
FC-BASED SEGMENTATION OF JAW TISSUES
Roberto Llor´ens, Valery Naranjo, Miriam Clemente, Mariano Alca˜niz and Salvador Albalat
∗
Instituto Interuniversitario de Investigaci´on en Bioingenier´ıa y Tecnolog´ıa Orientada al Ser Humano
Universidad Polit´ecnica de Valencia
Camino de Vera s/n, 46022 Valencia, Spain
Keywords:
Jaw tissues segmentation, Dental implantology, Fuzzy connectedness, Mathematical morphology.
Abstract:
The success of an oral implant surgery is subject to accurate advance planning. For this purpose, it is funda-
mental that a computer-guided program provides all the available information in a reliable way. Therefore,
to plan a suitable implant placement, an accurate segmentation of the tissues of the jaw is necessary. These
tissues are the cortical bone, trabecular core and the mandibular canal. The accurate segmentation of the
mandibular canal, along which the inferior alveolar nerve crosses the lower arch, is particularly important
since an injury to the canal can result in lip numbness. To this date, existing segmentation methods for the
jaw requires high human interaction and/or don’t achieve enough accuracy. Our overall aim is to develop an
automatic method for the segmentation of the whole jaw, focusing our efforts on achieving very high accuracy
and time efficiency. To this end, this paper presents an exhaustive evaluation of fuzzy connectedness object
extraction as a plausible segmentation core for this method, basing on the results achieved on 80 CT slices in
terms of detection and false alarm probability and merit factor.
1 INTRODUCTION
Dental implants are artificial roots, usually titanium-
made, that are inserted into the maxillary bone in or-
der to substitute the roots of the lost dental pieces,
providing better functionality and aesthetics. For a
long-term use, the placement of the implant must be
inferred precisely and therefore the biometric proper-
ties of the patient’s jaw must be known a priori. The
lower jaw is the densest and most prominent bone
of the face, and it is made up by three easily distin-
guishable tissues: a hard exterior cortical bone that
contains a softer osseous tissue filling its inner cav-
ity, the trabecular (or cancellous or spongy) bone, and
the mandibular canal (when present), which contains
the inferior alveolar nerve. This nerve runs along the
lower jaw, from the mandibular to the mental fora-
men, supplying sensation to the teeth. For this reason,
an injury to the canal might result in temporary or per-
manent lip numbness. All this gives rise to the need
for an accurate segmentation which provides precise
information to preoperative planning systems to as-
sure the success of the dental surgery. Previous works
∗
This work has been supported by the project MIRA-
CLE (DPI2007-66782-C03-01-AR07) of Spanish Ministe-
rio de Educaci´on y Ciencia.
in segmentation of dental tissues require high human
interaction and/or don’t achieve enough accuracy to
consider these approaches suitable for preoperative
planning systems. Our research, then, is based on de-
veloping a method which provides this segmentation
in an automatic and precise way.
The classical approach tries to plan the surgery
from panoramic X-ray views, but this resource has
a limited value due to the fact that it is an often-
distorted two-dimensional image. CT is the more
suitable evaluation method presenting 94% accu-
racy, whereas the periapical X-ray and the panoramic
images present 53% and 17% accuracy, respec-
tively (Reiser et al., 2004).
Many dental implant planning applications carry
out the process of 3D reconstruction from CT data
de-emphasizing tissue segmentation as in (Verstreken
et al., 1998), and many others delegate this task to
dentists or surgeons, providing tools with this pur-
pose (Galanis et al., 2007). (F¨utterling et al., 1998)
carry out a segmentation of hard tissues by threshold-
ing in strict sense while inner tissues are segmented
by assigning different material properties to the tetra-
hedral finite elements, depending on the density gray
values in the CT data-set. Obviously, any type of pre-
cision in the geometric model can therefore be omit-
409
Lloréns R., Naranjo V., Clemente M., Alcãniz M. and Albalat S. (2010).
FC-BASED SEGMENTATION OF JAW TISSUES.
In Proceedings of the Third International Conference on Bio-inspired Systems and Signal Processing, pages 409-414
DOI: 10.5220/0002698804090414
Copyright
c
SciTePress

ted. (W. Stein and Muhling, 1998) use Dijkstra’s al-
gorithm aided by 3D morphologyto trace the most fa-
vorable path between two nodes (here, the mandibular
and mental foramen) marked by an expert. (Krˇsek
et al., 2007) present a tissue segmentation process
which requires high human interaction assisted only
by basic morphological operations and threshold in
Hounsfield values. (Kang et al., 2007) use a fuzzy
C-means-based partition tree for segmenting tissues
with overlapped gray level range. (DeBruijne et al.,
2003) adapted active shape models (ASM) to tubu-
lar structures. ASM are landmark-based linear shape
models which try to fit a structure according to the
variation represented in a training set previously an-
notated by an expert. (Rueda et al., 2006) follow this
study and use active appearance models (AAM) for
the segmentation of jaw tissues. However, since ho-
mologous points can not be established among differ-
ent slices and some structures are unconnected or do
not even appear, the precision achieved is completely
insufficient.
Udupa et al. (Udupa and Samarasekera, 1996)
present a novel method based on fuzzy subset the-
ory with excellent results in different fields of med-
ical imaging. In (Saha et al., 2000), several functions
are proposed to represent intensity and homogeneity
components of affinity. Our efforts are focused on
evaluating all the configurations and validating them
for the segmentation of jaw tissues in slices like those
shown in figure 1, in order to create a complete au-
tomated 3D reconstruction valid for any preoperative
dental implant planning system.
2 METHOD
Fuzzy connectedness (FC) has proven to give suc-
cessful results in several medical applications such as
multiple sclerosis lesion detection, blood vessel def-
inition (Udupa et al., 1997a) and tissues segmenta-
tion (Udupa et al., 1997b). Our aim is to evaluate
FC object segmentation in dental CT slices, defined
transversally to the dental arch by means of a preop-
erative implant planning system, as shown in figure 1.
2.1 Theory Fundamentals
Fuzzy connectedness is a fuzzy subsets theory-based
methodology. The algorithm starts from a seed and
evaluates the affinity in a neighborhood of the pix-
els present in a queue. The queue is updated while
the affinity is still able to be refined. In this way, the
algorithm computes the connectivity map of the im-
age under study, where each pixel value represents the
Figure 1: Definition of transversal slices by means of aplan-
ning system.
affinity between the pixel and the seed. Consequently,
it is intuitive to define an object as those pixels whose
connectivity value is greater than a threshold.
The affinity describes the similarity between two
pixels and represents the power of the connection be-
tween them. For this reason the affinity is based on the
adjacency between the pixels and on the similarity of
their intensities. Adjacency represents the contiguity
between pixels. For this study, 4-adjacency is consid-
ered and can be defined, for the pixels c
i
and d
i
, as
follows:
µ
α
(c, d) =
1 ,if
p
∑
i
(c
i
−d
i
)
2
≤ 1
0 ,otherwise
(1)
Analytically, the affinity can be expressed as:
µ
κ
(c, d) = h(µ
α
(c, d), f(c), f(d), c, d) (2)
That is, the affinity between the pixels depends on
their adjacency, position and some function of them.
According to the fuzzy connectedness theory de-
scribed in (Saha et al., 2000), the affinity should con-
sist of two components: an object-feature-based com-
ponent and a homogeneity-based component. Both
components must be considered in the design of the
affinity, although in some applications it is more pro-
ductive to consider only one component.
Therefore we can design a great variety of func-
tions for each component independently and combine
them to obtain the desired affinity relation valid for
the application under study. Then, it is possible to
refine the affinity as follows
µ
κ
(c, d) = µ
α
(c, d)g(µ
Ψ
(c, d), µ
Φ
(c, d)) (3)
where µ
Ψ
and µ
Φ
represent the homogeneity-based
and the object-feature-based component, respectively.
The strength of relation Ψ represents the degree of the
BIOSIGNALS 2010 - International Conference on Bio-inspired Systems and Signal Processing
410
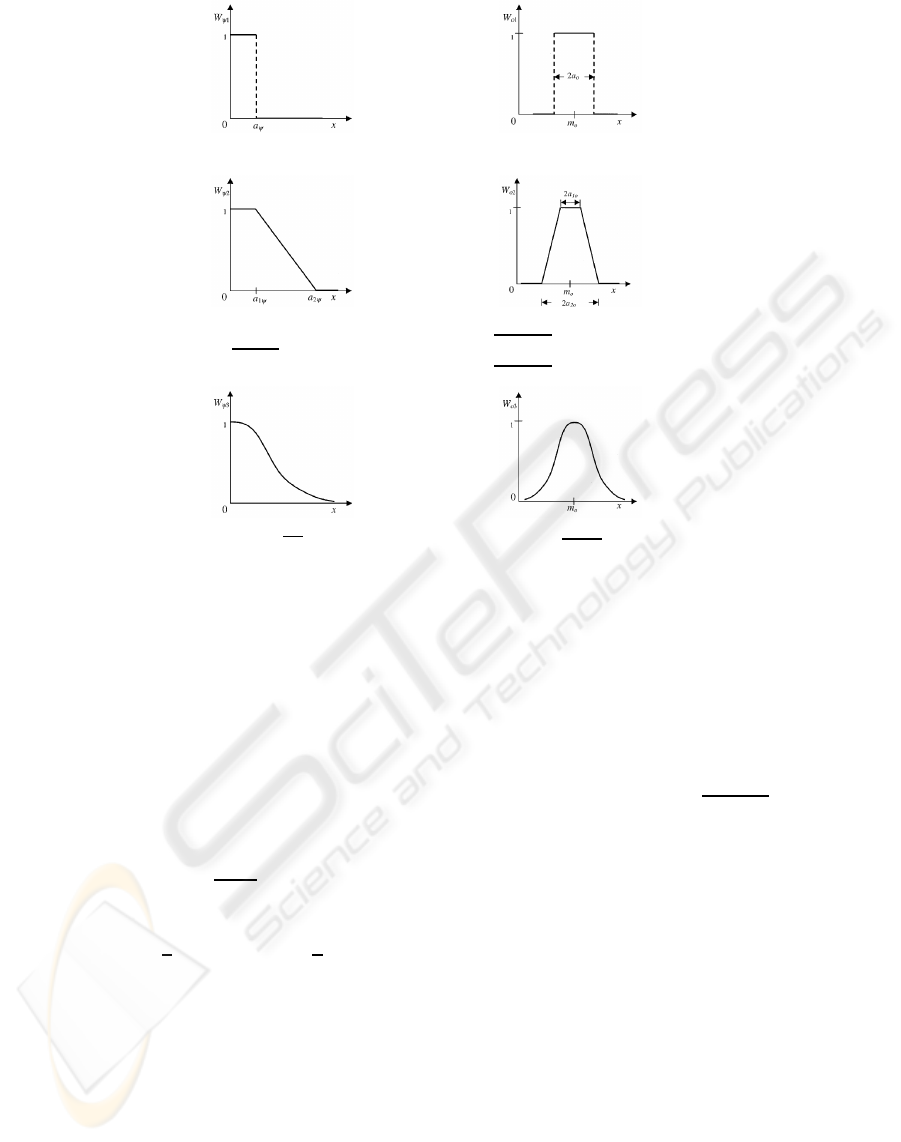
W
Ψ1
(x) =
1 ,si 0 ≤x ≤a
Ψ
0 ,si x > a
Ψ
W
o1
(x) =
(
0 ,si x < m
o
−a
o
1 ,si m
o
−a
o
≤x ≤ m
o
−a
o
0 ,si m
o
+ a
o
< x
W
Ψ2
(x) =
1 ,si 0 ≤x ≤a
1Ψ
a
2Ψ
−x
a
2Ψ
−a
1Ψ
,si a
1Ψ
≤ x ≤ a
2Ψ
0 ,si x > a
2Ψ
W
o2
(x) =
0 ,si x ≤m
o
−a
o
x−(m
o
−a
2o
)
a
2o
−a
1o
,si m
o
−a
2o
≤x ≤ m
o
−a
1o
1 ,si m
o
−a
1o
≤x ≤ m
o
+ a
1o
(m
o
+a
2o
)−x
a
2o
−a
1o
,si m
o
+ a
1o
≤x ≤ m
o
+ a
2o
0 ,si m
o
+ a
2o
< x
W
Ψ3
(x) = e
−
x
2
2k
2
Ψ
, k
Ψ
> 0 W
o3
(x) = e
−
(x−m
o
)
2
2k
2
o
, k
o
> 0
a) b)
Figure 2: Expressions of µ
Ψ
(column a) and µ
Φ
(column b) considered.
union of the pixels due to the similarity of their inten-
sity levels. The strength of relation Φ represents the
degree of the union due to the similarity of some spe-
cific characteristic.
So, every function which combines these two
affinity components, satisfying the theoretical restric-
tions described in (Saha et al., 2000), is a valid ex-
pression of g. The set of functions evaluated in the
presented study was:
µ
κ
= µ
α
√
µ
Ψ
µ
Φ
(4)
µ
κ
= µ
α
(ω
1
µ
Ψ
+ ω
2
µ
Φ
) (5)
µ
κ
= µ
α
((1−min(µ
Ψ
,
1
2
µ
Φ
))µ
Φ
+ min(µ
Ψ
,
1
2
µ
Φ
)µ
Ψ
) (6)
2.1.1 Homogeneity-based Component
The homogeneity-based component between two pix-
els c and d can be modeled by |f(c) − f(d)|, which
indeed represents their unhomogeneity , and conse-
quently µ
Ψ
can be expressed as a function of that dif-
ference.
µ
Ψ
(c, d) = W
Ψ
(|f(c) − f(d)|) (7)
The evaluated functions, extracted from the fuzzy
subsets theory, are shown in figure 2, where x =
|f(c) − f(d)| in all the cases.
2.1.2 Object-feature-based Component
The feature considered is the intensity of the pixels.
The functions for modeling this component are the
homologous of the homogeneity-based case, and are
shown in figure 2, where x =
f(c)+ f(d)
2
for this com-
ponent description.
2.2 Segmentation Process
The complete segmentation of the jaw is carried out,
as shown in figure 3, applying FC on the cortical
bone (upper branch) and the mandibular canal (lower
branch). The trabecular core is obtained as the inner
zone of the cortical which is not considered canal.
As a result of this definition, the cortical bone
must be a closed structure, hence a morphological
processing is implemented to add the boundary to the
cortical. This process is shown in figure 4.
For the cortical processing, the seed is selected as
the pixel with maximum value in the distance matrix,
which is extracted from a previous coarse estimation
of the cortical.
The initial estimation of the cortical bone is ob-
tained by thresholding the region of interest (ROI),
FC-BASED SEGMENTATION OF JAW TISSUES
411
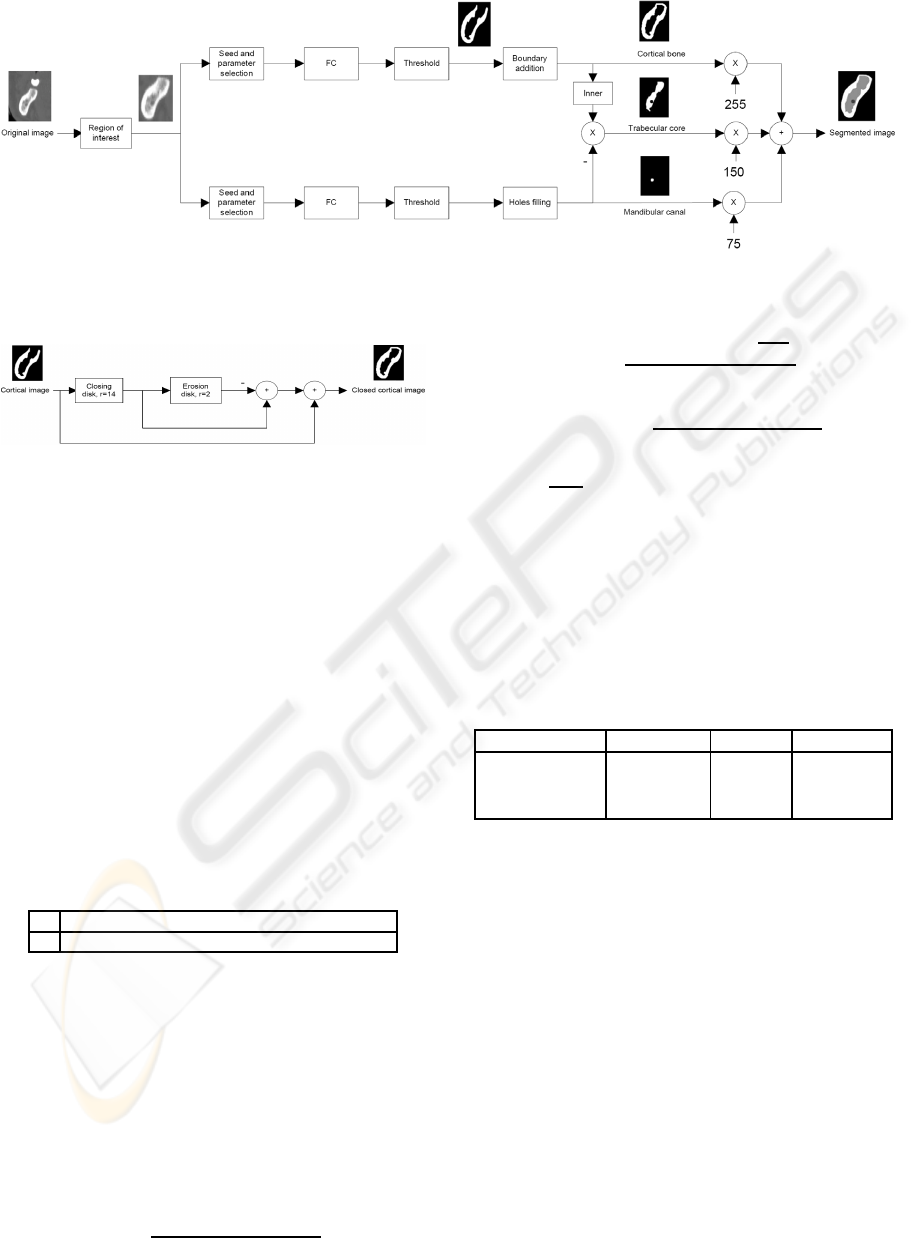
Figure 3: Segmentation process diagram.
Figure 4: Boundary addition diagram.
since it takes values saturated to 255, and is also used
to estimate the parameters needed for the FC segmen-
tation. For the canal processing, the seed is manually
selected and the parameters are estimated in a neigh-
borhood of it.
3 RESULTS
Defining g, µ
Ψ
and µ
Φ
it is possible to generate an
affinity family, for any given fuzzy relation κ, µ
κ
. Our
aim is to evaluate all the possible configurations ζ
lmn
to find the combination with the best performance for
the segmentation of jaw tissues.
l, m, n ∈[1, 2, 3] refer respectively to the three pos-
sible g, µ
Ψ
and µ
Φ
considered in section 2.1. The pa-
rameters used to define these components were
µ
Ψ
a
Ψ
= M
h
+tσ
h
, a
1Ψ
= 0, a
2Ψ
= M
h
+tσ
h
, k
Ψ
= M
h
+tσ
h
µ
Φ
m
o
= M
o
, a
o
= tσ
o
, a
1o
= 0 , a
2o
= tσ
o
, k
o
= tσ
o
where (M
o
,σ
o
) and (M
h
,σ
h
) are the mean and the
standard deviation of the intensities and the intensity
differences (respectively) of the defined cortical and
channel regions.
In all the cases, the results have been evaluated
by comparing the segmentation obtained with the
groundtruth set, consisting of 80 CT slices manually
segmented by a group of 5 experts. The resulting im-
ages have been evaluated using the detection and false
alarm probability and the merit factor defined as fol-
lows:
DP =
count(im
seg
AND im
gt
)
count(im
gt
)
(8)
FAP =
count(im
seg
AND im
gt
)
count(im
gt
)
(9)
MF = max(1−
count(im
seg
XOR im
gt
)
numpixels
) (10)
, where im
seg
refers to the segmented image, and
im
gt
and im
gt
refer to the groundtruth and inverted
groundtruth images, respectively. The process fol-
lowed can be summarized in fixing a possible function
g, and evaluating all combinations of µ
Ψ
and µ
Φ
. The
configurations with the best performance and their re-
spective merit factors are shown in table 1. It can be
deduced that the best configuration is ζ
323
, for both
the cortical and channel tissues.
Table 1: Best configurations comparison.
Combination function g Best configuration Cortical bone Mandibular canal
eq 4 ζ
123
96.185 99.717
eq 5 ζ
231
96.852 99.240
eq 6 ζ
323
96.962 99.739
Likewise, figure 5 shows the detection and false
alarm probability vs the threshold level used for bi-
narizing the connectivity map for the cortical bone
and mandibular canal. Finally, figure 6 shows the re-
sults obtained when the proposed segmentation pro-
cess was applied to some test set slices. The algorithm
processing time is approximately 1 second per image
of 153×180 pixel (using MATLAB on a Pentium IV
at 2.8 GHz and 1 GB of RAM).
4 DISCUSSION
In this paper a new segmentation method for den-
tal CT slices based on FC object extraction theory
and mathematical morphology has been presented.
For this purpose, all the possible combinations of the
BIOSIGNALS 2010 - International Conference on Bio-inspired Systems and Signal Processing
412

0 50 100 150 200 250 300
0
0.1
0.2
0.3
0.4
0.5
0.6
0.7
0.8
0.9
Threshold
Detection probability
Detection probability in cortical bone
ζ
231
ζ
323
ζ
123
0 50 100 150 200 250 300
0
0.02
0.04
0.06
0.08
0.1
0.12
Threshold
False alarm probability
False alarm probability in cortical bone
ζ
123
ζ
323
ζ
231
a) b)
0 50 100 150 200 250 300
0
0.1
0.2
0.3
0.4
0.5
0.6
0.7
0.8
0.9
Threshold
Detection probability
Detection probability in the mandibular canal
ζ
231
ζ
323
ζ
123
0 50 100 150 200 250 300
0
0.02
0.04
0.06
0.08
0.1
0.12
Threshold
False alarm probability
False alarm probability in the mandibular canal
ζ
123
ζ
323
ζ
231
c) d)
Figure 5: a) and b): Detection and false alarm probability
in the cortical bone. c) and d): Detection and false alarm
probability in the mandibular canal.
functions under study have been exhaustively evalu-
ated and the best configuration has been used for seg-
menting the tissues of the jaw in 80 dental CT slices,
achieving great accuracy in both cortical and canal
cases with merit factors of 96.962 and 99.739, respec-
tively. The trabecular core was also successfully ob-
tained as the inside of the cortical which is not con-
sidered canal. Furthermore, the presented method has
a very low computational cost, which makes it suit-
able for our overall purpose of segmenting and recon-
structing the whole jaw. Future research will focus on
adapting the presented method to this end and on dy-
namically adjusting the FC parameters to each slice
processed.
REFERENCES
DeBruijne, M., Ginneken, B. V., Viergever, M. A., and
Niessen, W. (2003). Adapting active shape models for
3d segmentation of tubular structures in medical im-
ages. In Information Processing in Medical Imaging.
Springer.
F¨utterling, S., Klein, R., Straer, W., and Weber, H.
(1998). Automated finite element modeling of a hu-
man mandible with dental implants.
Galanis, C. C., Sfantsikopoulos, M. M., Koidis, P. T.,
Kafantaris, N. M., and Mpikos, P. G. (2007). Com-
puter methods for automating preoperative dental im-
plant planning: Implant positioning and size as-
signment. Computer Methods and Programs in
Biomedicine, 86(1):30–38.
Kang, H., Pinti, A., Vermeiren, L., Taleb-Ahmed, A., and
Zeng, X. (2007). An automatic fcm-based method for
Figure 6: Results obtained with the proposed method.
tissue classification. In The 1st International Confer-
ence on Bioinformatics and Biomedical Engineering,
pages 510–514. IEEE Computer Society.
Krˇsek, P., Krupa, P., and Cernochov, P. (2007). Teeth and
jaw 3d reconstruction in stomatology. In Medical
Information Visualisation - BioMedical Visualisation.
IEEE Computer Society.
Reiser, G. M., Manwaring, J. D., and Damoulis, P. D.
(2004). Clinical significance of the structural integrity
of the superior aspect of the mandibular canal. Journal
of Periodontology, 75(2):322–326.
Rueda, S., Gil, J. A., Pichery, R., and niz, M. A. (2006). Au-
tomatic segmentation of jaw tissues in ct using active
appearance models and semi-automatic landmarking.
In Medical Image Computing and Computer-Assisted
Intervention MICCAI 2006. Springer.
Saha, P. K., Udupa, J. K., and Odhner, D. (2000). Scale-
based fuzzy connected image segmentation: Theory,
FC-BASED SEGMENTATION OF JAW TISSUES
413

algorithms, and validation. Computer Vision and Im-
age Understanding, 77(2):145–174.
Udupa, J. and Samarasekera, S. (1996). Fuzzy connect-
edness and object definition: Theory, algorithms, and
applications in image segmentation. Graphical Mod-
els and Image Processing, 58(3):246–261.
Udupa, J. K., Odhner, D., Tian, J., Holland, G., and Axel, L.
(1997a). Automatic clutter-free volume rendering for
mr angiography using fuzzy connectedness. In SPIE
Proceedings Medical Imaging, 3034:111119.
Udupa, J. K., Tian, J., Hemmy, D., and Tessier, P.
(1997b). A pentium pc-based craniofacial 3d imag-
ing and analysis system. J. Craniofacial Surgery,
3031(138):333339.
Verstreken, K., Cleynenbreugel, J. V., Martens, K., Mar-
chal, G., van Steenberghe, D., and Suetens, P. (1998).
An image-guided planning system for endosseous oral
implants. IEEE Transactions on Medical Imaging,
17(5):842–852.
W. Stein, S. H. and Muhling, J. (1998). Tracing of thin tubu-
lar structures in computer tomographic data. Com-
puter Aided Surgery, 3(2):83–88.
BIOSIGNALS 2010 - International Conference on Bio-inspired Systems and Signal Processing
414
