
Human Interface for a Neuroprothesis Remotely Control
Lucas M. Argentim
1
, Maria Claudia F. Castro
1
and Plinio A. Tomaz
2
1
Electrical Engineering Department, University Center FEI, Humberto de Alencar Castelo Branco,
Sao Bernardo do Campo, Brazil
2
Computer Science Department, University Center FEI, Humberto de Alencar Castelo Branco,
Sao Bernardo do Campo, Brazil
Keywords:
Electrical Stimulation, Electromyography, User Interface, Stroke, Hemiplegia.
Abstract:
Neuromuscular Electrical Stimulation (NMES) and Surface Electromyography (sEMG) have been widely ex-
plored by the scientific community for the rehabilitation of individuals with motor deficits due to stroke. The
literature shows the benefits of sEMG-activated NMES use in both motor rehabilitation and neural plasticity
stimulation. Currently, there is a strong tendency to expand the clinical environment, and the internet can be
used by healthcare professionals to do detailed follow-up and interact with their patients remotely. This work
presents a neuroprothesis activated by sEMG that allows configuration and monitoring of usage parameters re-
motely. Two control platforms were developed for different user profiles; health professionals (Web Interface)
and neuroprosthesis users (Smartphone Application).
1 INTRODUCTION
Stroke consists of a neurological deficit that occurs
when there is a lack of adequate blood flow in a par-
ticular brain region, either by obstruction (ischemic,
of 85% of the cases) or by rupture (hemorrhagic) of
the vessels resulting in a cerebral infarction (Sacco
et al., 2013). As a consequence, spasticity as a change
in skeletal muscle control, caused by an imbalance of
signals from the Central Nervous System to the nerve
endings of muscles, and a muscular hypertonia are
verified. As an example, the flexor pattern of the el-
bow, wrist and fingers is a commonly observed con-
dition in these individuals, which makes it impossible
to perform ordinary everyday tasks that use the move-
ment of these joints independently, such as combing
the hair, brushing teeth, typing in keyboard of a com-
puter, etc. (Carmo et al., 2015).
Approximately 15 million people worldwide have
a stroke each year; even where advanced technology
and facilities are available, 60% of those who suffer
a stroke die or become dependent, causing immediate
and devastating changes both in their lives and those
closest to them. Although the incidence of stroke
is declining in many developed countries, the abso-
lute number of strokes continues to increase because
of the ageing population. By 2050, the proportion
of the world’s population over 60 years is estimated
to be 22% reaching 2 billion, 80% living in low-
and middle-income countries (Mackay and Mensah,
2004a,b; WHO, 2015). It is important that such care
is taken to control, prevent and manage these events.
The techniques for rehabilitation of individuals
with motor deficiency, due to Stroke, are widely ex-
plored by the scientific community. The Neuromus-
cular Electrical Stimulation (NMES) is a widely used
technique in rehabilitation of individuals with mo-
tor dysfunctions. Over the years, works such those
developed by Kralj et al. (1993), Hara (2008), Shin
et al. (2008), Lin and Yan (2011), Hu et al. (2012),
Meadmore et al. (2013) and Hara (2013) have demon-
strated the effectiveness of this technique for reduce
the time needed to re-establish patients’ condition
from a stroke.
Kralj et al. (1993) showed that, since the 1970s,
a NMES have been applied as a therapeutic means
of effective treatment to improve the range of wrist
and finger extension motions and to prevent the con-
tractures caused by flexor spasticity. Hara (2008)
and Hara (2013) presented a home-based rehabilita-
tion program with power-assisted FES. However, the
system did not allowed configuration and monitoring
of usage parameters remotely by health professionals.
Instead, patients received training for equipment con-
figuration and electrode positioning and the exercise
protocol that they should accomplish. However, they
attained, using a multi-channel near-infrared spec-
troscopy (NIRS) study, a greater cerebral blood flow
Argentim, L., Castro, M. and Tomaz, P.
Human Interface for a Neuroprothesis Remotely Control.
DOI: 10.5220/0006719002470253
In Proceedings of the 11th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2018) - Volume 1: BIODEVICES, pages 247-253
ISBN: 978-989-758-277-6
Copyright © 2018 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
247

during an EMG-controlled NMES therapy. Shin et al.
(2008) showed, in a random experiment, that the use
of an EMG-triggered NMES to the wrist extensor
for two sessions (30 min/session) a day, five times
per week for 10 weeks could results in a signifi-
cant improvement of motor function and also in a re-
organization of the sensorimotor cortex. Lin and Yan
(2011) also applied NMES with sessions in the same
frequency, but for a period of only 3 weeks. They
observed not only significant improvement of motor
functions, but also the maintenance of it for at least 6
months. Promising results were also achieved by Hu
et al. (2012) using an EMG-driven electromechani-
cal robot system integrated with NMES and by Mead-
more et al. (2013) using a multi-channel NMES sys-
tem in goal-orientated tasks.
Other studies confirm that this practice not only
promotes functional recovery but also stimulates the
process of neurogenesis, that is, the sequence of
events leading to the formation of the nervous sys-
tem after traumatic events that may have damaged
it (Huang et al., 2015). The effects depends on the
severity of the impairments, the time since stroke, the
intensiveness of the NMES use and more than this the
chalenges for the Nervous System. However, there
is a trend towards the advantage of sEMG-triggered
NMES over cycling NMES, a better motor enhance-
ment due to task-oriented training, and patients re-
taining some degree of finger extension tend to shift
towards a focused activity in the ipsilesional corti-
cal site after NMES induced activity, whereas patients
who did not regain finger extension showed enhanced
involvement of the contralesional cortical site (Kem-
permann et al., 2000; Rushton, 2003; Schaechter,
2004; Shin et al., 2008; Quandt and Hummel, 2014).
In addition, a strong tendency to expand the clin-
ical setting for treatment of individuals may be noted
in recent years (Piron et al., 2004, 2009). The lit-
erature presents works such as Zhang et al. (2008)
and Buick et al. (2016) in which the Internet allows
healthcare professionals to make detailed follow-up
and interact with their patients remotely from their
homes.
Based on this context, this work presents a neu-
roprothesis activated by sEMG that allows configu-
ration and monitoring of usage parameters remotely.
Two control platforms were developed for different
user profiles; health professionals (Web Interface) and
neuroprosthesis users (Smartphone Application).
2 MATERIALS AND METHODS
2.1 Neurostim
Neurostim is a custom made neuroprothesis, based on
the application of Neuromuscular Electrical Stimula-
tion (NMES) with surface electrodes activated by sur-
face myoelectric signal (sEMG). Its use aims for the
rehabilitation of the upper limbs (hands and wrists)
of patients with hemiplegia due to stroke. Both
NMES and sEMG technologies are widely known,
widespread and used in the clinical area.
The differentials correspond to the proposal of
daily use in the home environment, and for this pur-
pose two control platforms was developed; one for the
user of the neuroprosthesis and another platform for
remote monitoring, allowing the health professional
to communicate with the device and to configure the
parameters of use, physiotherapy programs, and flow
monitoring.
2.2 Interface for the Neuroprosthesis
User
The user interface, in the form of a Android smart-
phone application was developed using Android Stu-
dio 2.3.3 is meant to be installed in Android versions
4.0 and superior. The application has the primary pur-
pose of configuring and controlling the neuroprosthe-
sis with the necessary instructions so that, with the use
of electric current, the extension of wrist and fingers
is promoted when the intent of movement by the user
is captured via sEMG.
As a secondary objective, the application should
be able to become a communication channel between
the user and the healthcare professional responsible
for the rehabilitation program so that, adjustments of
the parameters of use and information sending about
the usage can be done remotely through the internet.
Users who will be using the application have been
victims of a stroke. As a consequence, the motor
functions of one side of his body were affected, caus-
ing them to partially lose the movements of the wrist
and fingers. In the smartphone applications, users
must be able to:
• Use the neuroprosthesis simply and comfortably
• Use a smartphone application that has a simple
interface and allows navigation between features
with only one hand, which is possibly the non-
dominant hand.
Functionalities and tasks to be performed in the
application include:
BIODEVICES 2018 - 11th International Conference on Biomedical Electronics and Devices
248

• Log in to a profile that saves personal information
about your device’s usage Screen Layout
• Pair the neuroprosthesis with the application to be
able to communicate and exchange data
• Consult historical information on past uses of the
neuroprosthesis
• Choose mode of operation: rehabilitation pro-
gram or functional mode
• Setting the parameters of Intensity of stimulation
of neuroprosthesis
• Configure sEMG threshold parameters of neuro-
prosthesis activation
• Start the operation with exercises or daily activi-
ties
• Reconfigure the parameters, if necessary, during
use
• Send the data collected by the neuroprosthesis in
an automatic and transparent way to the health-
care professional responsible for their rehabilita-
tion program
• Receive updates of usage parameters from the
neuroprosthesis in an automatic and transparent
way, to be able to adapt the usage of the device ac-
cording to its evolutionary framework during the
rehabilitation program
All data must be stored locally, specially if the
smartphone does not have an internet connection
at the end of procedure and synchronization can
not happen at that time (will be trigger later on
whenever a stable connection is available).
2.3 Interface for the Health Professional
The interface for the health professional consists of
a web platform programmed in HTML5, PHP and
JavaScript to analyze and interact remotely with the
neuroprosthesis. The web interface should be able to
become a two-way communication instrument with
the neuroprotesis, promoting the necessary informa-
tion to configure its use, as well as an instrument of
interaction between the user and the health profes-
sional and data analysis. By synchronizing the cap-
tured neuroprosthesis data on the respective smart-
phones, the healthcare professional will get the de-
vice’s usage history information so progression anal-
ysis from a clinical standpoint can be done and re-
motely perform the necessary adjustments, as all the
data will be stored in a MySQL database.
The web interface must become a bi-directional
communication instrument with the neuroprosthesis,
promoting, through data packets, the necessary con-
figurations so that, with the use of electric current, the
extension of wrists and fingers can be promoted when
the intention of movement by the user is captured via
sEMG. The interface should also be able to become an
instrument of data analysis and interaction between
the user and the healthcare professional responsible
for the rehabilitation program so that, adjustments of
the parameters of use and the sending of information
about the use can be done remotely.
The interface should be able to configure the neu-
roprosthesis to the most diverse types of user char-
acteristics. For this, a database will store the pa-
rameters referring to each patient and their respec-
tive neuroprosthesis. When patients synchronize in-
formation with their smartphones, the healthcare pro-
fessional will get historical devices usage information
so that, analyzes of the clinical picture progression
can be made and the necessary adjustments are made
remotely.
Users who will use the web interface are health-
care professionals responsible for the rehabilitation
program for patients who have been victims of a
stroke. With the Web Interface, they must be able to:
• Log into a profile that has saved your personal in-
formation using the interface
With the users of the neuroprosthesis under
their responsibility registered
• Be able to register new users under your responsi-
bility
Name
Last name
Age
E-mail
Social Security Number
General address data
Contact
Patient ID
Rankin Scale score
Diagnosis
Responsible Health Professional ID
• Edit the existing user registry under their respon-
sibility
• Configure the neuroprosthesis remotely
Rise Time
Down time
Plateau Time
Activation threshold of sEMG
Intensity of the stimulus
Execution time or number of repetitions
Frequency of stimulus
Human Interface for a Neuroprothesis Remotely Control
249
• Analyze the usage data history sent from the
smartphone to the cloud database, shared between
both applications in a web interface
Dates of use
Time of use
Repetition count
Sampling of sEMG sensor readings
Time stamp of user parameter changes
• Set remotely usage schedule for configurable pa-
rameters
Relevant information can be printed and exported
from the web interface to further analysis on com-
plementary software, whenever is needed.
3 RESULTS
3.1 Neurostim
Neurostim uses an ATmega32u4 (Atmel Corporation)
microcontroller to produce symmetrical biphasic con-
stant current pulses with fixed frequency at 20 Hz,
two pulse width options 300 µs and 600 µs, and ad-
justable amplitude from 0 to 40 mA (resistive load of
1k Ω). These parameters have already been studied
by the scientific community and are considered to be
the most adequate for obtaining muscle contraction
patterns (Quandt and Hummel, 2014).
The stimulator is small in size and attached to an
armband where there are also the electrodes needed
for stimulation and sEMG, with the possibility of re-
location of position to allow customization and per-
sonalization according to the individualized motor re-
sponse. The stimulation electrodes are conductive
rubber.
The triggering of the stimulation depends on an
initial effort of the user captured by a sEMG device
called MyoWare (Advander Technologies LCC), de-
fined through the configuration of a threshold of the
myoelectric signal. Once the threshold is reached,
the stimulation is triggered, following an increase in
the amplitude of the graded stimulus to the config-
ured maximum (ramp modulation), remaining for the
pre-established time and then ceasing, also with the
gradual decrease of the stimulus. A new cycle will
only be started if the threshold of the sEMG signal is
again reached.
3.2 Interface for the Neuroprosthesis
User
Upon start, the application synchronizes all data avail-
able at the server side for the logged in user. All in-
formation regarding historical data and agenda of us-
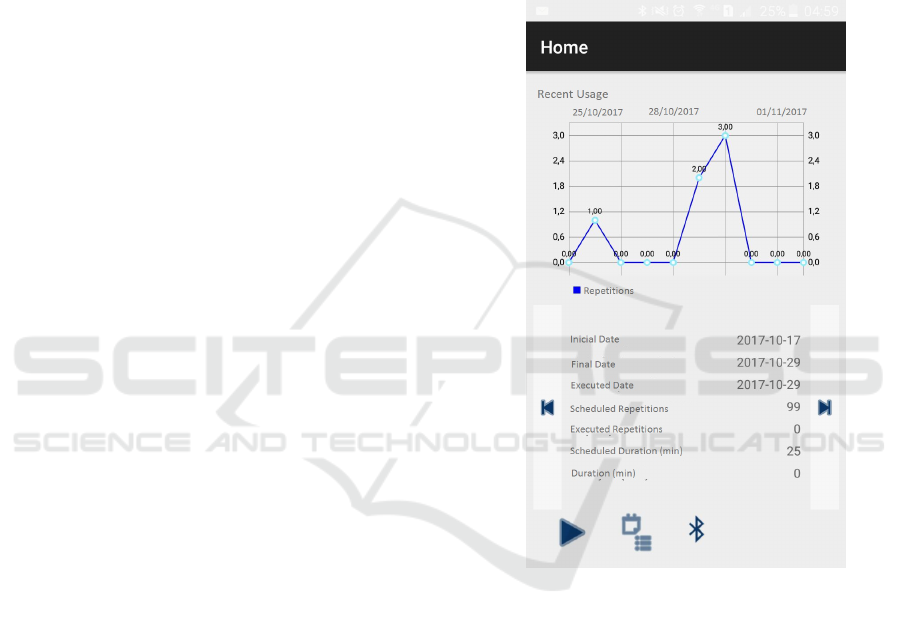
age will be available for consult, as shown in Figure
1. Depending on the setups made by the health pro-
fessional, the user will have the possibility to choose
between modes of operation, maximum intensity, etc.
Figure 1: Application screen showing historical usage data.
The communication with the smartphone applica-
tion is made through Bluetooth using the nRF51822
(Nordic Semiconductor) module, where the device
can receive and send information in real time accord-
ing to the setups made on the app (change of threshold
and intensity by the user), as shown in Figure 2.
3.3 Interface for the Health Professional
User can login with its credentials at the login page,
having access to the list of all the patients under its
responsibility as shown in Figure 3. Whenever is
needed, new patients can be added.
For each patient, the health professional can setup
an agenda containing the usage parameters accord-
ing to the rehabilitation program strategy, as shown
in Figure 4.
BIODEVICES 2018 - 11th International Conference on Biomedical Electronics and Devices
250
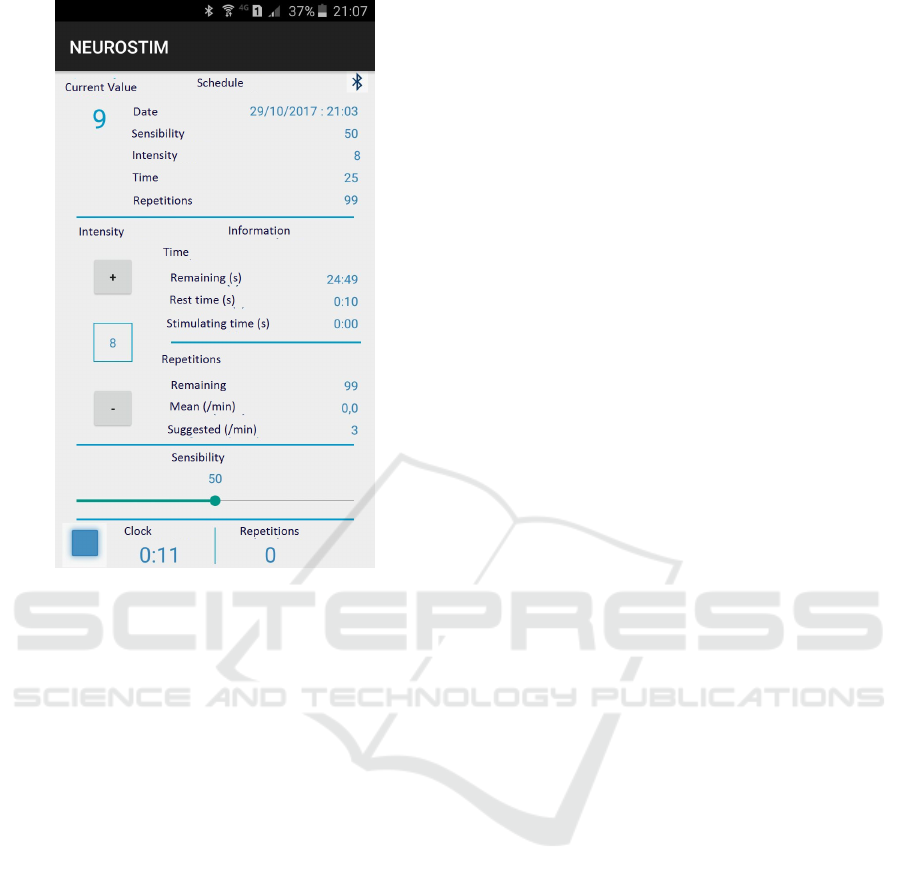
Figure 2: Application screen showing the control functions
and readings from the neuroprothesis.
Also, the health professional can visualize data
from all the procedures executed by each patient. In-
formation regarding sEMG and usage parameter will
be shown in a graph (Figure 5), along with a summary
with relevant information about the respective proce-
dure (Figure 6).
Relevant information can be printed and exported
from the web interface to further analysis on comple-
mentary software, whenever is needed.
3.4 Interfaces and System Evaluation
While awaiting the approval of the ethics commit-
tee to perform the usability and clinical performance
evaluation of the system, a pilot test was performed to
verify if users are able to use the interfaces and if the
data storage and communication are well performed.
Results were as expected, where an user was able to
successfully control the neuropothesis, as well as re-
trieve and send data to the server on the cloud contain-
ing usage setups and procedures informations auto-
matically. Additionaly, another user was able to suc-
cessfully setup remotely the neuropothesis, as well as
retrieve data containing usage setups and procedures
information automatically so further analysis and ad-
justments could be done without having the patient
physically present to do so.
4 CONCLUSION
The neuroprosthesis should be versatile enough for
the user to feel comfortable using it as a physiother-
apeutic element either in the rehabilitation program,
or in a functional way to assist in daily tasks. The
effectiveness of user interaction is a key to device ac-
ceptance.
The smartphone application was developed in a
way that simple interfaces allow the patient to per-
form all expected activities with ease, preventing er-
rors during the stimulation procedures and avoiding
non expected behaviors from the electric stimulation.
The web interface was also developed so the
health professionals can have significant information
about usage and perform analysis to execute eventual
adjustments to maximize effectivity to patients treat-
ment.
As the next steps usability and clinical evaluations
trials will be performed.
REFERENCES
Buick, A. R., Kowalczewski, J., Carson, R. G., and Proc-
hazka, A. (2016). Tele-supervised fes-assisted exer-
cise for hemiplegic upper limb. IEEE Transactions
on Neural Systems and Rehabilitation Engineering,
24(1):79–87.
Carmo, J. F., Morelato, R. L., Pinto, H. P., and Oliveira,
E. R. A. (2015). Disability after stroke: a systematic
review. Fisioterapia em Movimento, 28(2):407–418.
Hara, Y. (2008). Neurorehabilitation with new functional
electrical stimulation for hemiparetic upper extremity
in stroke patients. Journal of Nippon Medical School,
75(1):4–14.
Hara, Y. (2013). Rehabilitation with functional electrical
stimulation in stroke patients. International Journal
of Physical Medicine & Rehabilitation, 1:147.
Hu, X., Tong, K., Li, R., Xue, J., Ho, S., and Chen, P.
(2012). The effects of electromechanical wrist robot
assistive system with neuromuscular electrical stimu-
lation for stroke rehabilitation. Journal of Electromyo-
graphy and Kinesiology, 22:431–439.
Huang, Y., YeE Li, J. C., Zhou, H., and Tan, S. (2015). Elec-
trical stimulation elicits neural stem cells activation:
new perspectives in cns repair. Frontiers in human
neuroscience, 9.
Kempermann, G., van Praag, H., and Gage, F. H. (2000).
Activity-dependent regulation of neuronal plasticity
and self repair. Progress in brain research, 127:35–
48.
Kralj, A., Amovi
´
c, R., and Stani, U. (1993). Enhancement
of hemiplegic patient rehabilitation by means of func-
tional electrical stimulation. Prosthetics and Orthotics
International, 17(2):107–114.
Human Interface for a Neuroprothesis Remotely Control
251
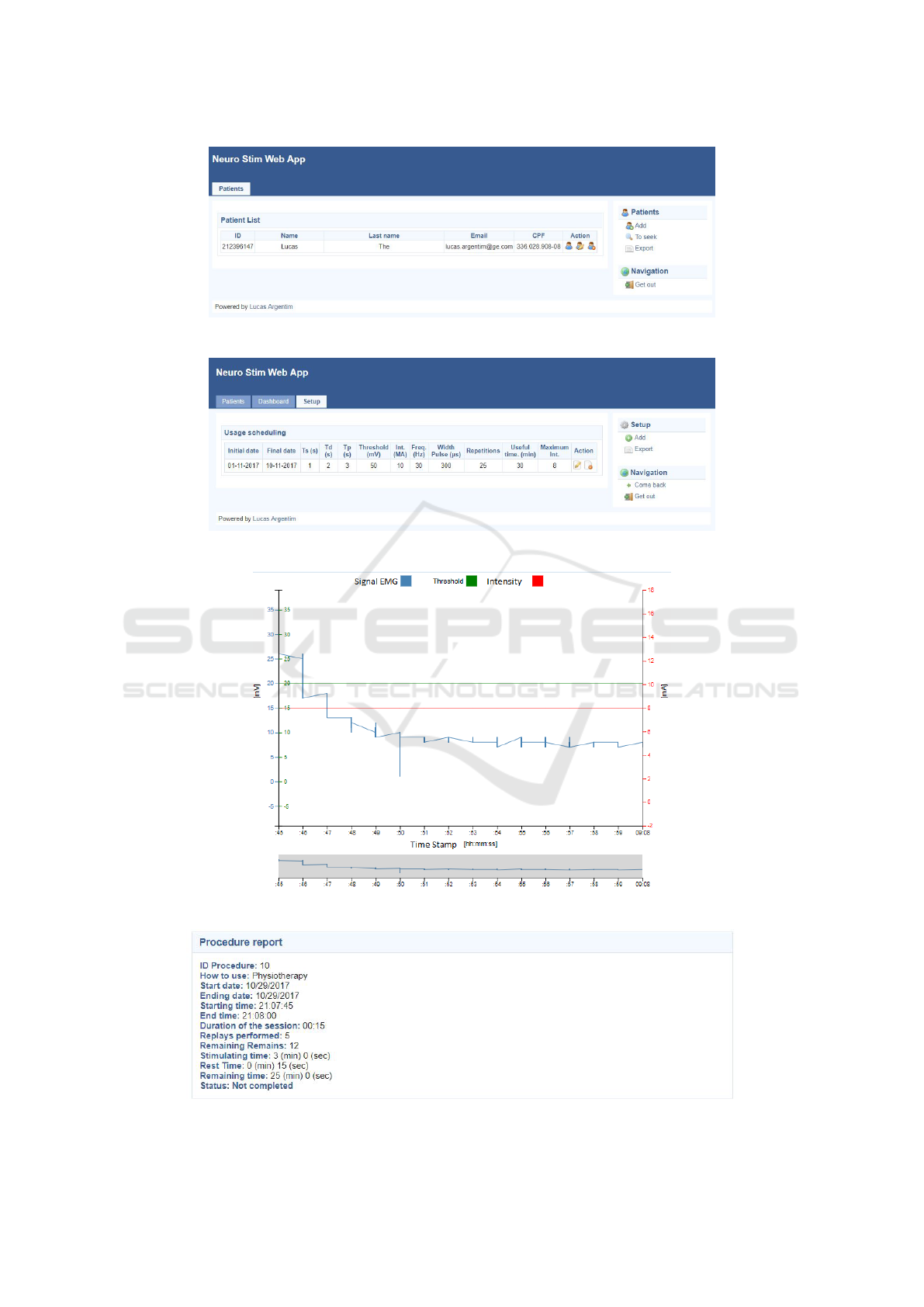
Figure 3: Web application screen showing list of Health Professionals patients.
Figure 4: Web application screen showing usage parameters agenda for a patient.
Figure 5: Web application screen showing sEMG and usage information of a procedure.
Figure 6: Web application screen showing summary information of a procedure.
BIODEVICES 2018 - 11th International Conference on Biomedical Electronics and Devices
252

Lin, Z. and Yan, T. (2011). Long-term effectiveness of neu-
romuscular electrical stimulation for promoting motor
recovery of the upper extremity after stroke. Journal
of rehabilitation medicine, 43(6):506–510.
Mackay, J. and Mensah, G. (2004a). Section 15: Global
burden of stroke. (Accessed on Oct/2017).
Mackay, J. and Mensah, G. (2004b). Section 16: Deaths
from stroke. (Accessed on Oct/2017).
Meadmore, K., Exell, T., Freeman, C., Kutlu, M., Rogers,
E., Hughes, A.-M., Hallewell, E., and Burridge, J.
(2013). Electrical stimulation and iterative learning
control for functional recovery in the upper limb post-
stroke. In 2013 IEEE International Conference on
Rehabilitation Robotics, Seattle, Washington USA.
IEEE.
Piron, L., Tonin, P., Trivello, E., Battistin, L., and Dam,
M. (2004). Motor tele-rehabilitation in post-stroke
patients. Medical informatics and the Internet in
medicine, 29(2):119–125.
Piron, L., Turolla, A., Agostini, M., Zucconi, C., Cortese,
F., Zampolini, M., Zannini, M., Dam, M., Ventura,
L., Battauz, M., et al. (2009). Exercises for paretic
upper limb after stroke: a combined virtual-reality
and telemedicine approach. Journal of Rehabilitation
Medicine, 41(12):1016–1020.
Quandt, F. and Hummel, F. C. (2014). The influence of
functional electrical stimulation on hand motor re-
covery in stroke patients: a review. Experimental &
Translational Stroke Medicine, 6:9.
Rushton, D. (2003). Functional electrical stimulation and
rehabilitationan hypothesis. Medical engineering &
physics, 25(1):75–78.
Sacco, R. L., Kasner, S. E., Broderick, J. P., Caplan, L. R.,
Connors, J. B., Culebras, A., Elkind, M. S., George,
M. G., Hamdan, A. D., Higashida, R. T., Hoh, B. L.,
Janis, L. S., Kase, C. S., Kleindorfer, D. O., Lee, J.-
M., Moseley, M. E., Peterson, E. D., Turan, T. N.,
Valderrama, A. L., and Vinters, H. V. (2013). An up-
dated definition of stroke for the 21st century. Stroke,
44(7):2064–2089.
Schaechter, J. D. (2004). Motor rehabilitation and brain
plasticity after hemiparetic stroke. Progress in Neuro-
biology, 73:61–72.
Shin, H. K., Cho, S. H., Jeon, H.-s., Lee, Y.-H., Song,
J. C., Jang, S. H., Lee, C.-H., and Kwon, Y. H.
(2008). Cortical effect and functional recovery by
the electromyography-triggered neuromuscular stim-
ulation in chronic stroke patients. Neuroscience let-
ters, 442(3):174–179.
WHO (2015). Ageing and health. (Accessed on Oct/2017).
Zhang, S., Hu, H., and Zhou, H. (2008). An interactive
internet-based system for tracking upper limb motion
in home-based rehabilitation. Medical & Biological
Eengineering & Computing, 46(3):241–249.
Human Interface for a Neuroprothesis Remotely Control
253
