
Versatile Low-cost Modular Microfluidic Arrays for Cancer
Diagnostics
James F. Rusling
1-4
, Colleen Krause
1
,Brunah Otieno
1
, Karteek Kadimisetty
1
,Chi Tang
1
,
Abhay Vaze
1
and Gregory Bishop
1
1
Department of Chemistry (U-3060), University of Connecticut, 55 N. Eagleville Rd., Storrs, Connecticut 06269, U.S.A.
2
Department of Cell Biology, University of Connecticut Health Center, Farmington, Connecticut 06032, U.S.A.
3
Institute of Materials Science, University of Connecticut, 97 N. Eagleville Road, Storrs, Connecticut 06269, U.S.A.
4
School of Chemistry, National University of Ireland, Galway, Ireland
Keywords: Cancer, Biomarkers, Multiplex, Protein Detection, Microfluidics, Nanoparticles.
Abstract: Accurate, sensitive, multiplexed detection of biomarker proteins in serum and tissue holds significant
promise for personalized cancer diagnostics and therapy monitoring. Here we describe fabrication details of
a modular microfluidic system featuring a small chamber for on-line protein capture from serum by
magnetic beads, positioned upstream of a nanostructured multi-sensor array chamber to achieve high
sensitivity for up to eight proteins, with the ability to expand to many more proteins. Microfluidic chambers
are made by templating PDMS channels on machined aluminum molds to avoid lithography, and mounted
in hard plastic housings equipped with inlet and outlet lines and interfaced with valves. Gold immunoarrays
fabricated by screen or ink-jet printing, or wet chemical etching of gold films utilize amperometry or
electrochemiluminescence (ECL) detection. These arrays are interfaced with microfluidics to achieve well-
controlled mass transport leading to excellent signal/noise and unprecedented sensitivities. With interest in
low cost point of care (POC) systems, we developed a module to also facilitate automated microfluidic
reagent and sample delivery utilizing an open source microcontroller and micropumps, with ECL detection
by camera.
1 INTRODUCTION
Measuring diagnostic panels of multiple proteins in
serum holds great promise for future personalized
cancer screening and therapy monitoring (Manne,
Srivastava and Srivastava, 2005) (Rusling, Kumar
and Gutkind, et al. 2010)
(Ludwig and Weinstein,
2005) (Rusling, 2012) (Rusling, Munge, et al. 2013).
It is necessary to measure multiple proteins in
samples from each patient because a single protein
biomarker is subject to too much individual
variability to give highly accurate predictions. Thus,
the potential to measure concentrations of panels of
biomarker proteins for cancer diagnostics has
created great interest in the biomedical community
for some time (Kulasingam and Diamandis, 2008).
(Hanash, Pitteri, and Faca 2008) (Giljohan and
Mirkin, 2009) (Hanash, Baik and Kallioniemi,
2011). Unfortunately, broad realization of such
diagnostic strategies has yet to be achieved. This is
due in a large part to the lack of suitable
inexpensive, sensitive devices to measure multiple
biomarker proteins in patient samples, as well as the
lack of fully validated panels for cancer diagnosis.
For clinical or point-of-care (POC) use, technical
simplicity of protocols and low cost are essential.
Emerging aspects of nanotechnology and materials
science combined with microfluidics provide
exciting new opportunities to design and fabricate
such devices (Rusling, Munge, et al. 2013).
This
paper will describe development of prototype
modular microfluidic systems capable of
ultrasensitive detection of multiple serum proteins
(Figure 1). Unlike our previous publications
describing multiplexed protein detection with some
of these systems, the present paper will focus on
fabrication, system optimization, and new
automation aspects of the devices.
72
F. Rusling J., Krause C., Otieno B., Kadimisetty K., Tang C., Vaze A. and Bishop G..
Versatile Low-cost Modular Microfluidic Arrays for Cancer Diagnostics.
DOI: 10.5220/0004707400720076
In Proceedings of the International Conference on Biomedical Electronics and Devices (BIODEVICES-2014), pages 72-76
ISBN: 978-989-758-013-0
Copyright
c
2014 SCITEPRESS (Science and Technology Publications, Lda.)
2 SYSTEMS FOR PROTEIN
DETECTION
For protein detection, a 100 L modular cylindrical
chamber for on-line protein capture from
serum on magnetic beads is positioned upstream
of a nanostructured multi-sensor array in a 60 L
chamber to achieve high sensitivity for up to eight
proteins (Figures 1 and 2) (Otieno et al., 2014) with
the ability to expand to many more proteins. The
microfluidic chambers are made by templating
PDMS channels on machined aluminum molds to
avoid lithography. The PDMS slabs are mounted in
hard plastic housings equipped with inlet and outlet
lines to construct the desired chambers, and
interfaced with valves. These modular microfluidic
immunoarrays utilizing amperometry or
electrochemiluminescence (ECL) detection
chambers interfaced with microfluidics achieved
well-controlled mass transport leading to excellent
signal/noise and unprecedented sensitivity.
Amperometric multi-sensor array chips were
fabricated by ink-jet printing of 4 nm alkylthiol gold
nanoparticles (€0.20/chip) (Krause, et al., 2013)
commercial screen printing of carbon and coating
with 5 nm glutathione-gold nanoparticles (€7/chip)
(Chikkaveeraiah, et al., 2011), wet-etching to
fabricate gold CD arrays (Tang, et al., 2012), or, for
ECL, microwell-patterning of pyrolytic graphite
chips (€0.20/chip) (Sardesai, et al., 2013). A simple
print/heat/peel method was developed to transfer
computer printed patterns onto the arrays to create
hydrophobic wells as small as 10 nL around each
sensor. Briefly, a toner pattern is printed by a laser
jet onto high gloss paper, and transferred onto the
array substrate in a heat press. These toner
nanowells can hold 1 L solutions to facilitate
building nanostructures and attaching antibodies on
sensor elements while avoiding cross-contamination.
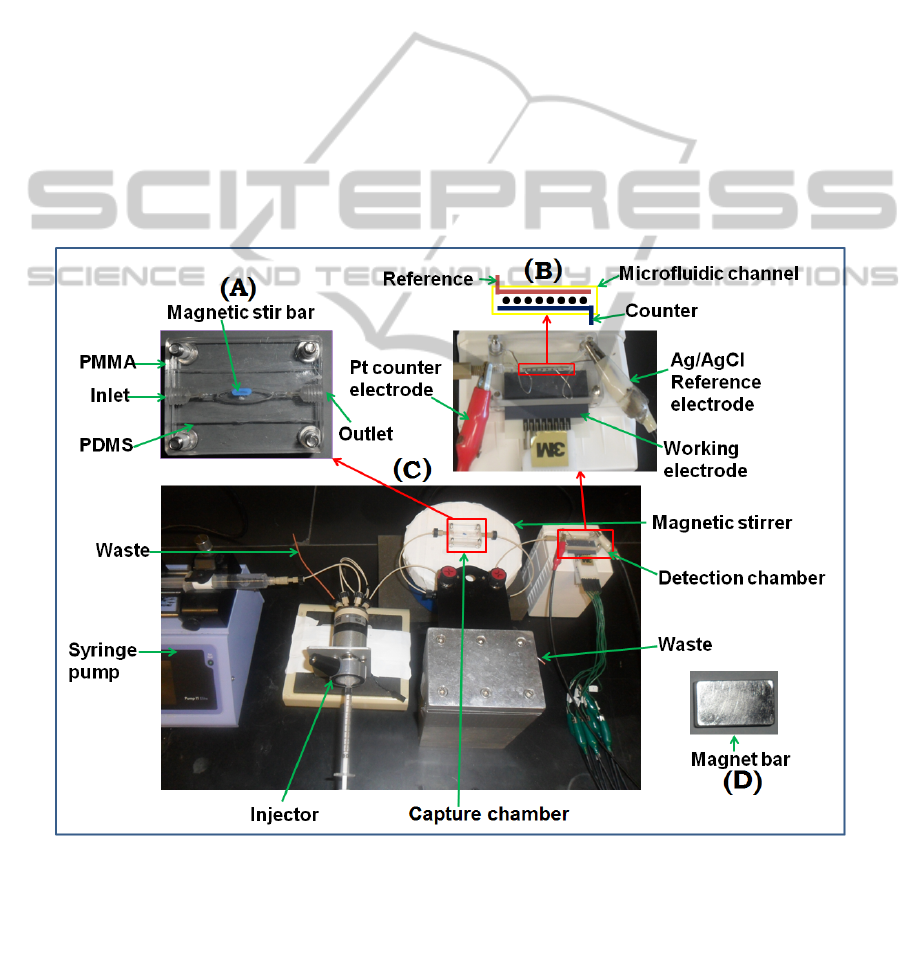
Figure 1: Prototype modular microfluidic system for on-line protein capture and amperometric detection using magnetic
beads. (A) Capture chamber in which target proteins are captured on-line from the sample by heavily labeled enzyme-
antibody-magnetic beads to form protein-bead bioconjugates. These are washed in the chamber while held magnetically,
then transported into the detection chamber (B) in the modular microfluidic system (C). The magnet (D) traps bioconjugate
beads in the channel during injection of sample and washing, and is removed for transfer of beads to the detection chamber.
Changes in the system for ECL detection include the replacement of multi-electrode array with a solid 2.5x2.5 cm pyrolytic
graphite chip with an array of microwells featuring carbon nanotube forests and replacement of the detection chamber with
a similar chamber that features a optical window on the top to facilitate detecting ECL light with a CCD camera.
VersatileLow-costModularMicrofluidicArraysforCancerDiagnostics
73
These arrays are fitted into the microfluidic PDMS
detection channel, which is attached to a syringe
pump or micropumps, with associated valves to
deliver reagents and samples to the correct
chambers. Magnetic control is used to hold magnetic
beads bioconjugated with antibodies and enzyme
labels in the capture chamber.
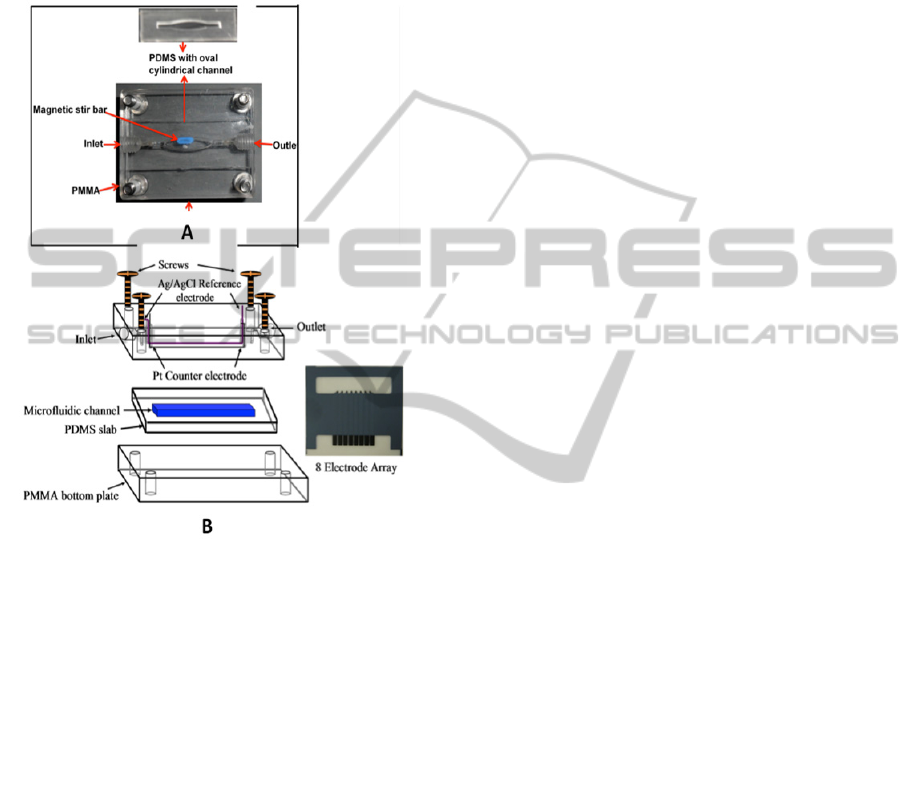
Figure 2: Modules of amperometric microfluidic arrays
with on-line protein capture on magnetic beads. (A)
Capture chamber featuring a PDMS-defined 100 L oval
cylinder sandwiched between two hard PMMA plates. A
tiny magnetic stir bar is included for mixing and
redispersing beads. The target proteins are captured on-
line from the sample in this chamber by heavily labeled
enzyme-antibody-magnetic beads to form protein-bead
bioconjugates. These are washed, and then transported
into (B) detection chamber housing an 8 AuNP-coated
electrode sensor array (on right, sensor elements on top,
contacts on bottom) coated with capture antibodies.
Subsequently, samples are injected and flow times
adjusted so as to fill the sample chamber. Washes
are done to minimize non-specific binding, and then
sent to waste. Then, magnetic control is released,
and the beads are transported to the detection
chamber, where a stop-flow incubation period
allows capture by antibodies on the sensors. After
washing, an enzyme activator/mediator solution is
injected to provide low noise, peak-shaped
responses, as shown for a mixture of interleukin (IL)
proteins IL-6 and IL-8 in serum (Figure 3).
Our multilabel approaches feature massively
enzyme- or ECL-dye-labeled particles that greatly
amplify signals for analyte proteins. Detection limits
as low as 5 fg mL
-1
(~200 aM) have been achieved
for simultaneous measurement of four oral cancer
biomarker proteins in a few L of serum in about 1
hr (Malhotra, et al., 2012). Applications to
biomarker proteins for oral cancer, metastasis, and
inflammation have been pursued. For detection of
metastasized cancer during surgery, high sensitivity
can be traded for speed to achieve immunoassays in
8 min (Krause, et al., 2013). The modular system
can be adapted to other measurements such as
oxidized DNA and metabolic toxicity screening by
changing the active films on the detectors.
3 AUTOMATED REAGENT
AND SAMPLE
INTRODUCTION
POC protein detection will require adding further
automation to the above prototypes. We have thus
most recently designed an automated reagent and
sample delivery module for ECL detection of
proteins. The system features six microfluidic
channels that lead to a detection chamber featuring a
patterned pyrolytic graphite-SWCNT chip (Figure
4). Detection is facilitated by an ECL dye embedded
into 100 nm silica nanoparticles and coated with
antibodies to provide amplification and low fg/mL
detection levels using a CCD camera. The entire
device costs ~€500, excluding the CCD camera.
Microwells were fabricated on the PG chip using the
print/heat/peel technology described above.
Integrated micropumps, one per channel, were
connected to a portable sample/reagent loading
cassette with preloaded, serum samples, wash
buffers and dye-silica nanoparticles equipped with
detection antibodies. These air-separated solutions
are pumped into the six-channel measurement
chamber chip with SWCNT wells containing capture
antibody). A microcontroller open-source electronics
prototyping platform (Arduino) interface provides
fully automated control of flow and incubation
times. A panel of 4 cancer biomarkers can be
measured at clinical levels using this approach.
4 CONCLUSIONS
We described inexpensive, versatile microfluidic
devices for multiple protein detection designed in a
BIODEVICES2014-InternationalConferenceonBiomedicalElectronicsandDevices
74
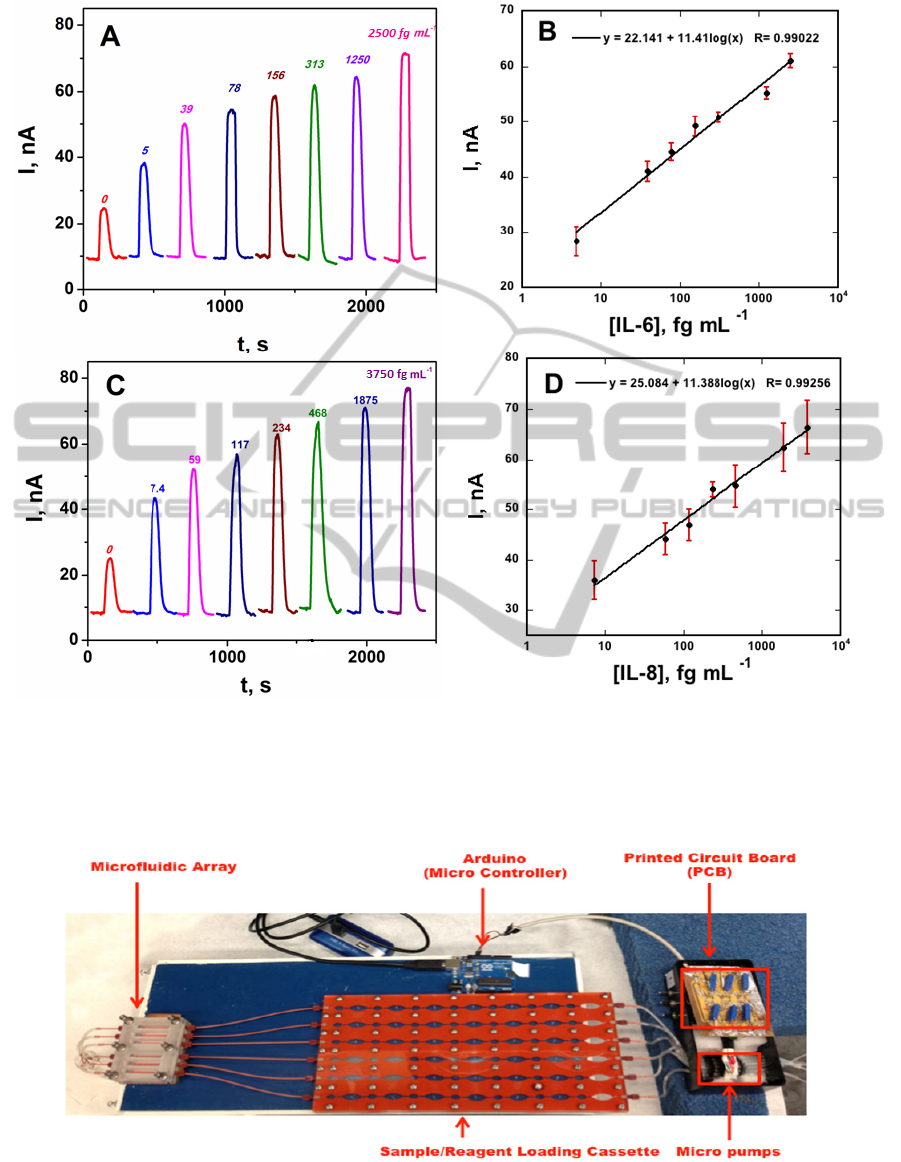
Figure 3: Amperometric immunoarray results for protein mixtures in undiluted calf serum using an 8-electrode screen-
printed carbon array with AuNP-coated sensors in the device in Figure 1 at -0.2 V vs. Ag/AgCl. Measurements were done
with an 8-electrode CH Instruments potentiostat. Peaks are developed by injecting a mixture of 1 mM HQ and 0.1 mM
H
2
O
2
to mediate and activate the horseradish peroxidase (HRP) labels on the magnetic beads for (A) IL-6 and (C) IL-8, and
calibration plots for (B) IL-6 and (D) IL-8. Error bars represent standard deviations (n=4) (Adapted from Otieno et al.,
2014).
Figure 4: Modular microfluidic array for ECL detection of proteins showing hardware including automated sample and
reagent loading cassette. The microfluidic array is placed under a CCD camera in a dark box and connected to a power
supply for ECL measurements.
VersatileLow-costModularMicrofluidicArraysforCancerDiagnostics
75

modular fashion with no lithography required, using
commercial valves and pumps when possible.
Printing, wet etching and print patterning have been
used to fabricate very inexpensive nanostructured
array chips. These devices can be set up in almost
any laboratory at a relatively low cost. However,
additional simplicity of operation is a goal for full
POC implementation. Our specific application is
detection of multiple proteins from serum and cell
lysates for cancer diagnostics, and this can be
achieved at detection limits down to 5 fg/mL
(attomolar levels), up to 200 times lower than
existing commercial multiplexed protein detection
systems (Rusling J. F., Kumar C. V., Gutkind J. S.,
et al. 2010).
ACKNOWLEDGEMENTS
This work is financially supported by grant
EB014586 from the National Institute of
Biomedical Imaging and Bioengineering (NIBIB),
NIH, USA.
REFERENCES
Chikkaveeraiah, B. V., Mani, V., Patel, V., Gutkind, J. S.,
Rusling. J. F. 2011. Microfluidic electrochemical
immunoarray for ultrasensitive detection of two cancer
biomarker proteins in serum, Biosensors &
Bioelectron. 26, 4477– 4483.
Giljohan D. A., Mirkin C. A. 2009. Drivers of
biodiagnostic development. Nature 462, 461-464.
Hanash S. M., Pitteri S. J., Faca V. M. 2008. Mining the
plasma proteome for cancer biomarkers. Nature 452,
571-579.
Hanash, S. M., Baik C. S., Kallioniemi, O. 2011.
Emerging molecular biomarkers—blood-based
strategies to detect and monitor cancer, Nature Rev.
Clin. Oncol. 8, 142–150.
Krause, C. E., Otieno, B. A., Latus, A., Faria R. C., Patel,
V., Gutkind, J. S., Rusling. J. F. 2013. Rapid
Microfluidic Immunoassays of Cancer Biomarker
Proteins using Disposable Inkjet-printed Gold
Nanoparticle Arrays, ChemistryOpen, 2, 141 – 145.
Kulasingam V, Diamandis E. P. 2008. Strategies for
discovering novel cancer biomarkers through
utilization of emerging technologies. Nature Clin.
Pract. Oncol. 5, 588-599.
Ludwig J. A., Weinstein J. N. 2005. Biomarkers in cancer
staging, prognosis and treatment selection. Nature
Rev. Cancer 5, 845-856.
Malhotra, R., Chikkaveeraiah, B. V., Patel, V. et al, 2012.
Oral Cancer Detection in the Clinic Using an
Ultrasensitive Microfluidic Array for a Panel of
Biomarker Proteins, Anal. Chem., 84, 6249–6255.
Manne U., Srivastava R. G., Srivastava S. 2005. Recent
advances in biomarkers for cancer diagnosis and
treatment. Drug Discov Today 10, 965-976.
Otieno, B. A., Krause, C. E., Latus, A., Chikkaveeraiah, B.
V., Faria R. C., Rusling. J. F. 2014. On-line Protein
Capture on Magnetic Beads for Ultrasensitive
Microfluidic Immunoassays of Cancer Biomarkers,
Biosens. & Bioelectron., 53, 268–274.
Rusling J. F., Kumar C. V., Gutkind J. S., et al. 2010.
Measurement of biomarker proteins for point-of-care
early detection and monitoring of cancer. Analyst 135,
2496–2511.
Rusling, J. F. 2012. Nanomaterials-based Electrochemical
Immunosensors for Proteins, Chem. Record, 12, 164–
176.
Rusling, J. F., Munge, B., Sardesai, N. P., Malhotra, R.,
Chikkaveeraiah, B. V. 2013. Nanoscience-based
electrochemical sensors and arrays for detection of
cancer biomarker proteins. Chapter 1, in Frank
Crespilho (Ed.), Nanobioelectrochemistry, Springer,
Berlin- Heidelberg, pp. 1-26.
Sardesai, N. P., Kadimisetty, K., Faria,
R., Rusling. J. F.,
2013. A microfluidic electrochemiluminescent device
for detecting cancer biomarker proteins, Anal.
Bioanal. Chem. 405, 3831-3838.
Tang, C. K., Vaze, A., Rusling. J. F. 2012. Fabrication of
immunosensor microwell arrays from gold compact
discs for ultrasensitive detection of cancer biomarker
proteins. Lab on a Chip, 12, 281-286.
BIODEVICES2014-InternationalConferenceonBiomedicalElectronicsandDevices
76
