
Development of a DNA Biodosimeter for UV Radiation
Telma S. Marques
1,2
, Filipa Pires
1
, Gonçalo Magalhães-Mota
1
, Paulo A. Ribeiro
1
, Maria Raposo
1
and Nigel Mason
2
1
CEFITEC, Departamento de Física, Faculdade de Ciências e Tecnologia,
Universidade Nova de Lisboa, 2829-516 Caparica, Portugal
2
Faculty of Science, Technology, Engineering and Mathematics, The Open University,
Walton Hall, Kents Hill, Milton Keynes MK7 6AA, U.K.
Keywords: DNA Calf Thymus, UV Radiation, Biodosimeters.
Abstract: Ultraviolet (UV) radiation has a strong influence in the damage of deoxyribonucleic acid (DNA). In this work,
the possibility of a DNA UV radiation dosimeter is evaluated. For that, calf thymus DNA samples, thin films
and aqueous solutions, were irradiated with 254 nm wavelength light during different periods of time, being
the damage caused by the irradiation analysed by both UV-visible and infrared spectroscopies. As the DNA
is a polyelectrolyte, the pH of the DNA samples was also considered as a variable. Results demonstrated that
damage in DNA takes place in both thin films and solutions when irradiated at 254 nm, as revealed by a
consistent decay in measured absorbance values. However, DNA solutions were seen to give more reliable as
the induced damage is easily measured. For this case, the absorbance at 260 nm was seen to exponentially
decrease with the irradiation time as a result of radiation damage with the kinetics damage strongly dependent
of pH. Consequently, the lifetime of such dosimeter device can be chosen by changing the pH of aqueous
solutions.
1 INTRODUCTION
The use of radiation for medical procedures, in
particular for diagnostic and therapy purposes, has
dramatically increased over the years (Yu, 2017).
Mechanisms of justification of procedures and
management of the patient dose are employed to
avoid unnecessary or unproductive radiation
exposure in diagnostic and interventional procedures.
Dose constrains are appropriated to comforters and
carers, and volunteers in biomedical research but
regarding the therapeutic applications, it is not
considered appropriate to apply dose limits or dose
constraints, because such limits would often do more
harm than good (ENEA2012).
The effects induced on biological systems by
electromagnetic radiation are due to the energy
transfer into the medium with absorption of the
radiation (Bernhardt, 1992, Bronzino, 1995, Moulder,
2007), and are characterized by a series of events
which differ (and are classified) according to their
reaction time scale, leading ultimately to biological
damage (Bernhardt, 1992). These events can thus be
divided into three groups: 1) Physical –interactions
between the charged particles and the tissues atomic
structures, which leads to ionization and concomitant
formation of ionic radicals, in an extremely short time
frame (around 10
-18
s); 2) Chemical – formation of ion
pairs through an ionization process, which leads to
formation of free radicals and chemical bonds rupture
(around 10
-6
s); and 3) Biological – follows from bond
rupture and is characterized by altering the proper
physiology of cells or even cells death (Moulder,
2007); the time that biological damage takes place
after chemical bonds rupture is usually long, ranging
from a few hours to several days, weeks, months, or
even years.
When a cell is irradiated there are two types of
changes which can occur, directly on the cellular
component molecules or indirectly on water
molecules, causing water-derived radicals. Radicals
react with nearby molecules in a very short time,
resulting in breakage of chemical bonds or oxidation
of the affected molecules. The major effect in cells is
DNA breaks (Gomes, 2014, Fretelde, 1993, Su, 1994,
Xu, 1994, Storhatf, 1999, Podgorsak, 2006). Ionizing
radiation can also lead to structural changes in several
macromolecules present in cells. In nucleic acids,
changes are essentially loss or damage of bases,
thymine dimmers formation, single or double strand
328
Marques, T., Pires, F., Magalhães-Mota, G., Ribeiro, P., Raposo, M. and Mason, N.
Development of a DNA Biodosimeter for UV Radiation.
DOI: 10.5220/0006732003280333
In Proceedings of the 6th International Conference on Photonics, Optics and Laser Technology (PHOTOPTICS 2018), pages 328-333
ISBN: 978-989-758-286-8
Copyright © 2018 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

breaks and also DNA-protein dimmers formation
(Kielbassa, 1997, Ravanat, 2001).
DNA is featured an interesting anionic
polyelectrolyte having a unique double helix structure
(Fretelde, 1993) that can be used for many purposes.
For example, on the basis of hydrogen bonding
properties of DNA base pairs, oligonucleotide probes
have been recently designed to detect tumour gene
and various biosensors were also proposed (Caruso,
1999, Lvov, 1993). Also, DNA aqueous solutions are
of special interest, mainly in the development of
biological sensors (Su, 1994, Xu, 1994).
Moreover, the DNA sequence defines the genetic
information that commands the development of any
living being and its main vital functions (Wilkins,
1953, Franklin, 1953, Gomes, 2014). Since DNA
plays an important role in the maintenance of the
genetic information, any modification in this
macromolecule has significant effects at the cellular
level (Lindahl, 1993; Beckman and Ames, 1997).
Thus many efforts have been taken to delineate the
mechanisms of formation and the chemical structures
of the DNA modifications produced by genotoxic
compounds, including also ionizing (X, gamma,
heavy ions) and non-ionizing (ultraviolet (UV) and
visible light) radiations (Ravanat, 2016).
The effects of ionizing radiation on DNA have
been investigated in detail during the last three
decades but one of the most common environmental
health hazards that cause highly toxic effects is the
UV radiation (Kielbassa et al., 1997, Ravanat et al.,
2001, Yu and Lee, 2017). It should be referred here
that UV radiation is classified as UVA (315-400 nm),
UVB (290-315 nm), and UVC (280-100 nm). Most
UVC is absorbed by the ozone layer, and only UVA
and UVB compose ground level UV radiation
(Kalinnowski, 1999, Caruso, 1999). This is because,
firstly, certain biomolecules such as proteins and
nucleic acids have chromophores that absorb in the
UV region of the spectrum. Under high UV fluxes,
these molecules are photo-chemically degraded or
transformed, resulting in impairment or even
complete loss of biological function. The magnitude
of damage caused by these so-called direct or primary
mechanisms is determined by the amount of radiation
absorbed (absorbance cross-section) and the quantum
yield of photo-damage (molecules damaged per
photon absorbed).
One class of UV toxicity effects is caused by a
series of indirect mechanisms. UV is absorbed by
some intermediate compound (photosensitising
agent) either inside or outside the cell to produce
reactive oxygen species (ROS) (Vincent, 2000). The
resulting high energy oxidants such as hydrogen
peroxide, superoxide or hydroxyl radicals can then
diffuse and react with other cellular components with
sites of damage that can be well away from the site of
photo-production. Regarding genetic damage, nucleic
acid bases absorb maximally in the UVC range, with
peak absorbance around 260 nm, and exhibit a tail
that extends well into the UVB (Vincent, 2000). This
absorbed energy results in the first excited singlet
state, with a lifetime of only a few picoseconds. Most
of this energy is dissipated by radiation less processes
inside the molecule, but a small fraction is available
for a variety of chemical reactions. This can result in
the photo-damage of nucleotides (Vincent, 2000),
with a two- to four-fold greater effect on pyrimidines
(thymine and cytosine) relative to purines (adenine
and guanine). In addition, three principal
photoproducts are formed by the UV-induced
reactions: (a) 5,6-dipyrimidines, which are
cyclobutane-type dimers, generally referred to simply
as pyrimidine dimers; (b) photohydrates; and (c)
pyrimidine (6—4) pyrimidones, often referred to as
(6—4) photoproduct (Vincent, 2000). For example,
skin aging, eye damage, and skin cancer are some of
the most harmful effects known. This is because of
increased production of cellular reactive oxygen
species and by direct DNA damage, and if the DNA
damage is not properly repaired, will lead to
mutations and interferes with many cellular
mechanisms (e.g. replication, transcription, and the
cell cycle) (Yu, 2017).
If one intends to develop a device which allows
the measurement of light dose based on biological
materials, it should be chear that there are three kinds
of biologic markers: exposure (dose), effect and
susceptibility markers. Biologic markers of effect
record biologic responses in individuals who have
been exposed to a genotoxic agent, but markers of
dose do not necessarily indicate effects.
Superimposed on this are susceptibility markers;
those that could be used to identify persons who are
at increased risk of developing a disease that could be
triggered by a radiation exposure. Included here
might be organisms whose ability to repair DNA
damage is limited (National Research Council, 1995).
Biological dosimetry does not measure the
exposure in real time but the biological changes
induced by radiation. There are both indicators of
exposure or effects. Often the two aspects overlap as
in the case of deterministic effects induced by high-
doses, as for the acute radiation syndrome clinic that
is characterized by damages in skin, haematopoietic,
gastrointestinal, and cerebrovascular systems. In the
case of stochastic effects, induced by low doses, the
biomarkers used to measure the absorbed dose, not
Development of a DNA Biodosimeter for UV Radiation
329

always imply a clear detriment of health. It has been,
however, often demonstrated that an increase in the
frequency of these indicators is associated with an
increased risk of radiation-induced cancer and may be
indicative of radio-sensitivity (Giovanetti, 2012).
According to Giovanetti et al, 2012, for a
biodosimeter to be effective the following features are
determinant: 1) measurement on tissues or fluids
easily obtainable; 2) the effect must be specific of
radiation; 3) response should vary directly depending
on the dose; 4) it has to measure also chronic or
repeated exposure; 5) it must be possible to measure
retrospectively exposure also after years and 6) the
measurement must be simple, fast or automated.
A simple method of analyse the effect of UV
radiation on DNA is the measurement of AC
electrical conductivity of DNA thin films (Gomes,
2012). Such study revealed that electrical conduction
arises from DNA chain electron hopping between
base-pairs and phosphate groups being the hopping
distance a value of 3.38990.0002Å which coincides
with the distance between DNA base-pairs.
Moreover, the loss of conductivity of DNA samples
follow the decrease in phosphates groups with
irradiation time, suggesting the use of DNA based
films for UV radiation sensors (Gomes, 2012). Based
in these achievements, in this paper, a new biological
dosimeter based radiation-induced lesions in DNA is
proposed, where the damage caused by radiation is
obtained by UV-visible (UV-Vis) and infrared
spectroscopies and related to radiation exposure.
2 MATERIALS AND METHODS
Ultra-pure water and DNA hydrophilized in sodium
salt form (DNA sodium salt from calf thymus, CAS
73049-39-5, acquired from Fluka®) was used for the
preparation of DNA aqueous solutions. Its dissolution
is favoured by the presence of sodium ion (counter-
ion), allowing the preparation of aqueous solutions
with anionic character. The concentration of the DNA
solutions was 0.025 mg/mL DNA. The pH value of
the DNA aqueous solution was 6, these solutions are
also designated as natural solutions or pHN. In order
to obtain DNA solutions with pH=9 and pH=3, the
pH was adjusted to basic or acid with NaOH (1M) and
HCl (1M), respectively.
Cast films were obtained by the drop casting
method, i.e, depositing some drops of the DNA
aqueous solutions with different pHs onto calcium
fluoride (CaF
2
) solid supports. These samples were
placed in a desiccator during several hours to dry.
Solutions and cast films were irradiated for
different periods of time by means of a 254 nm UVC
germicide lamp, model TUV PL-L 55W/4P HF 1CT
from Philips
®
, at an irradiance of 1.9W/m
2
, in a
ventilated chamber at room conditions.
The DNA damage was monitored in aqueous
solutions by measurements of UV-Vis spectra after
each irradiation period in a spectrometer (UV
2101PC, Shimadzu
®
) while the thin films were
characterized with a Fourier transform infrared
(FTIR) spectrometer Thermo Scientific Nicolet-
model 530 (Waltham, MA, USA).
3 RESULTS AND DISCUSSION
According to Schuch et al (Schuch 2013), to develop
a reliable system for measure the UV light dose, one
have to search for material that would present the
most adequate features: (i) high transmittance to UVB
and UVA wavelengths; (ii) resistance to
environmental adversities; (iii) possibility of framing
the shape of the template according to the aim of the
experiment; and (iv) low cost. Having into account
such advices and the conclusions achieved by Gomes
et al (Gomes, 2012), it seemed that the use of DNA
thin films should be interesting for the development
of a UV dosimeter. Consequently, DNA cast films
deposited onto CaF
2
and quartz were prepared from
DNA aqueous solutions with pH 3, 6(N) and 9. These
films were irradiated with 254 nm UV radiation for
different periods of time and the UV-vis and infrared
spectra were measured for the different irradiation
times. As expected, in the absence of water, the
changes caused by radiation are minimal as can be
inferred from the infrared spectra of the DNA cast
films prepared from DNA aqueous solutions (pHN)
before and after UV irradiation for 15 h, displayed in
Figure 1. The observed peaks in the spectra are in
accordance with Gomes et al (Gomes, 2009) where
the infrared absorbance peaks were systematically
assigned to the respective DNA groups. Accordingly
the range of wavenumbers contained between 1250
and 900 cm
-1
are associated with the phosphate
backbone region while 1500–1250 cm
-1
and 1800–
1500 cm
-1
wavenumber regions are associated to
DNA bases vibrations influenced by the sugar
component and to DNA bases, respectively (Gomes,
2009).
Since UV radiation has effect on DNA phosphates
groups as demonstrated by Gomes et al (Gomes,
2015), the values of absorbance at 1097 cm
-1
,
assigned to the presence of symmetric
stretching
of backbone in the DNA molecules (Gomes, 2009),
were plotted in Figure 2 as a function of the
AOMatSens 2018 - Special Session in Advanced Optical Materials, Sensors and Devices
330
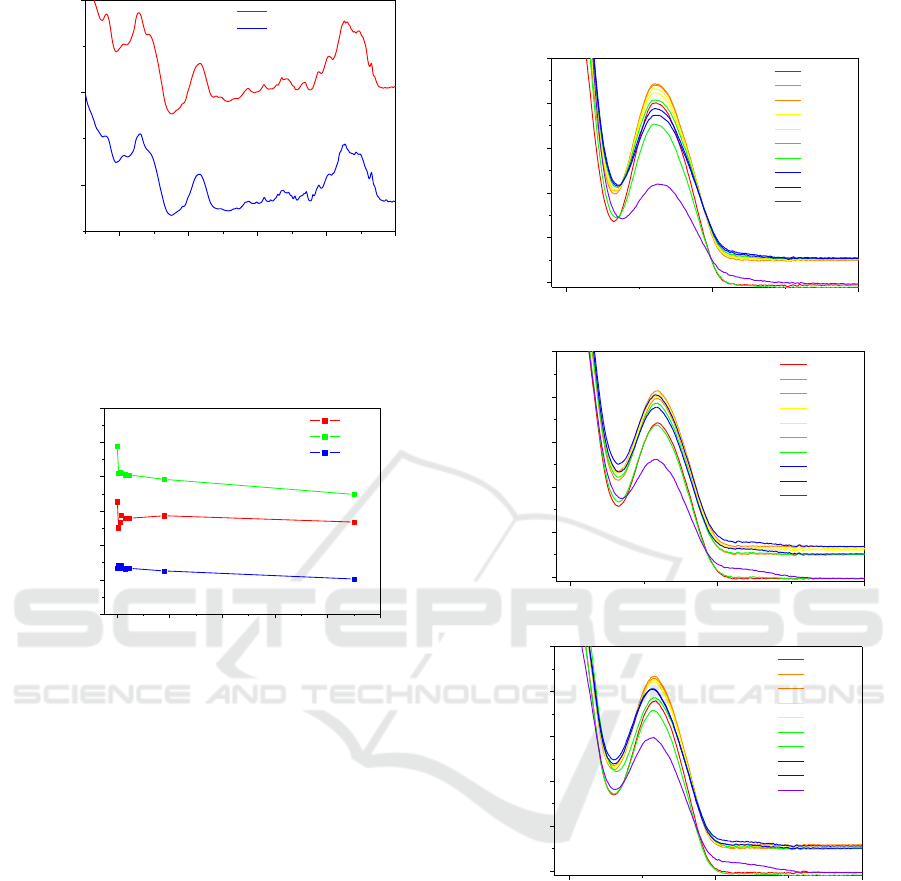
Figure 1: FTIR spectra of DNA casted films prepared from
solutions at natural pH (pH 6) conditions before and after
irradiation with UV-light at 254nm wavelength during 900
min (15 h).
Figure 2: Absorbance at 1097 cm
-1
after baseline
subtraction versus irradiation time for the different DNA
cast films prepared from aqueous solutions with different
pHs.
irradiation time for samples prepared from DNA
aqueous having different pH. Generally, an
absorbance decay is observed. However, these
measurements are always tricky due to baseline
fluctuations and also if the molecules concentration
seen by the beam is not identical–leading to
absorbance deviations. To circumvent this drawback,
the analysis of the effect of UV radiation at 254 nm
was carried out on DNA aqueous solutions prepared
at different pHs. Figures 3 a), b) and c) present the
UV-vis spectra obtained for the DNA aqueous
solutions with pH=3, pH=6 and pH=9, respectively,
irradiated during different periods of time. The
obtained results point out that the DNA solutions with
pH=3 (Figure 3 a) tend to be more sensitive to higher
times of UV light exposure since the absorbance at
260 nm for 900 minutes of irradiation was the lowest
value found for the different DNA solutions studied.
The baselines changes can be due to the light
scattering of smaller molecules, originated by the
cleavage of DNA molecule during the irradiation, as
demonstrated by Gomes et al, 2012.
Figure 3: Absorption spectra of DNA solutions with: a)
pH=3; b) pH=6 (natural) and c) pH=9; irradiated with 254
nm wavelength light for different periods of time.
The obtained results are in accordance with
literature as similar behaviours and patterns are
observed by Chen et al., 2009, where the disinfection
of water was studied and they present the effect of UV
radiation on the spores.
For a better comparison, the absorbance values at
260 nm, after removing the baseline (i.e. subtracting
the value of the absorbance at 350 nm), were
normalized, for each pH, and plotted as a function of
the irradiation time in figure 4. Several attempts have
1000 1200 1400 1600 1800
0.04
0.06
0.08
Absorbance
Wavenumber (cm
-1
)
0 (min)
900 (min)
0 200 400 600 800 1000
0,002
0,004
0,006
0,008
0,010
0,012
0,014
Absorbance
Irradiation Time (min)
pH=3
pH=N
pH=9
1097 cm
-1
200 300 400
0.0
0.1
0.2
0.3
0.4
0.5
Absorbance
Wavelenght (nm)
0 (min)
5 (min)
10 (min)
15 (min)
30 (min)
45 (min)
60 (min)
120 (min)
180 (min)
900 (min)
pH=3
200 300 400
0.0
0.1
0.2
0.3
0.4
0.5
Absorbance
Wavelenght (nm)
0 (min)
5 (min)
10 (min)
15 (min)
30 (min)
45 (min)
60 (min)
120 (min)
180 (min)
900 (min)
Natural pH
200 300 400
0.0
0.1
0.2
0.3
0.4
0.5
Absorbance
Wavelenght (nm)
0 (min)
5 (min)
10 (min)
15 (min)
30 (min)
45 (min)
60 (min)
120 (min)
180 (min)
900 (min)
pH=9
a)
b)
c)
Development of a DNA Biodosimeter for UV Radiation
331
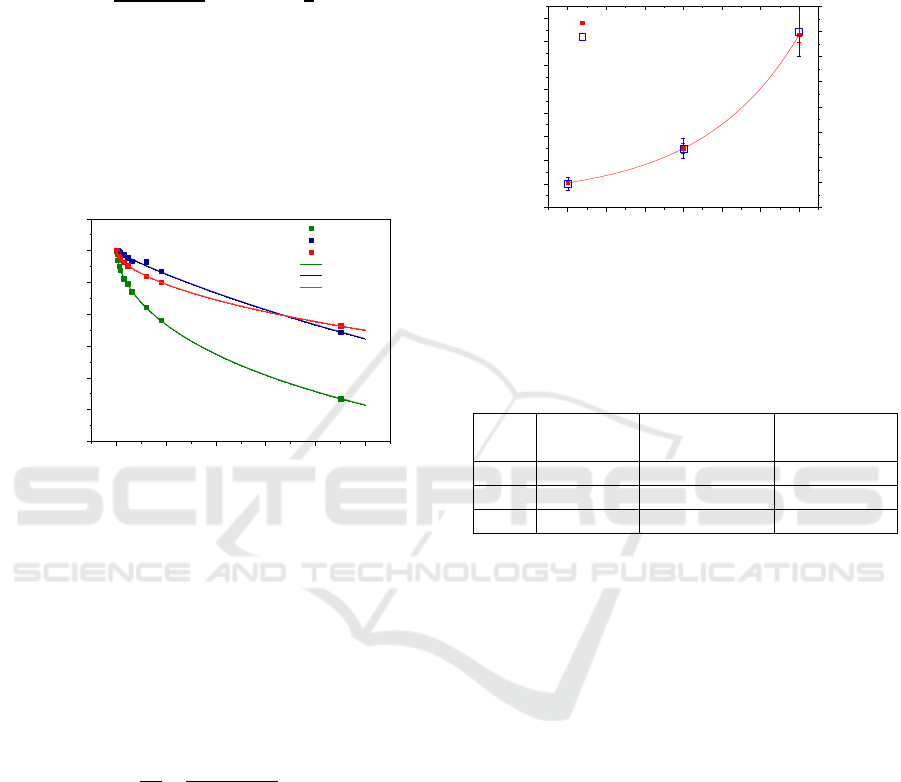
been done to find the best equation to model the
experimental data. The normalized experimental data
was found to be best fitted by an exponential
like expression as follows:
(1)
in which
is the absorbance at 260 nm,
corresponds to the initial (at the
beginning of the experiments) absorbance at 260 nm,
t the time in minutes, τ is the characteristic time or
time constant and n is a constant which can be related
with the order of the kinetics process (Raposo, 1997)
with respect to radiation damage.
Figure 4: Normalized absorbance at 260 nm after removing
the baseline versus irradiation time for the different
solutions. The lines correspond to the fitting with equation
(1).
Figure 5 shows the plot of the time constants in
minutes for each pH. The results show that DNA
solutions at higher pH (more basic) can be exposed to
UV light during more time. Moreover, from equation
(1) one can propose an expression for the dose level
to which the sample has been subjected, as follows:
(2)
in which D is the dose calculated by multiplying the
irradiance by the irradiation time, C
D
is the
characteristic dose constant and n is the order
parameter of the damage kinetics. These parameters
as well as the characteristic time constants are
presented in Table 1 for each pH investigated. From
the obtained results one can conclude that DNA
solutions can be suitable for the measurement of 254
nm wavelength light dose, being the lifetime of such
dosimeter device dependent of solution pH. To
develop a DNA based dosimeter device to cover also
UV A and UV B region, DNA damage has also to be
investigated in these UV regions. According with
previous results (Gomes, 2015), damage is expected
also take place with 300 nm wavelength light in such
a way that the same procedure described here should
be used to analyse the DNA damage when the
solutions are irradiated with higher wavelength light.
Figure 5: Time constant obtained by equation 1 versus pH
of the solutions irradiated and estimated dose for the
constant time for the solutions irradiated.
Table 1: Coefficients determined to each pH of DNA
solution irradiated.
pH
(min)
C
D
(Wm
-2
.min)
n
3
2050±40
3890±70
0.571±0.005
6
3500±200
6700±300
0.90±0.03
9
8300±300
15800±600
0.590±0.007
4 CONCLUSIONS
In this work it was demonstrated that aqueous DNA
solutions can be used to probe UV radiation at 254
nm and to evaluate the radiation dose at 254 nm,
through absorbance measurements. The absorbance
was seen to exponentially decrease with irradiation
time being the damage kinetics parameter dependent
of pH DNA aqueous solutions. This work also
evidenced that the lifetime of such DNA dosimeter
device can be chosen changing the pH of those
solutions. In the future we intent to 1) irradiate the
samples with a fixed wavelength of 300 nm in order
to check the new kinetics damage; 2) check if there is
a linear correspondence to the irradiation power; and
3) study the sensibility of the potential sensor.
ACKNOWLEDGEMENTS
The authors acknowledge the financial support from
FEDER, through Programa Operacional Factores de
0 200 400 600 800 1000
0.4
0.5
0.6
0.7
0.8
0.9
1.0
1.1
Normalized absorbance at 260 nm
Irradiation time (minutes)
pH3
pHN
pH9
Fitting (pH=3)
Fitting (pH=N)
Fitting (pH=9)
3 4 5 6 7 8 9
1000
2000
3000
4000
5000
6000
7000
8000
9000
Time constant
Dose at constant time
pH
Time constant (min)
2000
4000
6000
8000
10000
12000
14000
16000
18000
Dose (W/m
2
.min)
AOMatSens 2018 - Special Session in Advanced Optical Materials, Sensors and Devices
332

Competitividade—COMPETE and Fundação para a
Ciência e a Tecnologia—FCT, by the project
PTDC/FIS-NAN/0909/2014 and for the Portuguese
research Grants No. PEst-OE/FIS/UI0068/2011 and
UID/FIS/00068/2013. Telma Marques and Filipa
Pires acknowledge the fellowships
SFRH/BD/106032/2015 and PD/BD/106036/2015,
respectively from RABBIT Doctoral Programme
(RaBBiT, PD/00193/2012), Portugal.
REFERENCES
Bernhardt, J. H., 1992. Non-ionizing radiation safety:
Radiofrequency radiation, electric and magnetic fields.
Phys. Med. Biol. 37,807-844.
Bronzino, J.D., 1995. The biomedical engineering
handbook. CRC Press & IEEE Press. 2nd edition.
Caruso, F., Möhwald, H., 1999. Protein Multilayer
Formation on Colloids through a Stepwise Self-
Assembly Technique. Contribution from the Max
Planck Institute of Colloids and Interfaces, D-14424
Potsdam, Germany. J. Am. Chem. Soc., 1999, 121 (25),
6039–6046.
Chen, R.Z., Craik, S.A., Bolton, J.R. 2009. Comparison of
the action spectra and relative DNA absorbance spectra
of microorganisms: Information important for the
determination of germicidal fluence (UV dose) in an
ultraviolet disinfection of water. Water Research.
43(20):5087-96.
Franklin, R.E., Gosling, R.G., 1953. Evidence for 2-Chain
Helix in Crystalline Structure of Sodium
Deoxyribonucleate. Nature, 172, 156.
Giovanetti, A., Sgura, A., Aversa, G., 2012. Biological
Dosimetry - How to Measure the Absorbed Dose in
Different Scenarios. ENEA - Italian national agency for
new technologies, energy and sustainable economic
development. Rome. ISBN: 978-88-8286-264-0.
Gomes, P.J., 2014. Characterization of molecular damage
induced by UV photons and carbon ions on biomimetic
heterostructures. PhD Dissertation, FCT/UNL,
Portugal.
Gomes, P. J., Coelho, M., Dionísio, M., Ribeiro, P.A.,
Raposo, M., 2012. Probing radiation damage by
alternated current conductivity as a method to
characterize electron hopping conduction in DNA
molecules. Applied Physics Letters, 101, 123702.
Gomes, P.J., Ferraria, A. M., Botelho do Rego, A. M.,
Hoffmann, S.V., Ribeiro, P.A., Raposo, M., 2015.
Energy Thresholds of DNA Damage Induced by UV
Radiation: An XPS Study. The journal of Physical
Chemistry B, 119 (17) 5404- 5411.
Gomes, P.J., Ribeiro, P.A., Shaw, D., Mason, N.J. and
Raposo, M., 2009. UV degradation of deoxyribonucleic
acid. Polymer Degradation and Stability, 94(12) pp.
2134–2141.
Kielbassa, C., Roza, L., Epe, B., 1997. Wavelength
dependence of oxidative DNA damage induced by UV
and visible light. Carcinogenesis, 18, 811.
Lvov, Y., Decher, G., Sukhorukov, G., 1993. Assembly of
thin films by means of successive deposition of
alternate layers of DNA and poly(allylamine).
Macromolecules. 26 (20), pp 5396–5399.
Moulder, J., 2007. Power Lines, Cancer FAQ’s.
Electromagnetic Fields and Human Health. J.E. USA.
National Research Council, 1995. Chapter 6 - Biologic
Dosimetry and Biologic Markers: Growing public
concern about. Radiation Dose Reconstruction for
Epidemiologic Uses. Washington, DC: The National
Academies Press. ISBN: 978-0-309-05099-9.
Podgorsak, E.B., 2006. Radiation Physics for Medical
Physicist. Springer, Heidelberg. ISBN 978-3-642-
00875-7.
Raposo, M., Pontes, R.S., Mattoso, L.H.C., Oliveira
O.N.Jr., 1997. Kinetics of Adsorption of Poly (o-
methoxyaniline) Self-assembled films.
Macromolecules, 30, 6095-6101.
Ravanat, J.L., Douki, T., 2016., UV and ionizing radiations
induced DNA damage, differences and similarities.
Radiat. Phys. Chem.
https://doi.org/10.1016/j.radphyschem.2016.07.000.
Ravanat, J.L., Douki, T. and Cadet, J., 2001. Direct and
Indirect Effects of UV Radiation on DNA and Its
Components. Journal of Photochemistry and
Photobiology B: Biology, 63, 1-3, 88-102.
Schuch A.P., Garcia C.C., Makita K., Menck C.F., 2013.
DNA damage as a biological sensor for environmental
sunlight. Photochem. Photobiol. Sci., 2013, 12, 1259-
1272.
Su, H., Kallury, K.M.R., Thompson, M., Roach, A., 1994.
Interfacial Nucleic Acid Hybridization Studied by
Random Primer 32P Labeling and Liquid-Phase
Acoustic Network Analysis. Anal.Chem. 66, 769.
Vincent, W.F., Neale, P.J., 2000. 6 - Mechanisms of UV
damage to aquatic organisms. The Effects of UV
Radiation in the Marine Environment. Cambridge
University Press.
Wilkins, M.H., Stokes, A.R., Wilson, H.R., 1953.
Molecular Structure of Nucleic Acids: Molecular
Structure of Deoxypentose Nucleic Acids. Nature, 171,
738.
Xu, X.H., Yang, H.C., Mallouk, T.E., Bard, A.J., 1994.
Immobilization of DNA on an Aluminum (III)
Alkanebisphosphonate Thin Film with
Electrogenerated Chemiluminescent Detection.
J.Am.Chem.Soc. 116, 8386.
Yu, S.L., Lee, S.K., 2017. Ultraviolet radiation: DNA
damage, repair, and human disorders. Mol Cell Toxicol,
13:21-28.
Development of a DNA Biodosimeter for UV Radiation
333
