
and a better personality. PLOS ONE, 8(8):e72589.
Canning, C. and Scheutz, M. (2013). Functional near-
infrared spectroscopy in human-robot interaction.
Journal of Human-Robot Interaction, 2(3):62–84.
Chaminade, T., Zecca, M., Blakemore, S., Takanishi, A.,
Frith, C. D., Micera, S., Dario, P., Rizzolatti, G.,
Gallese, V., and Umilta, M. A. (2010). Brain response
to a humanoid robot in areas implicated in the percep-
tion of human emotional gestures. PLoS one, 5(7).
Cui, X., Bray, S., Bryant, D. M., Glover, G. H., and Reiss,
A. L. (2011). A quantitative comparison of NIRS and
fMRI across multiple cognitive tasks. NeuroImage,
54(4):2808–2821.
Cui, X., Bray, S., and Reiss, A. L. (2010a). Func-
UsingNearInfraredSpectroscopytoIndexTemporalChangesinAffectinRealisticHuman-robotInteractions
391

tional near infrared spectroscopy (NIRS) signal im-
provement based on negative correlation between
oxygenated and deoxygenated hemoglobin dynamics.
NeuroImage, 49(4):3039–3046.
Cui, X., Bray, S., and Reiss, A. L. (2010b). Speeded near in-
frared spectroscopy (NIRS) response detection. PLoS
ONE, 5(11).
Heger, D., Mutter, R., Herff, C., Putze, F., and Schultz, T.
(2013). Continuous recognition of affective states by
functional near infrared spectroscopy signals. In Proc.
ACII.
Heger, D., Putze, F., and Schultz, T. (2011). Online recog-
nition of facial actions for natural EEG-based BCI ap-
plications. In Proc. ACII, pages 436–446.
Hoshi, Y. (2011). Functional near-infrared spectroscopy:
current status and future prospects. J. of Biomed. Op.,
12(6).
Hoshi, Y. and Tamura, M. (1997). Near-infrared optical de-
tection of sequential brain activation in the prefrontal
cortex during mental tasks. NeuroImage, 5(4):292–7.
Kawaguchi, Y., Wada, K., Okamoto, M., Tsujii, T., Shibata,
T., and Sakatani, K. (2012). Investigation of brain
activity after interaction with seal robot measured by
fNIRS. In IEEE International Symposium on Robot
and Human Interactive Communication.
Lisetti, C. L. (2011). Believable agents, engagement, and
health interventions. In Proceedings of the Human
Computer Interaction International Conference.
Ochsner, K. N., Silvers, J. A., and Buhle, J. T. (2012). Func-
tional imaging studies of emotion regulation: a syn-
thetic review and evolving model of cognitive control
of emotion. An. of NY Acad. Sci., 1251:1–24.
Rosenthal von der Putten, A. M., Kramer, N. C., Hoffmann,
L., Sobieraj, S., and Eimler, S. C. (2013a). An exper-
imental study on emotional reactions towards a robot.
International Journal of Social Robotics, 5:17–34.
Rosenthal von der Putten, A. M., Schulte, F. P., Eimler,
S. C., Hoffmann, L., Sobieraj, S., Maderwald, S.,
Kramer, N. C., and Brand, M. (2013b). Neural corre-
lates of empathy towards robots. In Proc. HRI, pages
215–216.
Shibata, T. (2012). Therapeutic seal robot as biofeedback
medical device: Qualitative and quantitative evalua-
tions of robot therapy in dementia care. Proceedings
of the IEEE, 100(8):2527 –2538.
Solovey, E. T., Girouard, A., Chauncey, K., Hirshfield,
L. M., Sassaroli, A., Zheng, F., Fantini, S., and Ja-
cob, R. J. K. (2009). Using fNIRS brain sensing in
realistic HCI settings: experiments and guidelines. In
Proc. UIST.
Solovey, E. T., Lalooses, F., Chauncey, K., Weaver, D.,
Parasi, M., Scheutz, M., Sassaroli, A., Fantini, S.,
Schermerhorn, P., Girouard, A., and Jacob, R. J. K.
(2011). Sensing cognitive multitasking for a brain-
based adaptive user interface. In Proc. CHI.
Solovey, E. T., Schermerhorn, P., Scheutz, M., Sassaroli, A.,
Fantini, S., , and Jacob, R. J. K. (2012). Brainput: en-
hancing interactive systems with streaming fnirs brain
input. In Proc. CHI.
Strait, M., Briggs, G., and Scheutz, M. (2013a). Neural
correlates of agency ascription and its role in emo-
tional and non- emotional decision-making. In Affec-
tive Computing and Intelligent Interaction.
Strait, M., Canning, C., and Scheutz, M. (2013b). Limita-
tions of NIRS-based BCI for realistic applications in
human-computer interaction. In 5th International BCI
Meeting, pages 6–7.
Strait, M., Canning, C., and Scheutz, M. (2014). Inves-
tigating the effects of robot communication strategies
in advice-giving situations based on robot appearance,
interaction modality, and distance. In Conference on
Human-Robot Interaction (under review).
Strait, M. and Scheutz, M. (2014). Measuring users‘ re-
sponses to humans, robots, and human-like robots
with functional near infrared spectroscopy. In Confer-
ence on Human Factors in Computing (under review).
Szafir, D. and Mutlu, B. (2012). Pay attention!: designing
adaptive agents that monitor and improve user engage-
ment. In Proceedings of CHI, pages 11–20.
Szafir, D. and Mutlu, B. (2013). Artful: Adaptive review
technology for flipped learning. In Proc. of CHI.
Urry, H. L., van Reekum, C. M., Johnstone, T., Kalin, N. H.,
Thurow, M. E., Schaefer, H. S., Jackson, C. A., Frye,
C. J., Geischar, L. L., Alexander, A. L., and David-
son, R. J. (2006). Amygdala and ventromedial pre-
frontal cortex are inversely coupled during regulation
of negative affect and predict the diurnal pattern of
cortisol secretion among older adults. J. Neurosci.,
26(16):4415–4425.
Zander, T. O. (2009). Detecting affective covert user states
with passive brain-computer interfaces. In Proceeding
of Affective Computing and Intelligent Interaction.
Zander, T. O. and Kothe, C. (2011). Towards passive brain-
computer interfaces: applying brain-computer inter-
face technology to human-machine systems in gen-
eral. Journal of Neural Engineering, 8(2).
PhyCS2014-InternationalConferenceonPhysiologicalComputingSystems
392
