
VITAL-SIGN DATA FUSION MODELS FOR POST-OPERATIVE
PATIENTS
Marco A. F. Pimentel
1
, David A. Clifton
1
, Lei Clifton
1
, Peter J. Watkinson
2
and Lionel Tarassenko
1
1
Institute of Biomedical Engineering, Department of Engineering Science, University of Oxford, Oxford, U.K.
2
Nuffield Department of Anaesthetics, University of Oxford, Oxford, U.K.
Keywords:
Patient Monitoring, Early Warning Scores, Novelty Detection.
Abstract:
Deterioration in Patients who undergo upper-gastrointestinal surgery may be evident in the vital signs prior
to adverse events. A dataset comprising observational vital-sign data from 128 post-operative patients was
used to explore the trajectory of patients vital-sign changes during their stay in the post-operative ward. A
model of normality based on pre-discharge data from patients who had a “normal” recovery was constructed
using kernel density estimates, and tested with “abnormal” data from patients who deteriorate sufficiently to
be re-admitted to the Intensive Care Unit. The results suggest that the criticality of post-operative patients can
be evaluated by assessment of the distributions of their vital signs after their admission to the post-operative
ward.
1 INTRODUCTION
A large number of preventable and avoidable deaths
occur in hospitals each year in the UK, due to adverse
events such as cardiac arrest and unplanned admis-
sion into the ICU from other hospital wards (Bardell
et al., 2003; Kause et al., 2004). Patients who undergo
surgery for the removal of upper- gastrointestinal (GI)
cancer have a high incidence of post-operative com-
plications, often resulting in readmission to the In-
tensive Care Unit (ICU) several days after surgery
(Thompson et al., 2003; Veltkamp et al., 2002). Fail-
ure to identify such deteriorations in a timely manner
has led to the design of a two-phase clinical trial at
the Oxford Cancer Hospital, the “CALMS2” trial, in
which ambulatory post-operative cancer patients are
monitored using wearable sensors that are connected
by the hospital wireless network to patient monitors
and nursing display stations. Vital-sign measure-
ments are also made periodically by the nurses on the
ward as part of the usual post-operative care.
Our approach to monitoring in-hospital patients
relies on constructing models of normality based on
the vital signs acquired from a large population of
acutely-ill patients (Tarassenko et al., 2006; Hann,
2008). We propose to improve upon existing models
by investigating models tuned to specific patient pop-
ulations. Post-operative cancer patients are recover-
ing from surgery, so that they start in their most acute
state and then gradually stabilise. In this work, we
therefore adopt a novel approach in which the aim
is to learn the vital-sign trajectories associated with
“normal” recovery of these patients, allowing “abnor-
mal” trajectories to be identified in subsequent pa-
tients.
We present preliminary results of the analysis of
data acquired during Phase I of the CALMS2 clinical
trial. A multivariate model of the distribution of vital-
sign data from “normal” patients, which describe the
normal physiological trajectory, is constructed using
a non-parametric statistical approach, a kernel den-
sity estimate. This model is then tested on “abnor-
mal” data from patients who deteriorate sufficiently
after surgery to be re-admitted to the ICU.
2 DATASET
The dataset used for the work described by this paper
comprises measurements of heart rate (HR), respira-
tory rate (RR), peripheral arterial oxygen saturation
(SpO
2
), systolic blood pressure (SBP) and tempera-
ture acquired by ward staff every hour or every two
hours in the first days after patient admission (de-
pending on the patient’s condition), and every four
hours in the last days of the patient’s stay on the ward.
A set of 15, 029 observations X ∈ R
5
was obtained
from 169 patients. These patients were admitted to
410
A. F. Pimentel M., A. Clifton D., Clifton L., J. Watkinson P. and Tarassenko L..
VITAL-SIGN DATA FUSION MODELS FOR POST-OPERATIVE PATIENTS.
DOI: 10.5220/0003789104100413
In Proceedings of the International Conference on Bio-inspired Systems and Signal Processing (BIOSIGNALS-2012), pages 410-413
ISBN: 978-989-8425-89-8
Copyright
c
2012 SCITEPRESS (Science and Technology Publications, Lda.)
the Upper GI ward and the median length of stay on
the ward was 8 days (IQR 6-11). In order to select
a subgroup of patients with “normal” recovery from
surgery (class C
1
), we chose those patients who stayed
more than 4 days and less than 25 days on the ward,
and who were not (re-)admitted to the ICU. This re-
sulted in 128 patients being included in class C
1
.
It is assumed, a priori, that each of the D = 5 pa-
rameters has equal importance in the patient model.
Each parameter was therefore scaled to have the
same dynamic range using a zero-mean, unit-variance
transformation.
The vital-sign trajectory throughout patient stays
on the ward was evaluated by examining the 5 sub-
groups of data:
• G
1
: comprises the first clinical observation per-
formed when a patient was admitted to the ward;
• G
2
: is the average of all observations performed
on the first day of a patient’s stay on the ward;
• G
3
: comprises the average of all observations per-
formed on the day that corresponds to half of the
length of the patient’s stay on the ward (if the pa-
tient stayed 5 days on the ward prior to discharge,
this day would correspond to day three);
• G
4
: contains the average of all observations per-
formed on the day that corresponds to 75% of the
length of the patient’s stay on the ward;
• G
5
: contains the average of all observations per-
formed on the last day of the patient’s stay on the
ward.
These five subgroups were defined in this way be-
cause of the different lengths of patient stay on the
ward (from 4 to 25 days).
3 DATA VISUALISATION
3.1 Methodology
The first stage in constructing a model of normal-
ity for novelty detection usually consists in obtain-
ing more insight into the structure of the data. Pro-
cedures for visualisation of the data in their original
high-dimensional space are required. The relations of
proximity of the vital-sign data in their original space,
with D > 3, can be visualised through a non-linear
projection from R
D
to R
3
or R
2
.
A Sammon-mapping algorithm (Sammon, 1969)
was implemented and applied in the 640 mean vec-
tors contained in the 5 subgroups (G
1
, G
2
, G
3
, G
4
,
G
5
, with 128 data in each) from patients belonging to
class C
1
.
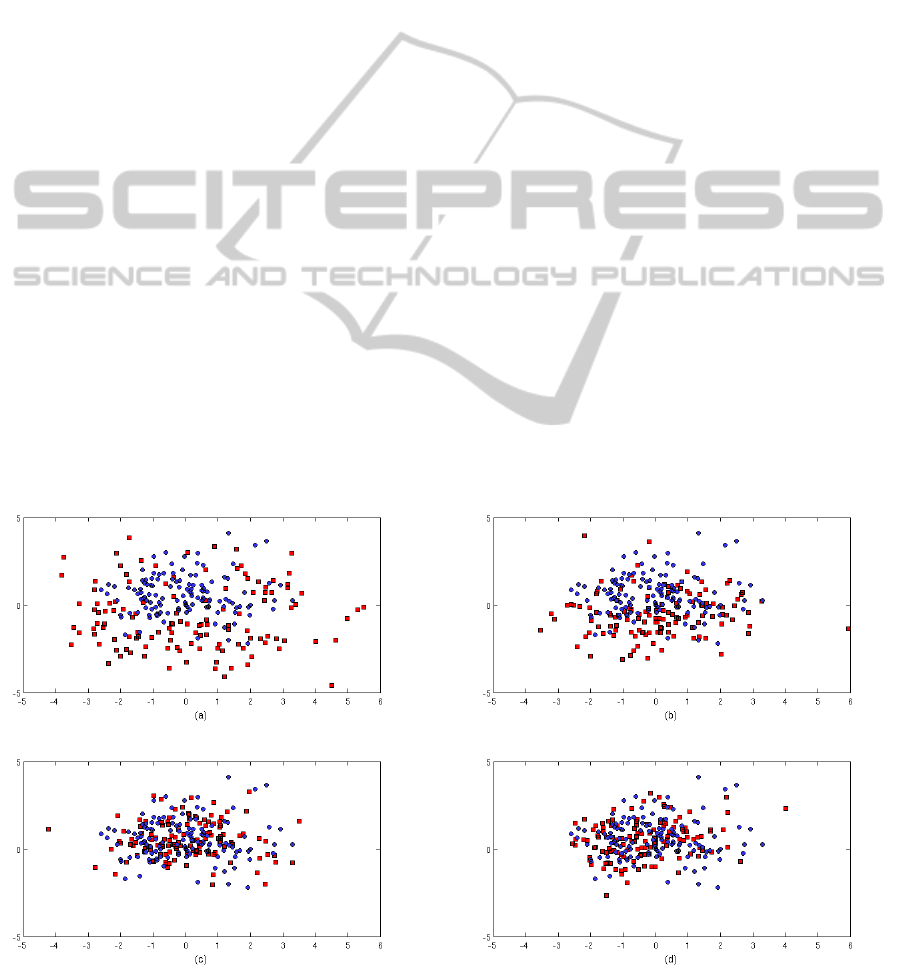
3.2 Results
Represented in each map of Figure 1 are the projected
data from G
1
, G
2
, G
3
, G
4
superimposed on the pro-
jected data points from G
5
(data from the last day on
the ward for each patient).
It may be seen from the plots that the groups form
Figure 1: Sammon maps obtained for the group dataset: (a) G
1
and G
5
, (b) G
2
and G
5
, (c) G
3
and G
5
, and (d) G
4
and G
5
; in
(a)-(d) G
1
, G
2
, G
3
and G
4
are represented by red {} and G
5
by blue {o}.
VITAL-SIGN DATA FUSION MODELS FOR POST-OPERATIVE PATIENTS
411
clusters with some overlap between them. If we con-
sider projected data from groups G
1
and G
5
(shown in
red and blue, respectively in the top-left plot), it may
be seen that the former are dispersed, while the latter
are more concentrated. It may also be seen that the
distributions of the data for G
3
(data that correspond
to the midpoint of patient’s stay) and G
5
groups are
very similar and evenly concentrated. This suggests
that for the 128 patients with a normal recovery pro-
cess from surgery, there are no large changes in the
vital-sign distributions after the midway point during
their stay on the ward. These results suggest the pos-
sibility that patients belonging to class C
1
could have
been discharged early, or provided with a lower level
of care from the halfway point of their stay, as they
had already been stabilised and no significant changes
in their vital-sign distributions subsequently occured.
4 MODEL OF NORMALITY
4.1 Methodology
For the construction of the model of normality, we
considered the G
5
subgroup of measurements as the
“normal” dataset. This contains the most stable phys-
iological variables (because these data are acquired
just before discharge, when the patient is most “nor-
mal” ). At discharge the patient is stable and deemed
well enough to go home. Therefore, the G
5
dataset
of N = 128 prototype vectors, X ∈ R
5
, was used for
construction of the model of normality.
A kernel density estimate (Bishop, 2007) is a tech-
nique that allows the underlying 5-D vital-sign proba-
bilty density function (pdf) to be estimated from train-
ing data. While other methods, such as Gaussian mix-
ture models, were considered, a kernel density esti-
mate was chosen as it has the advantage of being a
fully non-parametric method, so no a priori assump-
tions are made about the form of the probability dis-
tribution.
This method was initially used to estimate the pdf
of the 128 prototype vectors, x
1
,...,x
N
,
p(x) =
1
N(2π)
D/2
σ
D
N
∑
i=1
e
−
|x−x
i
|
2
2σ
2
(1)
which is a weighted sum of Gaussian kernels centred
on the 128 prototype vectors, x
i
, and where each ker-
nel is isotropic with variance σ
2
. The variance was
determined using the nearest-neighbour method pro-
posed by Bishop (Bishop, 2007).
In the final model, only those patients who were
discharged within 5 to 9 days were considered for
building the estimate of the pdf (N = 78). The ratio-
nale for this decision was to focus on the most “nor-
mal” patients from class C
1
(i.e. the patients who re-
cover entirely as expected after surgery). To identify
those patients, the mode of the lengths of stay on the
ward was calculated, and only the patients whose stay
on the ward was within the mode ± 2 days were taken
into account. This corresponds to the range [5 9] days.
The averages of the observations from each day for
each of these patients were calculated and the pdfs
p(x|θ), where θ corresponds to the model parameters,
were then determined from Equation (1) with N = 78.
In order to estimate the abnormality of a
previously-unseen test pattern, the departure from
normality is usually quantified using novelty defined
as z(x) = − log p(x|θ), where z(x) is the novelty
score. This process leads to “normal” data, which
have higher likelihoods p(x|θ), generating low nov-
elty scores, and “abnormal” data, which have lower
likelihoods, generating high novelty scores.
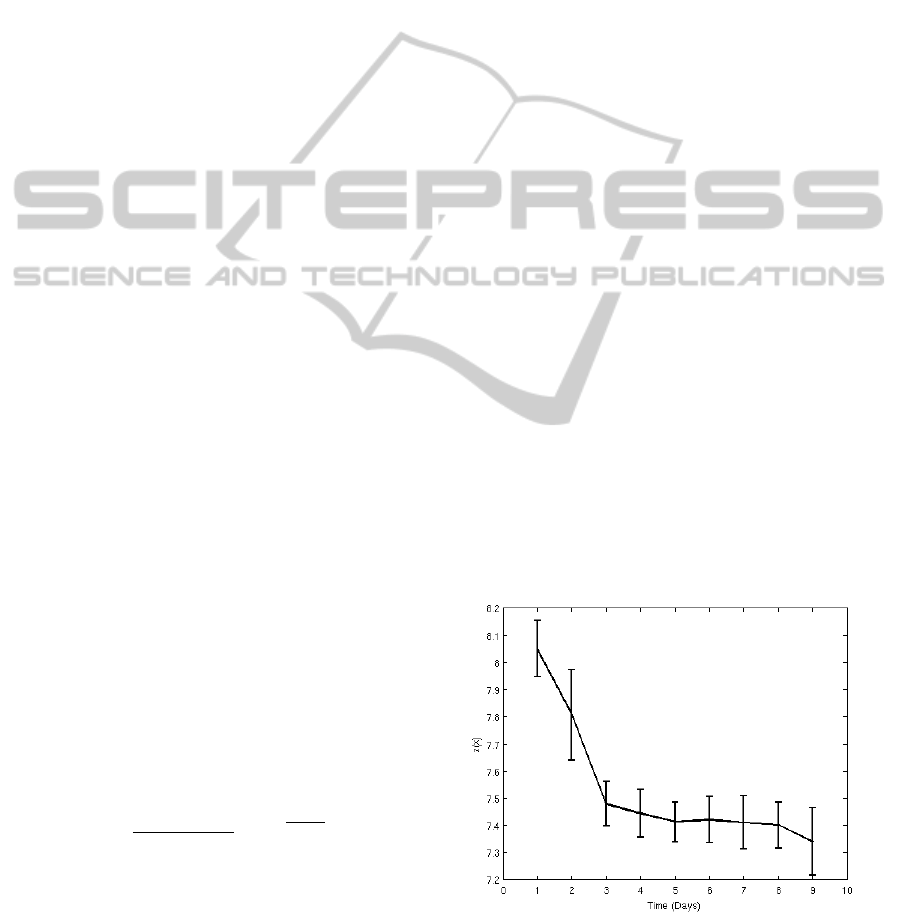
4.2 Results
The novelty scores z(x) from the most “normal” 78
patients using the 5-D pre-discharge data (G
5
) to con-
struct the model of normality are shown in Figure 2,
averaged for each patient for each day. These scores
confirm the results given by the Sammon maps. From
the trajectory of the novelty z(x) shown in Figure 2 we
can conclude that there is a significant decrease of the
value of z(x) during the first 3 days. From day 4 on-
wards, z(x) remains approximately constant. The first
3 days could be associated with the patient’s recov-
ery in the first post-operative days (Thompson et al.,
2003), whereas after day 4, the majority of patients
Figure 2: Representation of average of the novelty scores
z(x) = −log p(x|θ) with time for “normal” patients, where
the data probability density was constructed using pre-
discharge data from the same group of patients. Error bars
denoted one standard error of the group mean.
BIOSIGNALS 2012 - International Conference on Bio-inspired Systems and Signal Processing
412

included in this study appear to have fully recovered
from surgery and to be stable. They are well enough
to either be discharged or be provided with a lower
level of care for their remaining time on the ward.
The multivariate model of normality was also
tested on “abnormal” vital-sign data from two patients
who deteriorated sufficiently after surgery to be re-
admitted to the ICU. The model was able to identify
patient deterioration up to 12 hours before the pa-
tient’s readmission to the ICU. These results are omit-
ted for brevity.
5 CONCLUSIONS AND FUTURE
WORK
We have presented the preliminary analysis of data
acquired from patients who were admitted to the
post-operative Upper GI ward after cancer surgery.
We studied the vital-sign distributions at the time of
the patient’s admission to the ward, halfway through
their stay, and near the time of discharge. Although
changes in vital-sign distributions from “normal” pa-
tients between admission to the ward and subsequent
discharge were found, no significant changes in these
distributions were observed from halfway through
their stay to the time of discharge, which suggests that
these patients could have been discharged earlier or
provided with a lower level of care from the halfway
point of their stay on the ward.
A multivariate model of the distribution of vital-
sign data from “normal” patients, which describe the
normal trajectories, was constructed using a kernel
density estimate. The model confirmed the results ob-
tained with the Sammon maps.
Future work will concentrate on the refinement of
existing techniques for the target population group,
and on the improvement of model construction using
more complex dynamical modelling methods. How-
ever, there are a number of limitations that must be
overcome in future analysis. The dataset used in the
analysis described by this paper consisted of measure-
ments of vital signs acquired periodically (every 2 or
4 hours) by ward staff during Phase I of the clinical
trial. These infrequent patient observations combined
with lower nurse:patient ratios (typically, 1:4 to 1:10
in step-down wards), can lead to unnoticed clinical
deterioration and, consequently, to increased numbers
of adverse events. We are developing a continuous
vital-sign monitoring system to provide early warn-
ing of patient deterioration in a robust manner with
low numbers of false alarms.
ACKNOWLEDGEMENTS
The work described in this paper was funded by the
NIHR Biomedical Research Centre Programme, Ox-
ford. MAFP was supported by the RCUK Digital
Economy Programme grant number EP/G036861/1
(Oxford Centre for Doctoral Training in Healthcare
Innovation), and Dr David Clifton was supported by
the Wellcome Trust and the EPSRC under grant num-
ber WT 088877/Z/09/Z. The authors also wish to
thank the support of all clinical staff involved in the
collection of the data used in this investigation.
REFERENCES
Bardell, T., Legare, J. F., Buth, K. J., Hirsch, G. M.,
and Ali, I. S. (2003). ICU readmission after car-
diac surgery. European Journal of Cardio-Thoracic
Surgery, 23(3):354–359.
Bishop, C. M. (2007). Pattern Recognition and Machine
Learning. Springer.
Hann, A. (2008). Multi-parameter monitoring for early
warning of patient deterioration. Ph.D. thesis, Uni-
versity of Oxford.
Kause, J., Smith, G., Prytherch, D., Parr, M., Flabouris,
A., and Hillman, K. (2004). A comparison of an-
tecedents to cardiac arrests, deaths and EMergency in-
tensive care admissions in australia and new zealand,
and the united kingdom–the ACADEMIA study. Re-
suscitation, 62(3):275–282.
Sammon, J. W. (1969). A nonlinear mapping for data struc-
ture analysis. IEEE Transactions on Computers, C-
18(5):401– 409.
Tarassenko, L., Hann, A., and Young, D. (2006). Integrated
monitoring and analysis for early warning of patient
deterioration. British Journal of Anaesthesia, 97(1):64
–68.
Thompson, J. S., Baxter, B. T., Allison, J. G., Johnson,
F. E., Lee, K. K., and Park, W. Y. (2003). Temporal
patterns of postoperative complications. Arch Surg,
138(6):596–603.
Veltkamp, S. C., Kemmeren, J. M., van der Graaf, Y.,
Edlinger, M., and van der Werken, C. (2002). Pre-
diction of serious complications in patients admitted
to a surgical ward. The British Journal of Surgery,
89(1):94–102.
VITAL-SIGN DATA FUSION MODELS FOR POST-OPERATIVE PATIENTS
413
