
WEB TOOLS FOR CHEMICAL ENGINEERING EDUCATION
COUPLING FUNDAMENTALS WITH PROCESS DESIGN
The Distillation Case Study
M. G. Rasteiro, A. Ferreira and J. Granjo
Department of Chemical Engineering, University of Coimbra, Rua Sílvio Lima, Coimbra, Portugal
Keywords: On-line Tools, Simulators, Virtual Laboratories, Chemical Engineering, Distillation.
Abstract: In this paper the “Unit Operations and Separation Processes” area of a virtual platform called
LABVIRTUAL (http://labvirtual.eq.uc.pt) is presented. The objective is to support the study of students
engaged in a Chemical Engineering degree, especially in Portuguese-speaking countries. It is argued that
these web tools support the autonomous study of the students, contributing to develop their critical and
creative thinking, and enabling more practical approaches to the different chemical processes. Moreover, it
is shown how coupling, in the same platform, sections directed to basic concepts/mechanisms, with sections
directed to process design, contributes to knowledge integration and to a better understanding of the design
methodologies for each process.
1 INTRODUCTION
In recent years, there have been many changes in
teaching methodologies, namely in Engineering
Education. Throughout Europe, the Bologna process
required deep transformations in higher education
curricula and courses organization. One of the
consequences of the Bologna process is the
reduction of lecturing hours to allow more time for
the students to develop important skills such as: self
learning, critical thinking, teamwork and knowledge
integration between the different subjects.
Furthermore, in parallel to the scientific and
theoretical background, engineering students need to
develop a more practical training. These fast
changes and the rise of new technologies at the
world scale have been inducing changes in teaching
methodologies, having in mind the need to avoid
dependent learners in the classroom (Bell and Fogler
1998, Shacham et al. 2009 and Hasna 2010).
Additionally, it is also important to take advantage
of the new skills, namely competences on
informatics, the students possess nowadays. To
achieve these objectives a demand for web and
computational tools to support learning and teaching
activities in Engineering education is increasing.
Some authors (Bell and Fogler 1998, Henry
2005, Streicher et al. 2005, Felder 2006, Edgar 2006,
Henry et al. 2008, Shacham et al. 2009 and Garcia-
Herreros and Gomez 2010) support that these tools
contribute to engaging students and to the
development of active learning attitudes. Web tools
can facilitate the development of additional teaching
strategies for simulation, demonstration,
experimentation, operation, etc. Therefore, using
different and complementary resources, the
dynamics in the classes can increase (Bell and
Fogler 1998, Felder 2006, Shacham et al. 2009 and
Hasna 2010). The Chemical Engineering
Departments of both the Universities of Coimbra
and Porto have developed a virtual platform called
LABVIRTUAL (http://labvirtual.eq.uc.pt, Rasteiro
et al. 2009), with a wide scope, directed to the
teaching of Chemical Processes, aiming at
complementing and supporting the student’s study
and help in developing his autonomy. The
integration of the different Chemical Engineering
subjects was the main goal. LABVIRTUAL brings
together contents (multimedia libraries), simulators,
virtual experiments and remotely controlled
experiments, as well as links to other sources,
aiming at getting the student acquainted with the
subject under study.
The Chemical Processes area comprises four
sections: Unit Operations and Separations, Chemical
Reaction, Process Systems Engineering (PSE), and
Biological Processes. These sections present funda-
235
G. Rasteiro M., Ferreira A. and Granjo J..
WEB TOOLS FOR CHEMICAL ENGINEERING EDUCATION COUPLING FUNDAMENTALS WITH PROCESS DESIGN - The Distillation Case Study.
DOI: 10.5220/0003937102350240
In Proceedings of the 4th International Conference on Computer Supported Education (CSEDU-2012), pages 235-240
ISBN: 978-989-8565-06-8
Copyright
c
2012 SCITEPRESS (Science and Technology Publications, Lda.)

mental concepts and applications aimed at leading
the students to understand, for instance, how
different operating conditions result in different
process designs, or which alternatives are available
for a certain process. Whenever possible, process
integration is also addressed. Moreover, each section
includes case studies illustrating some of the features
of the applications developed. These tools can be
used in a wide range of disciplines in a Chemical
Engineering degree curriculum, and be accessed
either in classroom or at home.
This article focuses in more detail on the features
of the Unit Operations and Separation Processes area
of LABVIRTUAL. A special attention will be given
to the tools developed to study the Distillation
process, including the simulation and web
methodologies adopted. Moreover, we will stress
how fundamental insight of the underlying
mechanisms is addressed in this platform, and
integrated later to a better understanding of the
design methodologies of each chemical process.
Leading the students to establish this connection is
most important for the effective understanding of the
different chemical processes, and has been
accomplished with success in this platform.
2 WEB METHODOLOGIES
The Web infrastructure of the Virtual Laboratories
of Chemical Processes is based on standard open-
source software. This approach allows similar
functionalities to existing commercial software, with
good flexibility for the specific needs of the portal.
A Virtual Server is used that can integrate contents
from various sources and present these contents in a
consistent way to the user. Most of the materials
available are stored in a Content Management
System (CMS), for flexibility and to simplify the
inclusion of new material. Joomla! was used for this
task. Predefined presentation templates simplify the
addition of new contents and the management of
several kinds of information.
The integration of simulators and data
acquisition systems with the Web portal is a critical
point. The simulations are entirely run on the server
and the user just needs a regular browser to access
and interact with the platform. Since, as referred, the
CMS used allows the construction of customized
templates for the presentation of information, the
integration with the simulators is made by
developing forms to visualize and manage real-time
data, and for the insertion of input parameters used
by the simulators to run calculations and for
presentation of the results to the users. This layer
validates the user input and executes a system call to
autonomous simulation codes. As a result of a
successful simulation, graphical and numerical
results can be returned, to be displayed on the web
page. The communication between the
computational applications and the web platform
itself is done through a simulation gateway using a
CGI (Common Gateway Interface) protocol.
3 DESIGN OF DISTILLATION
PROCESSES
Distillation is one of the most used separation
processes in the manufacturing of chemical
products, generally speaking. Thus, this subject must
always be present in any Chemical Engineering
curriculum. The underlying principle of a separation
by distillation is the difference in volatility of the
different components in the feed mixture. Therefore,
a good understanding of vapour/liquid equilibrium is
essential to understand the design of distillation
equipment. In the Chemical Engineering curricula
this subject is usually taught in a course of Chemical
Thermodynamics.
In the platform LABVIRTUAL we have
integrated fundamental subjects with the design of
process equipment. This is the case in the section of
“Unit Operations and Separation Processes”, where
we start with the area of basic principles (Heat and
Mass Transfer and Chemical Thermodynamics) in
parallel with the area of Unit Operations design.
Figure 1 describes this arrangement.
Figure 1: Scheme of the Unit Operations and Separation
Processes area (from Granjo et al. 2009).
3.1 Basic Principles – Vapour/Liquid
Equilibrium
The “Chemical Thermodynamics” area addresses, in
CSEDU2012-4thInternationalConferenceonComputerSupportedEducation
236
general, the subject of the equilibrium of phases. In
the case of vapour/liquid equilibrium (VLE) it is
split in two parts: pure substances and mixtures. In
the VLE Fundamentals of pure substances’ section,
the significance of temperature and pressure
diagrams, the concepts of vaporization, vapour
pressure, substance’s fugacity and critical properties
are explained. Regarding the VLE of mixtures the
binary composition diagrams (Txy) and (Pxy) are
studied and common VLE problems are addressed:
bubble point pressure and temperature, BUBLP and
BUBLT; dew point pressure and temperature,
DEWP and DEWT, and isothermal flash.
Regarding VLE of mixtures, particularly important
for the design of distillation processes, homogeneous
and heterogeneous methods can be used. The VLE
simulator carries out calculations of BUBLP,
BUBLT, DEWP, DEWT and isothermal flash for
mixtures, from a database with 25 substances. The
following important thermodynamic model options
are included for the heterogeneous methods: the
ideal liquid and the perfect gas behaviour; the
UNIFAC method to model liquid non-ideality and
perfect gas; UNIFAC and the virial equation of state
to predict liquid and vapour non-idealities.
These simulators are directly connected to case
studies, to help the users to approach the simulator
in the correct way and to enhance their learning
experience. The main purpose is to allow discussing
the need of using methods of non ideal behaviour of
both liquid and vapour phases, which is mainly
determined by the type of mixture and the operating
pressure and temperature. For some of these case
studies the results obtained are compared to those in
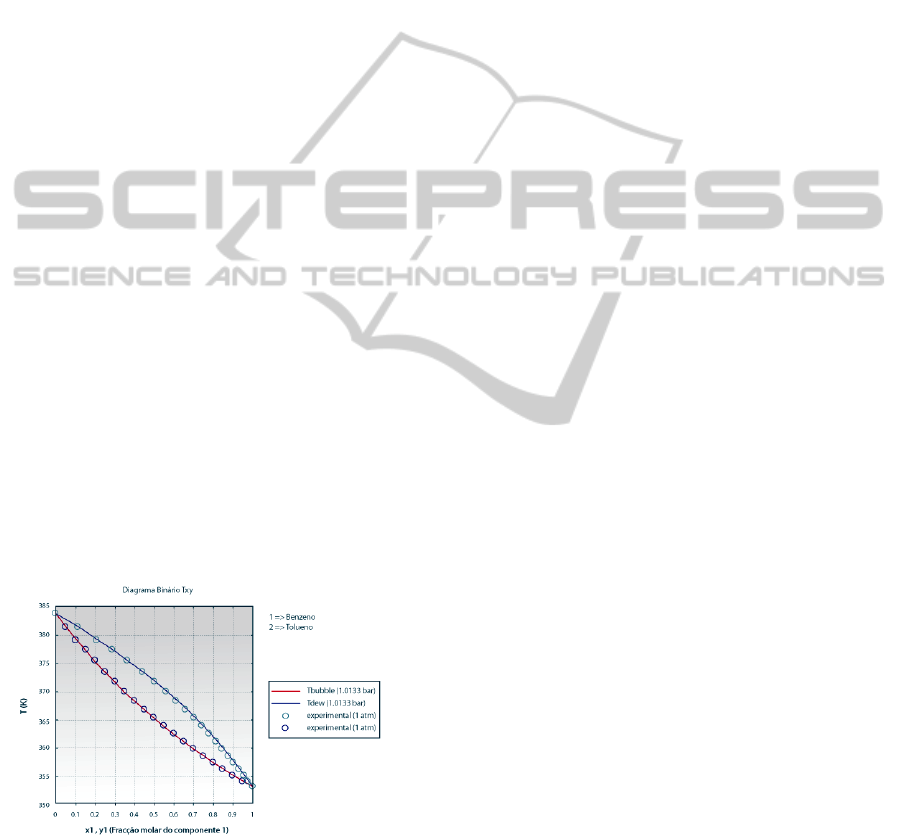
the literature, as shown in Figure 2.
Figure 2: Txy diagram for a binary mixture (benzene/
toluene) produced with the VLE simulator - comparison
with experimental data (legends in Portuguese).
3.2 Distillation Simulator
The area “Models Used in the Simulation” describes
the general case of distillation of a mixture with any
number of components. The shortcut methods are
discussed with reference to the Fensk, Underwood,
Gilliland and Kirkbride equations (FUGK) (Seader
and Henley 2006). As far as the rigorous methods of
calculation are concerned, the Wang-Henke (WH)
method is covered (Wankat 2007 and Seader and
Henley 2006). Empirical correlations and heuristics
incorporated in the Distillation simulator to design
and dimension tray distillation columns are also
addressed (Wankat 2007).
The Distillation Simulator, which has been
developed in MATLAB, allows the design of a
distillation column with one feed stream; two liquid
products (residue and distillate) streams; saturated
reflux; total condenser; adiabatic stages and constant
pressure. There are no limitations to the feed thermal
state and the user can specify the temperature (T
F
) or
the feed thermal condition. The feed components
database is the same as for the VLE simulator. Also,
the thermodynamic models options are the same as
for the VLE simulator. To carry out the simulation
calculations two strategies are available.
Three mixtures are treated in the Case studies
section: i-butane/n-butane/pentane/hexane; benzene/
methyl-cyclohexane/toluene and ethanol/water. With
the first two examples it is pretended to show the
influence of some of the distillation operating
variables (e.g. reflux ratio, pressure) for a system
with a non-ideal behavior, in the first example, and
for a system close to an ideal behavior, in the second
example. In the case of the ethanol/water mixture it
is examined the impact on the distillation process of
a mixture which exhibits an azeotropic point.
The user can experience as well the influence of
several variables on the column dimensions and
features, for instance, how changes in the operating
pressure or on the feed conditions influence the
column design and energy balance. Moreover, the
user is also directed to evaluate which is the best
thermodynamic model to describe VLE for each set
of components, that is, for each feed.
3.2.1 Example of the Use of the Distillation
Simulator – Distillation of a Mixture of
Hydrocarbons
This example corresponds to one of the case studies
in the distillation section and illustrates the use of
the distillation simulator to design a continuous
fractionating distillation column to separate a
mixture of hydrocarbons (n-pentane – nC
5
, n-hexane
- nC
6
and n-octane - nC
8
). The main separation
aimed at is between nC
5
and nC
6
, nC
8
being
considered a contaminant. The feed stream has got,
WEBTOOLSFORCHEMICALENGINEERINGEDUCATIONCOUPLINGFUNDAMENTALSWITHPROCESS
DESIGN-TheDistillationCaseStudy
237

Table 1: Summary of the design results for the distillation column to separate nC
5
, nC
6
and nC
8
.
x
D,LK
= 0.934; x
D,HK
=0.066;
x
B,LK
= 0.014; x
B,HK
=0.9;
LK recovery in distillate = 98%;
HK recovery in residue = 95 %
Residue molar flow rate (B) = 58 kmol·h
-1
Distillate molar flow rate (D) = 42 kmol·h
-1
Equilibrium stages, N = 22
Feed stage, N
F
= 10
Reflux ratio, R = 1.63
T
D
= 332 K; T
B
= 367 K.
Heat duty in condenser = -781 kW
Heat duty in reboiler = 810 kW
Column height = 21.4 m
Column diameter = 0.99 m
x
D,LK
, x
D,HK
are the mole fractions of light-key and heavy-key components in the distillate, respectively.
x
B,LK
, x
B,HK
are the mole fractions of light-key and heavy-key components in the residue, respectively.
T
D
, T
B
are the distillate and residue temperatures, respectively.
in mole percentages, 40, 55 and 5% of nC
5
, nC
6
and
nC
8
respectively (nC
5
, is considered as the light key
(LK) while nC
6
is the heavy key (HK)). The column
operates at 2 bar. In the results presented here it is
assumed that the feed stream enters the distillation
column as a saturated liquid, and that both the liquid
and vapour phases are taken as non-ideal (UNIFAC
and virial equation being used, respectively, for the
liquid and gas phases, Poling et al. 2001). The
recovery targets have been established as 98% of the
LK in the distillate and 95% of the HK in the bottom
product. The operating reflux ratio (R) is assumed to
be 1.2 times the minimum reflux ration (R
min
).
Figure 3 presents an image of the input form for the
distillation simulator for this case study.
Figure 3: Input form to the distillation simulator: example
for the case study (in Portuguese).
Table 1 shows the solution of this design
problem, giving the specifications of the resulting
distillation column, which must have 22 theoretical
stages (including the reboiler) the feed stream being
introduced in the 10th stage from top. The recoveries
of both the LK and HK have met the specifications
required. The column is expected to have a total
height of around 21 m with a diameter around 1 m.
Figures 4 and 5 are examples of the output
graphs from the simulator, showing how the liquid
composition and temperature vary along the column.
The students are guided to criticize these results by
observing, for instance, that the composition profiles
vary smoothly along the column, the same
happening to the temperature profile, which is an
indication of a well designed column where the feed
stream is being admitted at its optimal location.
Moreover, they can also verify that the heaviest
component (nC
8
) almost disappears in the
rectification section (stages above the feed). At a
later stage the students can be guided to evaluate,
easily, the influence of changing certain operating
parameters, such as the reflux ratio, operating
pressure, feed temperature or the feed location, on
the column design. Another feature that can also be
tested by the students is the influence of the
thermodynamic model selected to describe the liquid
and gas phases on the output results (design of the
distillation column). They can choose between ideal
gas and liquid phases, ideal gas and non-ideal liquid
phase and non-ideal gas and liquid phases. In this
way they can perceive better that for some mixtures,
as is the case for the example presented, the
hypothesis of ideality does not affect the final
equipment design. For other mixtures, like for
instance azeotropic mixtures (see Granjo et al.
2009), or for very severe operating conditions,
ideality is a completely invalid hypothesis.
CSEDU2012-4thInternationalConferenceonComputerSupportedEducation
238
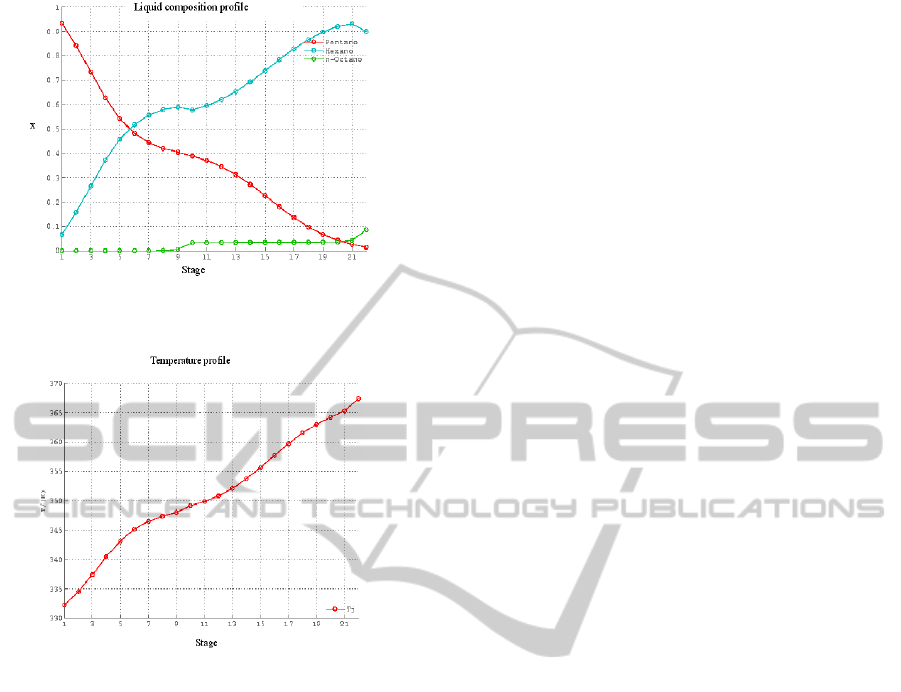
Figure 4: Liquid composition (mole fraction – x) profile –
optimal feed location (10
th
stage).
Figure 5: Temperature (T, K) profile – optimal feed
location (10
th
stage).
Furthermore, the students can also compare the
validity of different models for the design of a
distillation column, going from approximate and
short-cut methods to rigorous design methodologies
(Wankat 2007 and Seader and Henley 2006). This
case study can be used in the classroom to illustrate
the influence of the aforementioned aspects on the
design of a distillation column, serving as the basis
for a discussion with the students. Additionally, the
students can later go to the laboratory and perform
lab trials which they will be asked to compare with
the simulated results.
We have been monitoring the use of the portal by
the students and collecting their opinions about the
tools available, asking them to fill out an anonymous
questionnaire in each course.
The questionnaire is very similar for the different
courses. Figure 6 (a) presents the questionnaire
which is being used in the course of “Separation
Processes” of the Chemical Engineering Masters
degree of the University of Coimbra. The questions
addressed different aspects of the learning
experience, students frequency and purpose of use,
which facilities they had used, as well as their
evaluation of the interface and of the structure of the
portal. The results of the students’ responses to the
questionnaire handled during the three last editions
(since 2008), corresponding to a total of 78
respondents, are given in Figure 6 (b).The students’
response was, as a whole, very positive: 82% had
used the platform in the Process Separations I
discipline and about 91% had also used it in other
subjects. Moreover, it was observed that the majority
of the students had used LABVIRTUAL as a
support to their study, with most of them using the
platform several times. Another good point was that
the vast majority of the students considered the Unit
Operations and Separation Processes area of the
platform as useful, very easy to use and very well
structured. Globally, LABVIRTUAL had a very
good rate among the students enquired.
4 END NOTES
Web tools are important complementary instruments
for the teaching and learning process. They can be
accessed with maximum flexibility, from any place
at any time, and contribute to the students autonomy.
In the case of the multi-functional web platform
presented here, there are advantages from the
combination, in a single platform, of several
features: co-existence of teaching tools for a broad
range of Chemical Processes, which motivates
knowledge integration; diversity of approaches for a
deeper insight of each chemical process, going from
multimedia libraries, supplying the theoretical
concepts, to simulators and case studies, used for the
design of the different processes and, finally, virtual
experiments. The student should be able to:
understand and relate basic concepts and principles
associated to each process; establish analogies
between different processes; understand, apply and
know the limitations of the modelling methodologies
commonly used to interpret and troubleshooting
industrial problems; have an idea of the impact of
operational parameters on the equipment design,
dimensioning and cost. This makes it easier to
introduce the students to more practical and realistic
approaches to Chemical Engineering problems.
The experience of using this multi-purpose
platform, during three school years, has been
evaluated very positively by the students.
WEBTOOLSFORCHEMICALENGINEERINGEDUCATIONCOUPLINGFUNDAMENTALSWITHPROCESS
DESIGN-TheDistillationCaseStudy
239
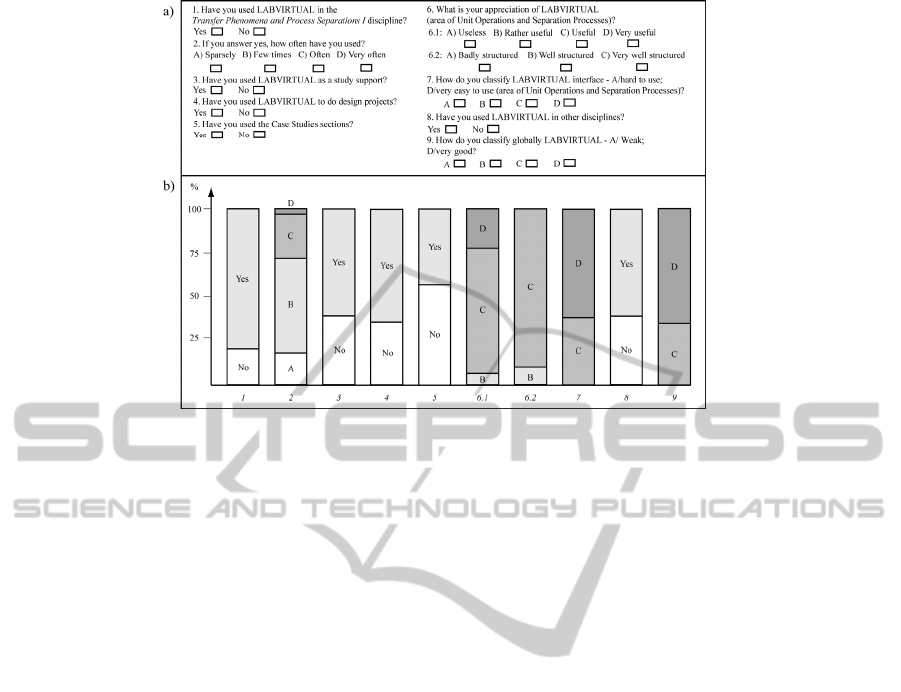
Figure 6: LABVIRTUAL assessment: a) enquiry; b) enquiry’s results (A- bad, D- very good).
ACKNOWLEDGEMENTS
The authors wish to acknowledge the receipt of
financial support from POSC, Portugal, contract
743/4.2/C/REG, which enabled the development of
the platform partially described here.
REFERENCES
Bell, J. T. and Fogler, H. S., 1998. The application of
virtual reality to chemical engineering and education.
In Proc. AIChE Annual Meeting, Miami, USA, session
170.
Edgar, T.F., 2006. Enhancing the undergraduate
computing experience. Chemical Engineering
Education, 40 (3) 231─238.
Felder, R.M., 2006. Teaching engineering in the 21st
century with a 12th-century teaching model: how
bright is that?. Chemical Engineering Education, 40
(2) 110─113.
Garcia-Herreros, P. and Gómez, J. M., 2010. Modeling
and optimization of a crude distillation unit: a case
study for undergraduate students. Comp. Appl. Eng.
Ed., DOI: 10.1002/cae.20469.
Granjo, J. F., Rasteiro, M. G., Gando-Ferreira, L. M.,
Bernardo, F. P., Carvalho, M. G., Ferreira, A. G.,
2009. A virtual platform to teach separation Processes.
Computer Appl. in Eng. Ed., DOI 10.1002/cae.20383.
Hasna, A.M., 2010. E-competence in chemical
engineering learning and teaching. In Proc. ICETC
2010 - 2nd. Int. Conf. Ed. Tech. and Computers,
China, art. no. 5529669, V4330-V4336.
Henry, J., 2005. Real laboratories at a distance. In Proc.
AIChE annual meeting, Cincinnati, USA, 3640.
Henry, J., Miletic, M., DiBiasio, D., Clark, W., 2008.
Varieties of ways for learning about distillation. In
Proc. ASEE annual meeting, Pittsburg, USA.
Poling, B. E., Prausnitz, J. M., O'Connell, J. P., 2001.
Properties of Gases and Liquids. McGraw-Hill, N. Y.,
5
th
edition.
Rasteiro, M. G., Ferreira, L. M., Teixeira, J. C., Bernardo,
F. P., Carvalho, M. G., Ferreira, A. G., Ferreira, R. Q.,
Garcia, F. P., Baptista, C. G., Oliveira, N. M., Quina,
M. M., Santos, L. O., Saraiva, P. A., Mendes, A. M.,
Magalhães, F. M., Almeida, A. S., Granjo, J. F.
Ascenso, M., Bastos, R. M., Borges, R., 2009.
LABVIRTUAL – a virtual platform to teach chemical
processes. Education for Chemical Engineers, 4 (1)
e9─19.
Seader, J. D. and Henley, E. J., 2006. Separation Process
Principles. John Wiley & Sons, Hoboken, NJ, 2
nd
edition.
Shacham, M., Cutlip, M. Brauner, N., 2009. From
numerical problem solving to model-based
experimentation – incorporating computer based tools
of various scales into the ChE curriculum, Chem. Eng.
Ed., 43 (4).
Streicher, S. J., West, K., Fraser, D. M., Case, J. M.,
Linder, C., 2005. Learning through simulation ─
student engagement, Chem. Eng. Ed., 39 (4),
288─301.
Wankat, P. C., 2007. Separation Process Engineering.
Prentice-Hall, Upper Saddle River, NJ, 2
nd
edition.
CSEDU2012-4thInternationalConferenceonComputerSupportedEducation
240
