
IMAGE SEGMENTATION TO EVALUATE
ISLETS OF LANGHERANS
C. Grimaudo, D. Tegolo, C. Valenti
Dipartimento di Matematica e Applicazioni, Universit`a di Palermo, via Archirafi 34, 90123, Italy
F. Bertuzzi
Istituto Mediterraneo per i Trapianti e Terapie ad Alta Specializzazione, via Tricomi 1, 90127, Italy
Keywords:
Islets of Langherans, implantation advisability, image segmentation.
Abstract:
This contribution deals with an unsupervised system to process digital photomicrographs in order to locate
and analyze islets of Langherans in human pancreases. The experiment has been conducted on real data and,
though we are still going to complete the evaluation of the whole method, we expect to define a set of proper
features (e.g. area, perimeter, fractal dimension, shape complexity, texture and entropy) useful for a fast and
reliable counting of healthy cells. In particular, this research aims to measure the advisability of a possible
implantation in patients affected by type 1 diabetes mellitus.
1 INTRODUCTION
This paper introduces a new system for the auto-
matic analysis of high power magnification photomi-
crographs of the human islets of Langherans. The
cells that make these clusters can be divided into a
few classes which include the α cells, that secrete
glucagon, and the β cells, responsible for the pro-
duction of insulin. This research field is of particular
interest because of the demand to evaluate the state
of these endocrine tissue for preoperative planning in
patients that suffer from severe type 1 diabetes melli-
tus, otherwise scarcely treatable by conventional ther-
apies (Ryan et al., 2005; Shapiro et al., 2006). It has
been verified that the probability of obtaining a fa-
vorable implantation increases when a large number
of viable and purified islets is transplanted in to the
patients (Bertuzzi and Ricordi, 2007). In a multivari-
ate analysis aimed to identify some in vitro parame-
ters for islet quality or function predictive of in vivo
graft function of the same islets after their transplan-
tation in diabetic patients, islet morphology (in terms
of the maintenance of their round shape profile, sim-
ilar to what they showed in the native pancreas) was
demonstrated to be correlated with 1 month recipi-
ent c-peptide production (Ricordi et al., 2001); islet
morphology therefore should be considered an indi-
rect parameter of islet viability. These results call
for the identification of some standardized strategies
to characterize islet morphology and to quantify their
degree of maintenance of their native round morphol-
ogy (Nano et al., 2005).
At present, the analysis is also performed by im-
proving the appearance through image processing
softwares or ad hoc systems (Metamorph). A grid is
laid on the slide so to fix the islets and to let easily
count their different typologies (see Figure 1). This
process is done by hand to separate those cells useful
to the implantation and obviously it is slow, subjec-
tive and liable to errors; an environment to help the
expert analyst is therefore desirable both to enhance
the quality of the digital photos and to elaborate the
images in order to locate automatically the zones of
interest.
A variety of methods is already present in liter-
ature for both supervised and unsupervised segmen-
tation of photomicrographs depicting cells (Coelho
et al., 2002; Tripodo et al., 2006; Montseny et al.,
2004; Bak et al., 2004). Usually these techniques
are taken back to the elaboration of histograms, appli-
cation of mathematical morphology, texture analysis,
Fourier and wavelet transforms to extract the shapes
of the components that have been found. Often the
images have noise due to the presence of small arti-
facts, distortions and blurring introduced by the op-
tical system, inherent inaccuracies due to the lattice
72
Grimaudo C., Tegolo D., Valenti C. and Bertuzzi F. (2008).
IMAGE SEGMENTATION TO EVALUATE ISLETS OF LANGHERANS.
In Proceedings of the First International Conference on Bio-inspired Systems and Signal Processing, pages 72-76
DOI: 10.5220/0001056800720076
Copyright
c
SciTePress
(e.g. the thickness of the sample that must be ana-
lyzed), imperfections of the coloring of the contrast
agent (e.g. due to variations of exposure time and to
the quantity of the marker itself).
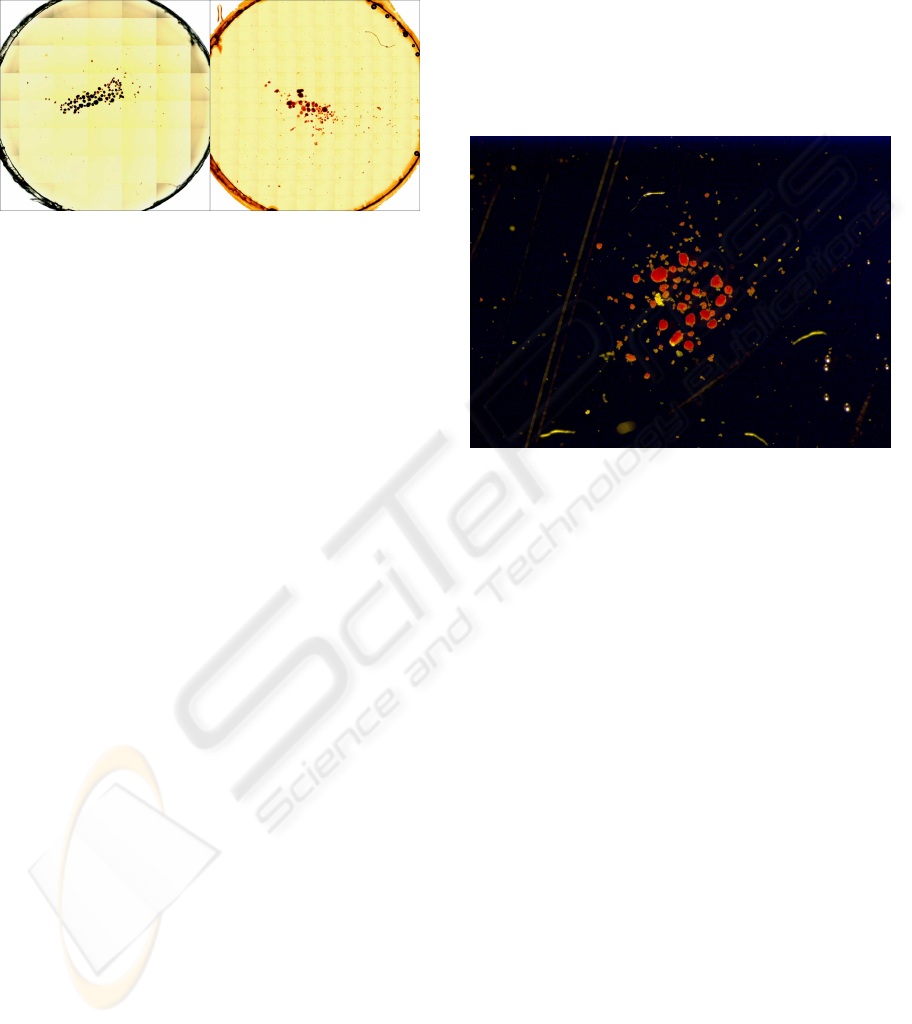
Figure 1: Two slides that put in evidence the presence of a
grid to easily count the number of the islets.
We have realized a system that acts through a li-
brary of tools to preprocess the data; the segmenta-
tion of the various components in the images often re-
quires the intervention of an expert user who locates
the promising clusters of cells. This approach can be
applied not only to the islets of Langherans, but also
to analyze other vital cells (e.g. hepatocytes, bone
marrow cells). Finally it should be verified the pos-
sibility to apply this strategy also in fixed tissue after
different immunohystochemistry staining.
The following Section 2 describes the new en-
vironment to elaborate and classify the islets of
Langherans. Experimental results are presented in
Section 3, while remarks and possible future works
are introduced in Section 4.
2 SEGMENTATION OF THE
ISLETS
In this paper, we aimed to describe the system which
has been developed to provide an unsupervised anal-
ysis of the human islets of Langherans (see Figure 2).
Different techniques have been implemented to en-
hance the quality of the images, to segment all com-
ponents, to distinguish among the cells and to evalu-
ate their conditions in order to quantify the advisabil-
ity of the implantation.
The photos in our database have been acquired
through a digital tool; they suffer from artifacts due
to the equipment (e.g. only the center of the image is
correctly in focus and a few impurities can be present
on the lenses). Predetermined threshold values result
in a poor separation between the components of the
images, but we have experimentally verified that the
Otsu method (Otsu, 1979) is able to compute these
optimal values in order to locate the imperfections on
the red and green channels of the RGB color space.
We have carried out a statistical examination on both
the background and foreground to determine their
starting threshold values; should the input image be
very different from the database we have considered,
then, to better calibrate the values, the user can select
some regions of interest, representative of the differ-
ent parts of the islets. Figure 3 shows the previous
input image soon after the preprocessing step.
Figure 2: A sample photomicrograph of a cluster of the hu-
man islets of Langherans.
We have successfully applied the same adap-
tive self-tuning technique that has been introduced
in (Tripodo et al., 2006) to discriminate between the
pure β cells, or the mixed β and exocrine/ductal cells
that are highlighted by the marker as red and orange
zones respectively, while the yellow parts correspond
to dead cells or impurities or simply exocrine/ductal
cells. This usually leads to a rough representation of
the cells, but a simple median filter is sufficient to
remove all small objects (5×5 kernel) and pointlike
noise (3×3 kernel). The shape of the cells so far ob-
tained can be further enhanced by the use of a math-
ematical morphology opening with a structuring ele-
ment represented by discrete disk of radius 2 (Soille,
2003). In such a way the cells of the islets are bet-
ter separated and, moreover, we can safely delete all
components that are too small (the allowed number of
pixels has been pre-defined according to the present
magnification power of the microscope). Figure 4
shows the final result obtained on the reference im-
age; another example is reported in Figure 5. We have
highlighted the final contour just to easily check the
segmentation of the relevant islets.
IMAGE SEGMENTATION TO EVALUATE ISLETS OF LANGHERANS
73
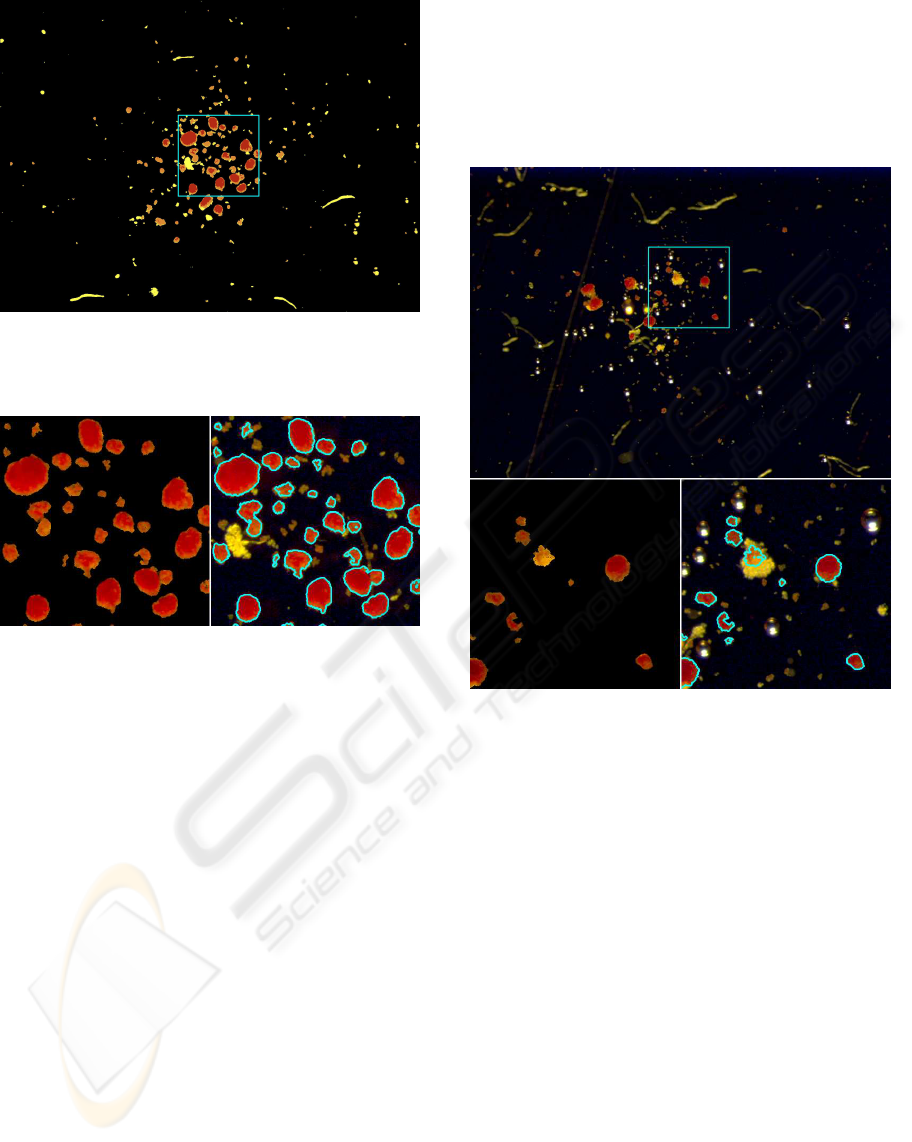
Figure 3: Some artifacts present in Figure 2 have been re-
moved. Due to the huge field of view, in the following we
will propose the results relative to the superimposed box.
Figure 4: Left: the remaining artifacts and dead cells have
been automatically removed from Figure 3. Right: the final
contour has been plotted on the input image of Figure 2.
3 EXPERIMENTAL RESULTS
Images have a size of 2088×1550 pixels and were ac-
quired at a sample dilution equal to 2500×, by a stere-
omicroscope Leica MZ12-5 with a 2× zoom magni-
fication and equipped with a digital camera, able of
a 4.34765µm/pixel picture calibration. The set of im-
ages we have studied has been obtained by isolating
the islets through the automated method from multi-
organ donors (Ricordi et al., 1989). After pancreas
digestion the islets from 3 preparations have been pu-
rified by COBE processor (Vargas et al., 1996) and
placed in a culture media for additional 48 hours at
24
◦
C. The islets have been finally stained with dithi-
zone (a vital stain that cross-reacts with zinc) and
therefore it has been used to recognize the α, exocrine
and ductal cells (in which zinc is absent) from the β
cells (rich in zinc).
A set of parameters that describe each kind of
cluster of cells has been extracted from the segmented
images. The area, the perimeter, the compactness (i.e.
the normalized ratio between the area and the squared
perimeter) and the eccentricity of the ellipse which
approximates the shape of the islet and the measures
of convexity/concavity of its edges return a quanti-
tative esteem of its aspect. In particular, compactness
and eccentricity measure the roundness: healthy islets
should not have protrusions.
Figure 5: The edges of the islets within the box have been
marked in blue.
The amount of information directly deducible
from the luminosity of the pixels is another useful
characteristic: the more homogenous an islet is, the
smaller its local entropy is. We are still investigat-
ing on the ability of operators that return marks about
the value of local sharpness and textures (which are
closely connected to the presence of luminosity gra-
dients).
For each islet I
i
we compute the product g
i
be-
tween its average luminosity ℓ
i
and its entropy e
i
.
If we indicate with µ
g
and σ
g
respectively the mean
and the standard deviation of all g = ℓ×e, then the
islets with a score |g
i
−µ
g
| < 2σ
g
can be considered
as promising candidate. A further important charac-
teristic is given by the compactness κ (Rangayyan,
2005): with an analogous approach, the islets till now
accepted with a compactness value κ
i
< µ
κ
+σ
κ
are
definitely classified as reasonably good. For the sake
of clarity, an islet is classified as good if it passes the
test on g and then on κ. The final evaluation of the
whole input photo of Figure 5 is summarized in Ta-
BIOSIGNALS 2008 - International Conference on Bio-inspired Systems and Signal Processing
74
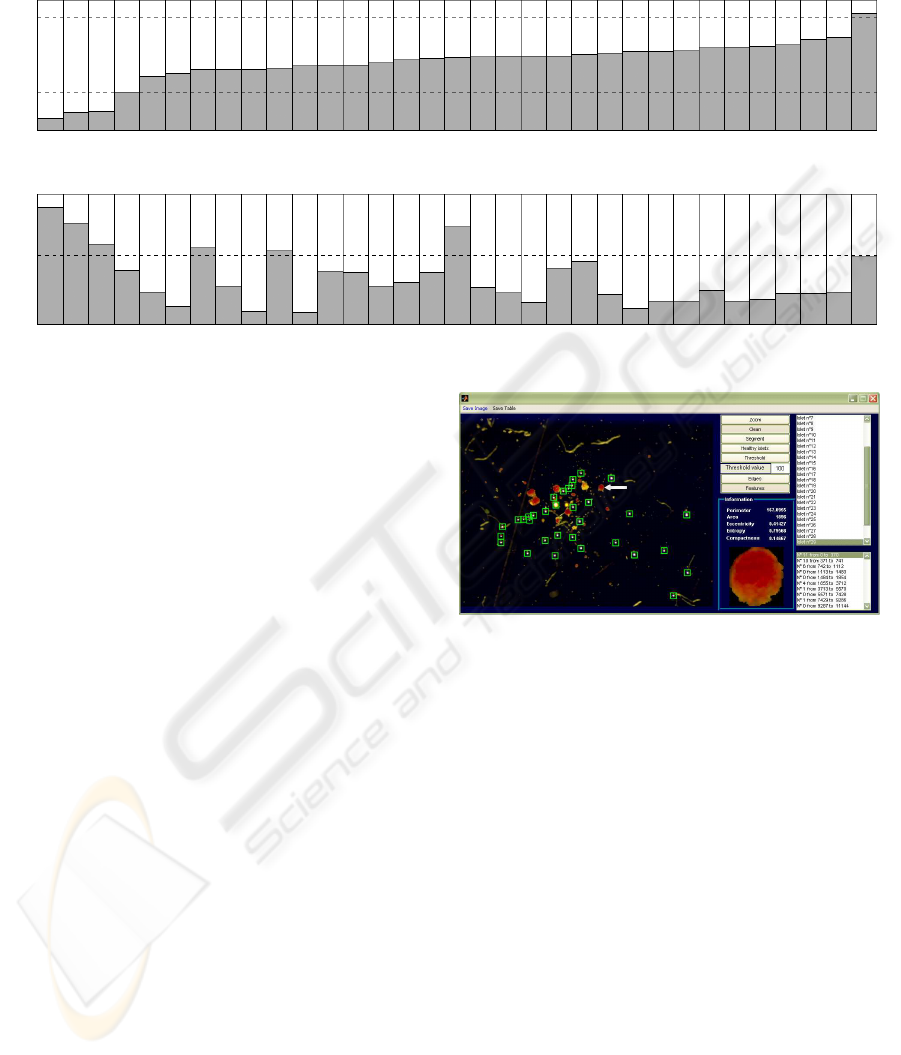
Table 1: The features of each islet (33 in this example) have been represented by two columns (top: g and bottom: κ). The
threshold values are represented by dashed lines and both tests have to be passed: κ reduces the number of candidates already
obtained by g (good islets have been marked with a H).
1 2
3
4
5 6 7 8 9 10
11 12
13
14
15 16
17
18 19 20
21 22
23
24
25 26
27
28 29 30 31 32 33
µ
g
+2σ
g
µ
g
−2σ
g
27.2
32.5
33.1
49.2
64.5
67.1
70.4
70.5
70.8
72.0
73.7
74.6
74.7
76.9
79.6
80.6
81.5
81.8
82.7
82.8
82.8
84.0
85.3
86.9
87.0
87.5
89.8
90.9
91.6
93.0
97.6
99.2
120.8
H H H H H H H H H H H H H H H H H H H H H H H H H H
µ
κ
+σ
κ
0.86
0.74
0.58
0.37
0.20
0.09
0.55
0.25
0.05
0.52
0.05
0.36
0.36
0.25
0.28
0.35
0.71
0.23
0.19
0.12
0.39
0.44
0.18
0.07
0.13
0.13
0.21
0.13
0.15
0.19
0.19
0.20
0.48
ble 1. Figure 6 shows how the system highlights a
single islet and proposes its features.
The percentage of the area of the yellow zones
(more precisely, the ratio between red and yellow)
indicates the purification of islet preparation and the
eventual presence of embedded islets, that means
islets surrounded by exocrine tissue (Ricordi et al.,
1995). The final ratio between the area of good islets
and the area of all islets summarizes the goodness of
the inspected photomicrograph. Several parameters
have been therefore available now by an automated
method of analysis for the characterization of an islet
preparation in terms of:
• islet number (the number of red clusters);
• islet dimension (the red area);
• islet purification (the ratio between yellow and red
areas in the whole preparation);
• percentage of embedded islets (the ratio between
red and yellow areas within an islet);
• islet morphology.
4 REMARKS AND FURTHER
WORKS
We have introduced an unsupervised system to locate
the human islets of Langherans in photomicrographs.
These clusters of cells have been characterized in or-
der to define some parameters representative of their
number and morphology. The predictive role of these
features towards their in vivo graft function should be
matter of further studies.
Figure 6: A screenshot of the graphical interface of the
system. Small green boxes automatically delimit bubbles
(present as artifacts in the photo). A selected islet is pointed
out by a white arrow and the values of the relevant features
are presented to the user.
From a computer science point of view, the effi-
ciency of the proposed method is still at the testing
stage (Altman, 1999) and our system should be con-
sidered as a tool to help the experts in obtaining a
quantitative esteem of the reliability of the islets in fa-
vorable implantation. The final results have been val-
idated by biologists involved in implantations to treat
patients affected by severe forms of type 1 diabetes
mellitus. It is interesting to note that the methodolo-
gies we have applied to segment the components of
the photos are quite standard and general enough and
that the extracted features can be extended to differ-
entiate between the α and β cells which compose the
islets; this is to correlate their peculiarities with infor-
mation of the state of the patients. Moreover, though
preliminary results are encouraging, we are improv-
ing the segmentation procedure by including further
algorithms based on mathematical morphology and
IMAGE SEGMENTATION TO EVALUATE ISLETS OF LANGHERANS
75

watershed/level sets.
To the best of our knowledge, our environment
is the first attempt to automatically analyze islets of
Langherans for implantations. Previous works rely
on manual segmentation of their photomicrographsor
are too general, thus to require to be adapted in or-
der to process images containing these kind of cells.
Therefore, a comparison of the results obtained by our
system is still desirable.
Additional projects should be the in vitro char-
acterization of the human islet preparations after the
staining with vital probes (i.e. propidium iodide, flu-
orescein diacetate (Barnett et al., 2004; Miyamoto
et al., 2000) and probes for apoptosis (Ichii et al.,
2005). This should allow the direct quantification of
vital, apoptotic and necrotic islets. Finally the auto-
mated system for imaging analysis should be applied
in fixed tissues after immunostaining for insulin and
glucagon thus allowing a complete characterization of
islet cell composition (Ichii et al., 2005; Street et al.,
2004).
ACKNOWLEDGEMENTS
The authors wish to thank Doctor Domenico Bosco
of the Hˆopitaux Universitaires de Gen`eve for useful
discussions and his kind contribution in providing the
input images.
REFERENCES
D.Altman (1999). Practical statistics for medical research.
Chapman & Hall/CRC.
E.Bak, K.Najarian, J.P.Brockway (2004). Efficient segmen-
tation framework of cell images in noise environ-
ments. Proceedings of the 26th Annual International
Conference of the Engineering in Medicine and Biol-
ogy Society, 3:1802–1805.
M.J.Barnett, D.McGhee-Wilson, A.M.Shapiro, J.R.Lakey
(2004). Variation in human islet viability based on
different membrane integrity stains. Cell Transplant,
13:481–488.
F.Bertuzzi, C.Ricordi(2007). Prediction of clinical outcome
in islet allotransplantation. Diabetes Care, 30:410–
417.
R.C.Coelho, V.Ges`u, G.Bosco, J.S.Tanaka, C.Valenti
(2002). Shape-based features for cat ganglion retinal
cells classification. Real-Time Imaging, Special Issue
on Imaging in Bioinformatics, 8:213–226.
H.Ichii, L.Inverardi, A.Pileggi, R.D.Molano, O.Cabrera,
A.Caicedo, S.Messinger, Y.Kuroda, P.O.Berggren,
C.Ricordi (2005). A novel method for the assess-
ment of cellular composition and beta-cell viability in
human islet preparations. Am J Transplant, 5:1635–
1645.
M.Miyamoto, Y.Morimoto, Y.Nozawa, A.N.Balamurugan,
B.Xu, (2000). Establishment of fluorescein diacetate
and ethidium bromide (fdaeb) assay for quality assess-
ment of isolated islets. Cell Transplant, 9:681–686.
E.Montseny, P.Sobrevilla, S.Romani (2004). A fuzzy ap-
proach to white blood cells segmentation in color bone
marrow images. Proceedings of the IEEE Interna-
tional Conference on Fuzzy Systems, 1:173–178.
R.Nano, B.Clissi, R.Melzi, G.Calori, P.Maffi,
B.Antonioli, S.Marzorati, L.Aldrighetti, M.Freschi,
T.Grochowiecki, C.Socci, A.Secchi, V.Carlo,
E.Bonifacio, F.Bertuzzi (2005). Islet isolation for
allotransplantation: variables associated with suc-
cessful islet yield and graft function. Diabetologia,
48:906–912.
N.Otsu (1979). A thresholding selection method from gray-
scale histogram. IEEE Trans. on System, Man, and
Cybernetics, 9:62–66.
R.M.Rangayyan (2005). Biomedical Image Analysis. CRC
Press.
C.Ricordi, P.E.Lacy, D.W.Scharp (1989). Automated islet
isolation from human pancreas diabetes. Diabetes,
38(1):140–142.
C.Ricordi, R.Alejandro, H.H.Rilo, P.B.Carroll, A.G.Tzakis,
T.E.Starzl, D.H.Mintz (1995). Long-term in vivo
function of human mantled islets obtained by incom-
plete pancreatic dissociation and purification. Trans-
plant Proc., 27(6):3382.
C.Ricordi, J.R.Lakey, B.J.Hering (2001). Challenges to-
ward standardization of islet isolation technology.
Transplant Proc., 33(1–2):1709.
E.A.Ryan, B.W.Paty, P.A.Senior, D.Bigam, E.Alfadhli,
N.M.Kneteman, J.R.Lakey, A.M.Shapiro (2005).
Five-year follow-up after clinical islet transplantation.
Diabetes, 54(7):2060–2069.
A.M.Shapiro, C.Ricordi, B.J.Hering (2006). International
trial of the edmonton protocol for islet transplan-
tation. The New England Journal of Medicine,
355(13):1318–1330.
P.Soille (2003). Morphological Image Analysis. Springer-
Verlag, New York, 2nd edition.
C.N.Street, J.R.Lakey, A.M.Shapiro, S.Imes, R.V.Rajotte,
E.A.Ryan, J.G.Lyon, T.Kin, J.Avila, T.Tsujimura,
G.S.Korbutt (2004). Islet graft assessment in the ed-
monton protocol: implications for predicting long-
term clinical outcome. Diabetes, 53:3107–3114.
The metamorph system,
c
molecular devices and
universal imaging corporation, WWW.UNIVERSAL-
IMAGING.COM.
C.Tripodo, C.Valenti, B.Ballar`o, Z.Rudzki, D.Tegolo,
V.Ges`u, A.M.Florena, V.Franco (2006). Megakary-
ocytic features useful for the diagnosis of myeloprolif-
erative disorders can be obtained by a novel unsuper-
vised software analysis. Histology and Histopathol-
ogy - Cellular and Molecular Biology, 21:813–821.
F.Vargas, M.Vives-Pi, N.Somoza (1996). Advantages of us-
ing a cell separator and metrizamide gradients for
human islet purification. Transplantation, 61:1562–
1566.
BIOSIGNALS 2008 - International Conference on Bio-inspired Systems and Signal Processing
76
