
Expansive Growth of Atherosclerotic Plaques Assessed by VH-IVUS
Association with TNF-α and OX-LDL Levels in Circulation
C. Ramos
1
, P. Napoleão
2
, C. Fondinho
3
, M. Selas
3
, M. Mota Carmo
3,4
, R. Cruz Ferreira
3,4
and T. Pinheiro
1
1
IST/ITN, Instituto Superior Técnico, Universidade Técnica de Lisboa, E.N. 10, Sacavém, Portugal
2
Instituto de Medicina Molecular, Av. Prof. Gama Pinto, Lisboa, Portugal
3
Serviço de Cardiologia, Hospital de Santa Marta CHLC, Lisboa, Portugal
4
CEDOC, Faculdade de Ciências Médicas, Universidade Nova de Lisboa, Campo Santana, Lisboa, Portugal
Keywords: VH-IVUS, Biomarkers, Expansive Plaque, Plaque Vulnerability.
Abstract: The identification of a vulnerable plaque through the quantification of soluble biomarkers would improve
the diagnosis and treatment of coronary artery disease. Inflammation and LDL oxidative modification have
been implicated in CAD. Disease severity and plaque vulnerability have recently been associated to
expansive plaque growth, rather than constrictive growth which results in vessel stenosis.
Forty CAD patients were admitted prospectively. VH-IVUS was performed and TNF-α and ox-LDL were
quantified in the serum and plasma, respectively. Expansive plaques characterized by large EEL diameters
and preserved luminal measures were associated to STEMI patients. Larger EEL diameter (≥4.6 mm
2
) was
significantly associated to increases of TNF-α concentrations whereas larger plaque areas (≥13.0 mm
2
)
associated with ox-LDL increases in the circulation. Hence, TNF-α and ox-LDL may be indicators of
plaque vulnerability.
1 INTRODUCTION
Coronary artery disease (CAD) has been studied for
decades but many of the mechanisms underlying
both the establishment and the development of this
disease are yet to be understood. (Fayard and Fuster,
2001); (Choi et al., 2008); (Greco et al., 2010) It is
generally accepted that oxidized low density
lipoproteins (ox-LDL) and tumour necrosis factor -
α (TNF-α) are involved in CAD. Oxidative
modification of LDL plays a part in the formation of
the fatty streak which constitutes the first step in
atheroma formation. During the progress of
atherosclerosis the inflammatory process is highly
activated, involving many types of cells and
cytokines, namely TNF-α. (Goldstein and Ross,
1987); (Hansson, 2005)
The identification of vulnerable coronary
atherosclerotic plaque is one of the ultimate goals of
coronary imaging. There is increasing evidence
suggesting the most vulnerable plaques are not
associated with constrictive growth which causes
vessel stenosis, but with outwards, expansive growth
– positive remodelling. (Hong et al., 2012) IVUS is
a widespread modality used for the direct
visualization of coronary lumen, vessel wall, and
atherosclerotic plaque. It allows the measurement of
plaque area and any thickening of arterial walls
(Amato et al., 2007); (Böse et al., 2007), which is an
important advantage over coronary angiography, the
golden standard method of coronary disease
assessment. In addition to lumen diameter, IVUS
provides plaque measures and histological structure
by analysing the radiofrequency spectra.
Noninvasive identification of rupture-prone
plaques would dramatically improve risk
stratification of both symptomatic and asymptomatic
patients. Therefore, associations between the
expansive growth of the atherosclerotic plaque and
soluble bioindicators may provide important
information that enhances the precision of clinical
and laboratory variables used to assess patients at
risk of CAD or of plaque rupture (Ramos et al.,
2013); (Hong et al., 2012).
90
Ramos C., Napoleão P., Fondinho C., Selas M., Mota Carmo M., Cruz Ferreira R. and Pinheiro T..
Expansive Growth of Atherosclerotic Plaques Assessed by VH-IVUS - Association with TNF-α and OX-LDL Levels in Circulation.
DOI: 10.5220/0004664500900094
In Proceedings of the International Congress on Cardiovascular Technologies (VisualCardio-2013), pages 90-94
ISBN: 978-989-8565-78-5
Copyright
c
2013 SCITEPRESS (Science and Technology Publications, Lda.)
2 OBJECTIVES
The aim of this study was to explore the possible
association of the atherosclerotic plaque
characteristics, assessed by VH-IVUS, and both the
inflammatory response and LDL oxidative
modification, evaluated systemically. In particular,
we investigated measurements for the plaque
expansive growth and the soluble markers TNF-α
and ox-LDL.
3 METHODS
Forty patients were enrolled in this prospective study
at the Cardiology Service of Santa Marta Hospital
(CHLC, Lisbon, Portugal). All patients underwent
standard diagnostic procedures and were treated
accordingly.
Peripheral blood was drawn from all patients into
blood collection tubes (Vacuette) with appropriate
anti-coagulant. Biochemical analyses were routinely
performed in the hospital, including the
measurement of troponin T, N-terminal pro-brain
natriuretic peptide (NT-proBNP) and C-reactive
protein (CRP). Serum and plasma were also
collected for ox-LDL and TNF-a determination by
enzyme-linked immunosorbent assays (ELISA).
Samples were stored at -80ºC until analysis, for a
period not exceeding 6 months. The concentration of
ox-LDL was measured in plasma and TNF-α in
serum using ELISA commercial kits (R&D
Systems). Patients characterization is summarized in
Table 1.
VH-IVUS was conducted in all patients and data
was recorded. VH-IVUS acquisition was performed
using an EagleEye catheter (20 MHz) at pullback
speed of 0.5 mm/sec. For each lesion, vessel, lumen
and atheroma measurements were obtained based on
total number of cross-sections analysed throughout
the region of interest. Lesion borders were
established using the leading edges of external
elastic lamina (EEL) and the luminal contour. The
radiofrequency backscatter information was
reconstructed using In-Vision gold commercial
software (Volcano Corporation, USA). The
percentages of fibrotic, fibro-fatty, calcified and
necrotic core were assessed.
The relationship between plaque characteristics
and biomarkers was evaluated by correlation. Plaque
measurements were categorized according to median
value (below or above) to assess biomarker
dependence on plaque morphology and content
changes. Differences between categories were
assessed using a Mann-Whitney test. The results
were considered significant for p<0.05.
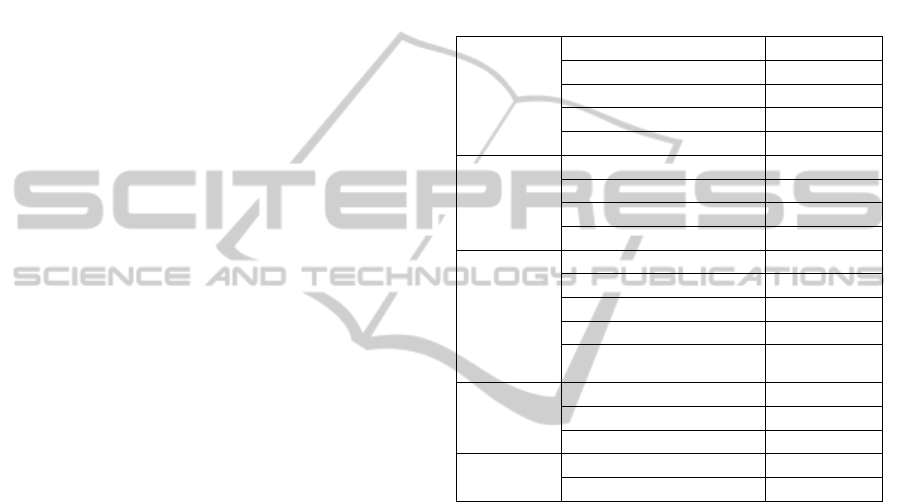
Table 1: Patients clinical, demographic and biochemical
characterization. Results are presented in median (Q25 –
Q75), unless specified otherwise. STEMI – ST-elevation
myocardial infarction; NSTEMI – non-ST-elevation
myocardial infarction; SA – stable angina; UA – unstable
angina; SI – silent ischemia; CRP – C-reactive protein;
NT-proBNP – N-terminal pro-brain natriuretic peptide;
ox-LDL – oxidized low density lipoprotein; TNF-α –
tumour necrosis factor- α.
Clinical
STEMI 5, 13
NSTEMI 7, 18
SA 13, 33
UA 11, 28
SI 3, 8
Demographics
Male sex (n, %) 26, 67
Age (y) 64 (57 – 71)
Weight (kg) 74 (67 – 80)
Height (m) 1.7 (1.6 – 1.7)
Risk factors /
Co-morbidities
Smoking (n, %) 6, 16
Obesity (%) 23, 62
Hypercholesterolemia (n, %) 25, 76
Arterial hypertension (n, %) 28, 72
Diabetes mellitus
(n, %)
17, 44
Biochemical
analysis
CRP (mg/l) 4.3 (1.8 – 16.5)
Troponin (ng/ml) 0.06 (0.01 – 0.15)
Pro-BNP (pg/ml) 203 (64 – 916)
Soluble
parameters
ox-LDL (U/l) 50.8 (38.4 - 66.2)
TNF-α (pg/ml) 2.5 (0.9 - 5.8)
4 RESULTS
The plaques in coronary segments of interest were
evaluated for morphological characteristics. The
minimum, maximum and median values of plaque
and lumen dimensions were determined. Plaque
composition was assessed in sections of the major
stenosis region.
The plaque characteristics measured are
summarized in Table 2.
Plaque morphology measurements were
determined in all patients and results were compared
among the different groups. STEMI patients showed
larger plaques than:
a) NSTEMI patients (100% STEMI patients with
EEL diameter ≥4.6mm against 29% NSTEMI
patients, p=0.028; 100% STEMI patients with EEL
area ≥17.0mm
2
against 29% NTEMI patients,
p=0.028; 100% STEMI patients with plaque area
≥13.0mm
2
against 15% NSTEMI patients, p=0.047);
ExpansiveGrowthofAtheroscleroticPlaquesAssessedbyVH-IVUS-AssociationwithTNF-αandOX-LDLLevelsin
Circulation
91
b) SA patients (100% STEMI patients with EEL
diameter ≥4.6mm against 46% SA patients, p=0.041;
100% STEMI patients with EEL area ≥17.0mm
2
against 46% SA patients, p=0.041; 100% STEMI
patients with plaque area ≥13.0mm
2
against 38%
NSTEMI patients, p=0.022);
c) UA patients (median EEL diameter of 4.9mm for
STEMI patients and of 4.6mm for UA patients,
p=0.005; median EEL area of 19.6mm
2
for STEMI
patients and of 16.6mm
2
for UA patients, p=0.003;
median plaque area of 12.5mm
2
for STEMI patients
and of 11.0mm
2
for UA patients, p=0.027).
Table 2: Atherosclerotic plaque measurements obtained by
VH-IVUS. Results of the plaque morphology and
composition are presented in median (Q25 – Q75).
Plaque characteristics
Stenosis (%) 76.8 (65.6 – 83.7)
Fibrotic tissue (%) 58.4 (48.8 – 70.1)
Fibro-fatty tissue (%) 9.7 (6.7 – 20.7)
Calcified tissue (%) 11 (3.1 – 18.3)
Necrotic core (%) 15.8 (10.3 – 21.7)
Lumen
area (mm
2
) 2.2 (1.9 – 2.7)
diameter (mm) 3.6 (2.9 – 5.0)
External elastic
lamina
diameter (mm) 4.6 (4.2 – 4.9)
area (mm
2
) 17.0 (14.3 – 19.3)
Plaque area (mm
2
) 13.0 (10.0 – 15.0)
Plaque burden (%) 77.0 (66.3 – 83.6)
The atherosclerotic plaque physical
characteristics obtained by VH-IVUS were studied
and related with oxidative and inflammation
bioindicators measured in the blood circulation, e.g.
ox-LDL and TNF-α.
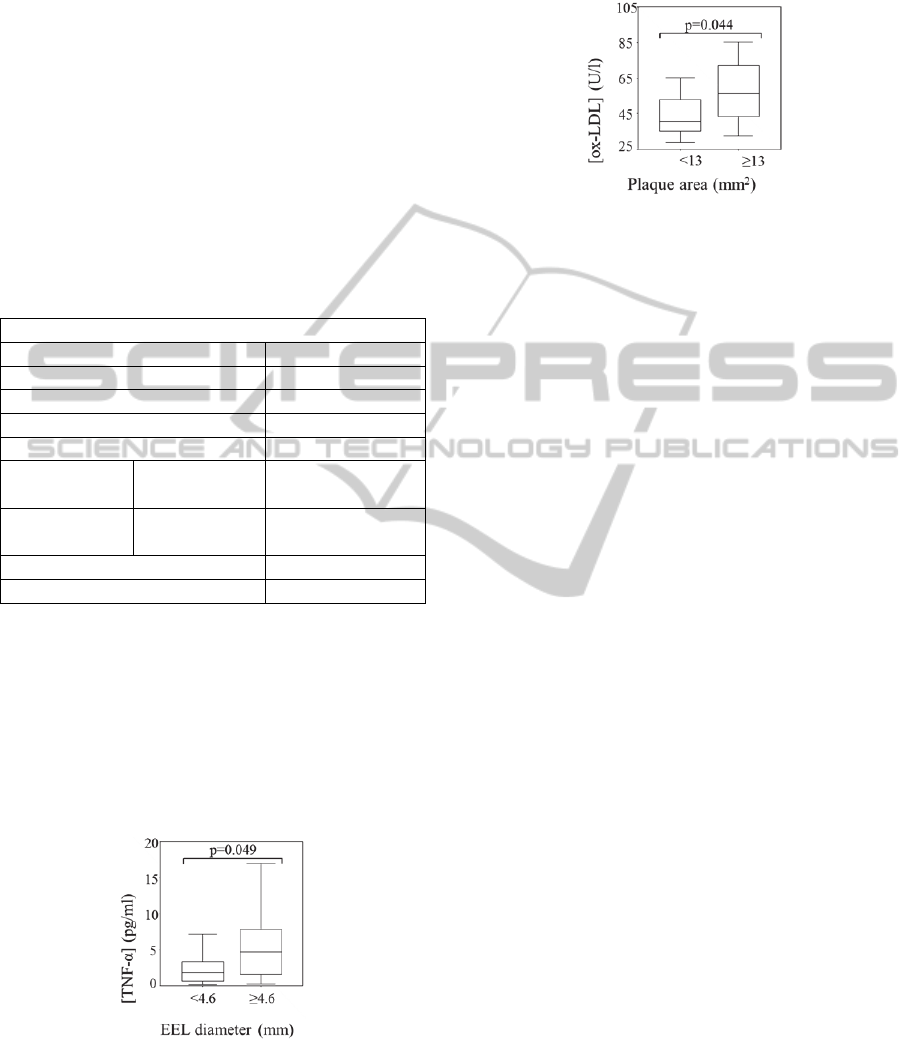
Figure 1 shows that the concentration of TNF-α
significantly increased for large plaques, as
expressed by EEL diameter (Figure 1) (p=0.049).
Figure 1: Box-plot representation of TNF-α concentration
relative to EEL diameter <4.6mm and ≥4.6mm.
Figure 2 shows that the concentrations of ox-
LDL were also significantly associated with the
atheroma dimensions. Higher ox-LDL
concentrations are associated to large plaque areas,
above median value (area ≥13 mm
2
) (p=0.044).
Figure 2: Box-plot representation of ox-LDL
concentration relative to plaque area <13mm
2
and
≥13mm
2
.
5 DISCUSSION
Atherosclerosis primarily affects the arterial wall
and there is increasing evidence that supports the
idea that positive remodelling, more than vessel
stenosis, is associated to plaque vulnerability.
(Hoffman et al., 2006); (Böse et al., 2007);
(Napoleão et al., 2011); (Hong et al., 2012). VH-
IVUS allows the observation of the vessel wall,
accounting for positive remodelling, or plaque
expansive growth.
STEMI patients had significantly larger plaques
with large EEL diameter and plaque area compared
to the other groups of patients. No significant
differences in lumen dimensions or area
measurements were observed, suggesting that
plaques of STEMI patients have an outward growth.
Hence, plaque rupture appears to be related to
plaque expansive growth, rather than to vessel
stenosis. Similar observations were reported by
Hong et al., (2012). Positive plaque remodelling was
associated to thin cap fibroatheroma and plaque
greater percentage of necrotic core, which were
indicated as indexes of plaque vulnerability.
We also intended to evaluate the possible
association between plaque morphological
characteristics and TNF-α and ox-LDL circulation
levels. TNF-α is involved in endothelial cell
activation and in the inflammatory response.
Increasing levels of this pro-inflammatory cytokine
promotes a continuous systemic inflammatory
stimulation that can trigger and amplify local
inflammatory responses, hence expressing the extent
of vascular inflammation. (Sano et al., 2006); (Böse
et al., 2007) The association of TNF-α with EEL
diameter – and not with lumen measurements –
suggests that this cytokine may be a good indicator
of plaque positive remodelling.
CARDIOTECHNIX2013-InternationalCongressonCardiovascularTechnologies
92

Extensive experimental data shows that ox-LDL
is formed in the arterial wall contributing to the
plaque progression. It is accepted that ox-LDL in
circulation is originated in the vessel wall, being its
circulating levels strongly associated to
angiographically documented CAD (Napoleão et al.,
2012). Increases in plaque area may favour plaque
outflow and exposure to shear stress may contribute
to endothelial denuding and plaque cap erosion,
which leads to plaque rupture. (Greco et al., 2009);
(Choi et al., 2010) Hence, the positive association of
plaque area with ox-LDL concentrations in plasma
can also be considered a marker of plaque
instability.
6 CONCLUSIONS
The association of ox-LDL and TNF-α circulating
levels with characteristics of plaque expansive
growth indicate that these biomarkers may have a
role in plaque activity expressing plaque
vulnerability. The results suggest that these
biomarkers have clinical implications for identifying
vulnerable patients. Further studies are needed to
evaluate the impact of ox-LDL, TNF-, and VH-
IVUS derived measures on clinical presentation.
ACKNOWLEDGEMENTS
The study was carried out under Fundação para a
Ciência e Tecnologia PIC/IC/82734/2007 research
contract.
REFERENCES
Amato, M., Montorsi, P., Ravani, A., Oldani, E., Galli, S.,
Ravagnani, P. M., Tremoli, E., Baldassarre, D., 2007.
Carotid intima-media thickness by B-mode ultrasound
as surrogate of coronary atherosclerosis: correlation
with quantitative coronary angiography and coronary
intravascular ultrasound findings, European Heart
Journal 28: 2094–2101.
Böse, D., von Birgelen, C., Erbel, R., 2007. Intravascular
Ultrasound for the Evaluation of Therapies Targeting
Coronary Atherosclerosis, J Am Coll Cardiol 49:925–
32.
Choi, S. H., Chae, A., Miller, E., Messig, M., Ntanios, F.,
DeMaria, A. N., Nissen, S. E., Witztum, J. L.,
Tsimikas, S., 2008. Relationship Between Biomarkers
of Oxidized Low-Density Lipoprotein, Statin Therapy,
Quantitative Coronary Angiography, and Atheroma
Volume, J Am Coll Cardiol 52: 24-32.
Fayad, Z. A., Fuster, V., 2001. Clinical imaging of the
high-risk or vulnerable atherosclerotic plaque, Circ.
Res.; 89: 305-316.
Goldstein, J. L., Ross, M. S., 1987. Regulation of low-
density lipoprotein receptors: implications for
pathogenesis and therapy of hypercholesterolemia and
atherosclerosis, Circulation 76: 504-507.
Greco, T. P., Conti-Kelly, A. M., Anthony, J. R., Greco,
Jr. T., Doyle, R., Boisen, M., Kojima, K., Pharm, B.
A., Matsuura, E., Lopez, L. R., 2010. Oxidized-
LDL/β2-Glycoprotein I Complexes Are Associated
With Disease Severity and Increased Risk for Adverse
Outcomes in Patients With Acute Coronary
Syndromes, Am J Clin Pathol 133:737-743.
Greco, T. P., Conti-Kelly, A. M., Greco, Jr. T., Doyle, R.,
Matsuura, E., Anthony, J. R., Lopez, L. R., 2009.
Newer Antiphospholipid Antibodies Predict Adverse
Outcomes in Patients With Acute Coronary Syndrome,
Am J Clin Pathol 132: 613-620.
Hansson, G. K., 2005, Inflammation, Atherosclerosis, and
Coronary Artery Disease, N Engl J Med 352:1685-95.
Hoffmann, U., Moselewski, F., Nieman, K., Jang, I. K.,
Ferencik, M., Rahman, A. M., Cury, R. C., Abbara, S.,
Joneidi-Jafari, H., Achenbach, S., Brady, T.J., 2006.
Noninvasive Assessment of Plaque Morphology and
Composition in Culprit and Stable Lesions in Acute
Coronary Syndrome and Stable Lesions in Stable
Angina by Multidetector Computed Tomography, Am
Coll Cardiol. 47: 1655– 62.
Hong, Y. J., Jeong, M. H., Choi, Y. H., Song, J. A.,
Ahmed, K., Lee, K. H., Kim, D. H., Lee, M. G., Park,
K. H., Sim, D. S., Yoon, N. S., Yoon, H. J., Kim, K.
H., Park, H. W., Kim, J. H., Ahn, Y., Cho, J. G., Park,
J. C., Kang J. C., 2012. Positive remodeling is
associated with vulnerable coronary plaque
components regardless of clinical presentation: Virtual
histology-intravascular ultrasound analysis. Int J
Cardiol (published online).
Napoleão, P., Selas, M., Ramos, C., Turkman, A.,
Andreozzi, V., Mota Carmo, M., Viegas-Crespo, A.
M., Cruz Ferreira, R., Pinheiro, T., 2011. The Role of
Inflammatory Biomarkers in the Assessment of
Coronary Artery Disease. In: Coronary Angiography -
Advances in Noninvasive Imaging Approach for
Evaluation of Coronary Artery Disease: Ed: Baskot
B.G., InTech. p281-314.
Napoleão, P., Selas, M., Freixo, C., Mota Carmo, M.,
Viegas-Crespo, A. M., Cruz Ferreira, R., Pinheiro, T.,
2012. T lymphocytes alterations are associated with
oxidized LDL, troponin T, white blood cells and C-
reactive protein during acute myocardial infarction.
Clinical Hemorheology and Microcirculation
(published online).
Ramos, C., Napoleão, P., Cruz Ferreira, R., Fondinho, C.,
Selas, M., Mota Carmo, M., Crespo, A. M. Pinheiro,
T., 2013. Relationship Between Ox–LDL, Immune
Cells, Atheroma Dimensions and Angiographic
Measurements Assessed by Coronary Angiography
and Intravascular Ultrasound. In What Should We
Know About Prevented, Diagnostic, and
ExpansiveGrowthofAtheroscleroticPlaquesAssessedbyVH-IVUS-AssociationwithTNF-αandOX-LDLLevelsin
Circulation
93

Interventional Therapy in Coronary Artery Disease,
Ed: Baskot B.G., InTech.
Sano, K., Kawasaki, M., Ishihara, Y., Okubo, M.,
Tsuchiya, K., Nishigaki, K., Zhou, X., Minatoguchi,
S., Fujita, H., Fujiwara, H., 2006. Assessment of
Vulnerable Plaques Causing Acute Coronary
Syndrome Using Integrated Backscatter Intravascular
Ultrasound, J Am Coll Cardiol 47: 734–41.
CARDIOTECHNIX2013-InternationalCongressonCardiovascularTechnologies
94
