
Monitoring Muscle Stem Cell Cultures with Impedance Spectroscopy
Yaiza Yuste
2
, Juan A. Serrano
1
, Alberto Olmo
1
, Andrés Maldonado-Jacobi
1
, Pablo Pérez
1
,
Gloria Huertas
1
, Sheila Pereira
2
, Fernando de la Portilla
2
and
Alberto Yúfera
1
1
Instituto de Microelectrónica de Sevilla (IMSE), Universidad de Sevilla, Av Americo Vespuccio, S/N, Sevilla, Spain
2
Instituto de Biomedicina de Sevilla (IBIS), Campus Hospital Universitario Virgen del Rocío, Sevilla, Spain
Keywords: Impedance Spectroscopy, Oscillation-Based Test (OBT), Skeletal Muscle, Myoblast, Stem Cell
Differentiation.
Abstract: The aim of this work is to present a new circuit for the real-time monitoring the processes of cellular growth
and differentiation of skeletal myoblast cell cultures. An impedance spectroscopy Oscillation-Based
technique is proposed for the test circuit, converting the biological system into a voltage oscillator, and
avoiding the use of very high performance circuitry or equipment. This technique proved to be successful in
the monitoring of cell cultures growth levels and could be useful for determining the degree of
differentiation achieved, of practical implications in tissue engineering.
1 INTRODUCTION
Impedance spectroscopy is currently used for real
time monitoring of different biological processes,
such as cell toxicity, cell invasion or inflammation
(Giaever and Keese, 1984; Daza et al., 2013; Pérez
et al., 2017). Impedance spectroscopy has the
advantage of being a non-invasive technique
(current intensity can be kept at minimum levels)
and is relatively non-expensive (only one sample or
petri plate is required for a performance curve).
Different circuits have been used for these
applications, using different topologies and
electrodes depending on the application.
The use of impedance spectroscopy in the
monitoring of the growth and differentiation of stem
cells is recently being studied for different tissue
engineering applications. Human mesenchymal stem
cells (hMSCs) development has been studied with
impedance spectroscopy in different works (Eun et
al., 2011; Hildebrandt et al., 2010). The impedance
spectra of osteogenic treated hMSCs reported a
significant increase of the magnitude of impedance
compared to controls cultivated in normal growth
medium (Hildebrandt et al., 2010). In this work, it is
concluded that impedance spectroscopy is an
appropriate method for non-invasive
characterization of osteogenic differentiation of
hMSCs, which is relevant for quality control of cell-
based implants and cell-based test systems for drug
development (Hildebrandt et al., 2010).
Other interesting and recently used stem cell
lines in tissue engineering are adipose stem cells
(Nordberg et al., 2017) or myoblasts (Liao and
Zhou, 2009). Skeletal muscle tissue engineering
holds great promise for regenerative medicine.
However, ex vivo cultivation methods typically
result in a low differentiation efficiency of stem cells
as well as graft that resemble the native tissues
morphologically, but lack contractile function.
In this work, a new circuit is proposed to apply
the impedance spectroscopy technique in myoblast
assays, to see whether this technique is useful in the
study of growth and differentiation of these cells
into muscular structures, in a similar way as it was
studied for others (Hildebrandt et al., 2010). The
circuit is based on the Oscillation-Based Test
technique, with amplitude and frequency values
obtained dependant on the cell culture
bioimpedance.
In section 2, the description of this circuit is
presented, together with the description of the initial
experiments performed.
96
Yuste, Y., Serrano, J., Olmo, A., Maldonado-Jacobi, A., Pérez, P., Huertas, G., Pereira, S., Portilla, F. and Yúfera, A.
Monitoring Muscle Stem Cell Cultures with Impedance Spectroscopy.
DOI: 10.5220/0006712300960099
In Proceedings of the 11th International Joint Conference on Biomedical Engineer ing Systems and Technologies (BIOSTEC 2018) - Volume 1: BIODEVICES, pages 96-99
ISBN: 978-989-758-277-6
Copyright © 2018 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
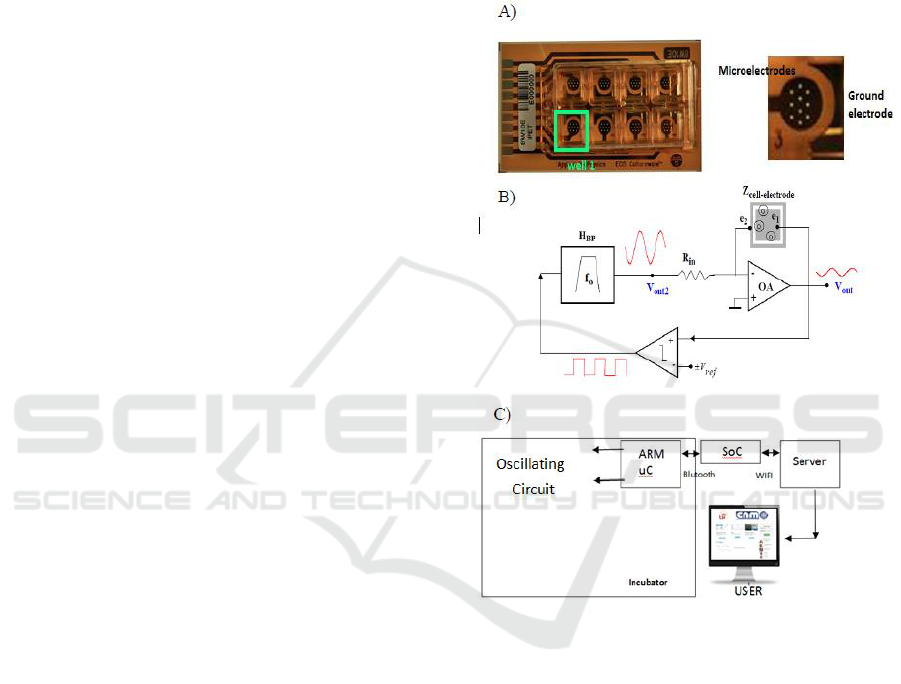
2 MATERIALS AND METHODS
2.1 Impedance Spectroscopy
Monitoring System
For cell culture assays, commercial electrodes
8W10E PET, from Applied Biophysics (Applied
Biophysics) were employed. The multi-well is
composed of eight separated wells; each one
contains ten circular biocompatible gold
microelectrodes of 250 µm diameter in parallel, and
a surrounding reference electrode.
The proposed circuit for bioimpedance
measurements is based on the well-known technique
of Oscillation-Based Test (Huertas et al., 2015). It
avoids the use of very high performance circuitry or
equipment, as well as accurate current/voltage
generators, Instrumentation Amplifiers (IA) and
exact precise demodulation circuits, converting the
bioimpedance in a voltage oscillator, whose
oscillation parameters (fosc, aosc) are dependent of
the biological sample under test. This circuit
performance is fully described in (Huertas et al.,
2015; Pérez et al., 2017). Simplified circuit diagram
is illustrated in Fig. 1C. Cell cultures are
incorporated to circuit analysis through the
electrode-cell impedance value Zcell-electrode. This
circuit works as a voltage oscillator, being
characterized by its oscillation parameters:
frequency of oscillation (fosc) and amplitude of
oscillation (aosc) at the output voltage signal (Vout).
The aim, in this application, is to characterize cell
growth and differentiation level through these
parameters. For the proposed circuit, has been
observed that the amplitude of oscillation is more
sensible to changes in bioimpedance (Huertas et al.,
2015). A limited maximum current intensity of 10
µA was applied to the cell cultures.
2.2 Cell Cultivation and Experiments
Performed
Rat skeletal myoblasts were obtained from Rattus
Norvegicus L6 cell line (ATCC® CRL-1458™) and
were cultured at 37˚C in a CO
2
incubator at 5% on
the Instituto de Biomedicina de Sevilla (IBIS). The
growth medium used was Minimum Essential
Medium α (12571-063, Gibco) supplemented with
10% fetal bovine serum (F7524, Sigma) and 1%
penicillin-streptomycin (15140-122, Gibco).
After the cells reached 85-90% of confluence,
they were sub-cultured using trypsin-EDTA at
0.05% (25300-062, Gibco) and seeded 10
4
cells in
the appropriate wells of the multi-well used (wells 2,
3, 4, 6, 7, 8) with growth medium. When the specific
wells reached 70% of confluence, after rinse with
phosphate buffered saline (L0615, Linus), the
medium was changed to differentiation medium,
MEMα supplemented with 2% horse serum (S0910,
Biowest) and 17.8mM NaHCO
3
(S6297, Sigma-
Aldrich). Microscope images were taken with the
Olympus IX-71 inverted phase microscope.
Figure 1: Impedance Spectroscopy monitoring system. A)
8W10E PET cultureware from Applied Biophysics (Pérez
et al., 2016) with 8 wells of 0.8 cm
2
. Detail of each well:
cells are measured on top of the 10 circular gold electrodes
in parallel, with 250 m diameter. The ground electrode
can be seen on the right. B) Simplified block diagram
proposed for measurements, composed by a bioimpedance
converter, comparator and bandpass filter. Oscillation
parameters: amplitude (a
osc
) and frequency (f
osc
) can be
measured over the signal V
out
. C) Communication circuit
used.
All cell cultures (wells 2, 3, 4, 6, 7, 8) were held
in growth medium for control the first days, starting
form 10.000 cells. The differentiation in myotubes
(wells 6, 7, 8) was initialized by treatment with
differentiation medium, whereas the rest were held
in growth medium for control (wells 2, 3, 4). Cell
culture growth medium and differentiation medium
were measured by the impedance system (wells 1,
Monitoring Muscle Stem Cell Cultures with Impedance Spectroscopy
97
5), in order to differentiate any possible effect of the
medium used. The medium was replaced every 2–3
days. Of the two group of cells, one well was left
without measuring impedance (wells 4, 8), as a
control, in order to detect any possible effect of
current intensity on stem cells. Table 1 summarizes
the wells used in the 8W10E PET cultureware.
Temperature and humidity values were also
monitored during all the experiment. In each
medium change, cells were seen under the
microscope, and photographs of all wells were
taken. Two experiments were performed, in order to
compare results.
3 RESULTS AND DISCUSSION
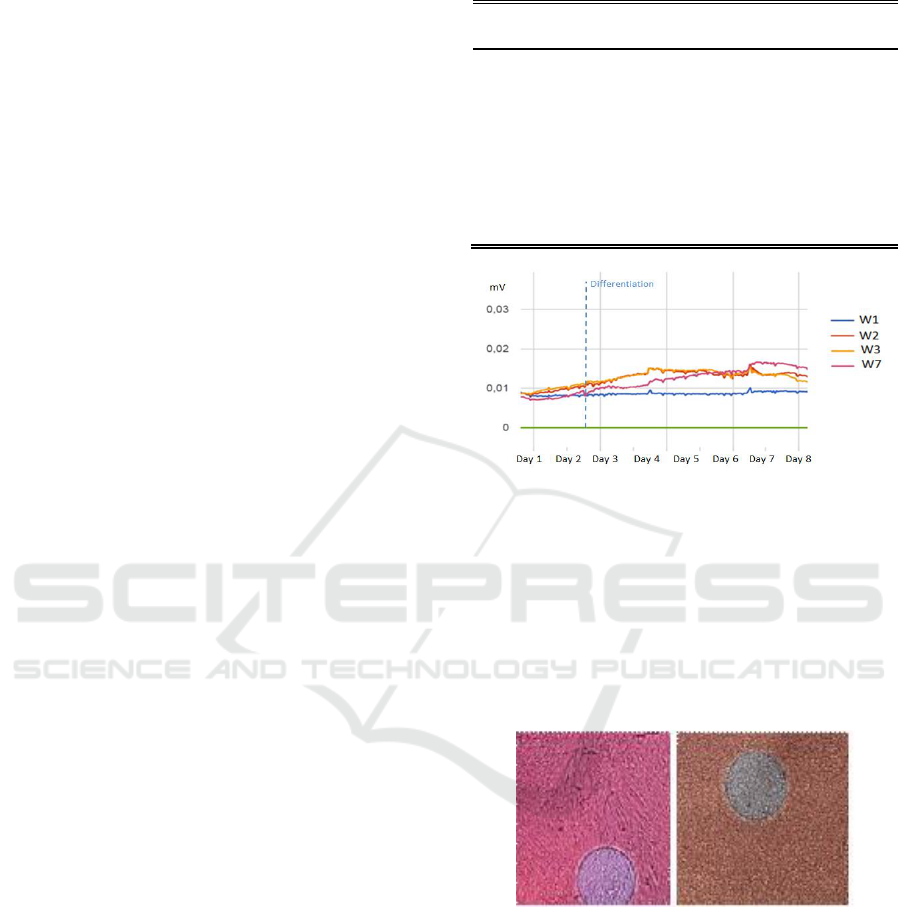
Similar results were obtained in both experiments.
Fig. 2 shows the signal registered for the different
cell cultures, corresponding to the amplitude of the
oscillations of the circuit (a
osc
), together with the
measurement of the temperature and humidity
values. The OBT circuit used successfully detected
the initial cell growth, in a similar way as in other
cell types (Daza et al., 2013; Pérez et al., 2017). The
amplitudes observed are useful parameters to
determine the confluence level of the cell culture or
fill factor (defined as the area occupied by cultured
cells divided by the total culture area). A practical
threshold could be set at a fill factor of 70%, as cells
should change to differentiation medium at this
moment. In Fig. 2 it can be seen the behaviour of
muscular stem cells without differentiation (wells 2,
3), following a similar behaviour to other cell
cultures (Eun et al., 2011; Hildebrandt et al., 2010),
in comparison with muscular stem cells that have
followed a differentiation process (well 7). Stem
cells cultures that changed to the differentiation
medium show an initial decrease in the amplitude
values (day 4), as growth is then limited. This initial
decrease in cell proliferation is in accordance with
other works (Eun et al., 2011). However, after a few
hours, Fig. 2 shows a typical linear increase in the
monitored amplitude, corresponding to the
differentiation process (contrasted with microscope
images), reaching final higher amplitude levels than
cell cultures that don´t differentiate, similar to
reported in (Eun et al., 2011; Hildebrandt et al.,
2010; Bagnaninchi and Drummond, 2011).
Cellular growth and differentiation was observed
with microscope images, as shown in figure 3. In
each medium change, cells were seen under the
microscope, and photographs of all wells were
taken.
Table 1: Wells used in the 8W10E PET cultureware.
Well
Culture
Measurement
of impedance
Well 1
Growth medium
Yes
Well 2
Stem cells without differentiation
Yes
Well 3
Stem cells without differentiation
Yes
Well 4
Stem cells without differentiation
No
Well 5
Differentiation medium
Yes
Well 6
Stem cells for differentiation
Yes
Well 7
Stem cells for differentiation
Yes
Well 8
Stem cells for differentiation
No
Figure 2: A) Amplitude signals monitored for stem cells
without differentiation (W2 W3) and for differentiation
(W7). Cell culture medium was also measured (W1). An
electrical error was found in W6. After an initial transient
regime, all signals corresponding to cell cultures started to
rise, corresponding to cellular growth. After differentiation
medium was used (Day 2) and after a transitory stop in the
measured amplitude corresponding to a decrease in cell
proliferation, stem cells following the differentiation
process (W7), showing a higher increase in the monitored
amplitude.
Figure 3: Microscope images of the cell cultures. In each
medium change, cells were seen under the microscope,
and photographs of all wells were taken. Left) Well 7
(stem cells for differentiation) on the eighth day from the
start of differentiation. Tubular structures corresponding to
muscle myotubes can be observed. Right) Well 2 (stem
cells without differentiation) at the eighth day. Differences
between these two different wells are significant, although
a more quantitative work should be carried out in the
future.
A good level of differentiation was observed at
the end of the differentiation process. Tubular
structures corresponding to myotubes were clearly
BIODEVICES 2018 - 11th International Conference on Biomedical Electronics and Devices
98

observed at the end of the differentiation process, as
shown in Fig. 3.
These results suggest the differentiation in cell
lines correspond to differences in bioimpedance
measured, although the work should be completed in
the future with more quantitative analysis.
4 CONCLUSIONS
A new oscillating circuit based on Impedance
Spectroscopy has been presented for the real-time
monitoring of the cellular growth and differentiation
processes of stem cells. The technique has been first
applied to muscle stem cells.
The circuit proved to be useful for monitoring
the processes of cell growth and estimating the fill
factor of muscular stem cell cultures. The
oscillation-based circuit proposed successfully
detected this cell growth, in a similar way as in other
cell types. A useful threshold for the fill factor of
70% has been positively tested on stem cell-cultures,
to activate them towards differentiation by changing
the medium.
Real-time monitoring of cell differentiation can
be also enabled with the proposed impedance
spectroscopy method. An initial decrease in cell
proliferation was detected at the change of medium
to differentiation medium. However, after a few
hours, a linear increase in the monitored amplitude
was recorded, corresponding to the differentiation
process, which was contrasted with microscope
images. A final higher amplitude levels in
differentiated cell cultures were detected. The
technique could be useful for determining the degree
of differentiation achieved, although more detailed
tests would be needed.
No significant differences between cell cultures
where electrical impedance was used and the control
ones. However, higher levels of intensity could be
used, which could influence the process of cellular
differentiation and facilitate the development of
cells, or even facilitate the contraction of muscular
structures, what could be of importance in the design
of new bioreactors for tissue engineering.
ACKNOWLEDGMENT
This work was supported in part by the Spanish
founded Project: Integrated Microsystems for cell
culture test (TEC2013-46242-C3-1-P): Spanish, co-
financed with FEDER program.
REFERENCES
Applied Biophysics, http://www.biophysics.com/.
Bagnaninchi, P. O., and Drummond, N., 2011. “Real-time
label-free monitoring of adipose-derived stem cell
differentiation with electric cell-substrate impedance
sensing”. Proceedings of the National Academy of
Sciences of the United States of America 108 (16),
6462-6467.
Cheema, U., Yang, S. H., Mudera, H., Goldspink, G. G.,
Brown, R. A., 2003. “3-D in vitro model of early
skeletal muscle development”. Cell Motil.
Cytoskeleton 54, 226–23.
Daza, P., Olmo, A., Cañete, D., Yúfera, A., 2013.
“Monitoring living cell assays with bio-impedance
sensors”. Sensors and Actuators B: Chemical. 176,
605-610.
Eun, P. H., Donghee, K., Sook, K. H., Cho, S., Jung-Suk,
S., K., Young, K. J., 2011. “Real-time monitoring of
neural differentiation of human mesenchymal stem
cells by electric cell-substrate impedance
sensing,” Journal of biomedicine & biotechnology. ID
485173.
Giaever, I., Keese, C. R., 1984. “Monitoring fibroblast
behavior in tissue culture with an applied electric
field,” Proc Natl Acad Sci USA. 81, 3761–3764.
Hildebrandt, C., Büth, H., Cho, S., Impidjati, Thielecke,
H., 2010. “Detection of the osteogenic differentiation
of mesenchymal stem cells in 2D and 3D cultures by
electrochemical impedance spectroscopy” Journal of
Biotechnology 148, 83–90.
Huertas, G., Maldonado-Jacobi, A., Yúfera, A., Rueda,
Huertas, J- L., 2015. “The Bio-Oscillator: A Circuit
for Cell-Culture Assays,” IEEE Transactions on
Circuits and Systems. Part II: Express Briefs. 62, 164-
168.
Liao, H., Zhou, G. H., 2009. “Development and progress
of engineering of skeletal muscle tissue” Tissue Eng.
Part B Rev. 15, 319–331.
Nordberg, R. C., Zhang, J., Griffith, E. H., Frank, M. W.,
Starly, B., Loboa, E. G., 2017. “Electrical Cell-
Substrate Impedance Spectroscopy can monitor age-
grouped human adipose stem cell variability during
osteogenic differentiation,” Stem Cells Translational
Medicine 6 (2), 502–51.
Pérez, P., Maldonado, A., Yúfera, A., Huertas, G., Rueda,
A., Huertas, J. L., 2016. “Towards Bio-impedance
Based Labs: A Review”. Journal of Electrical
Engineering. 4 (3), 116-127.
Pérez, P., Maldonado, A., López, A., Martínez, C., Olmo,
A., Huertas, G. and Yúfera, A., 2017. “Remote
Sensing of Cell Culture Assays. Cell Culture,”
Chapter 4 In: New Insights in Cell Culture
Technology. 135-155. InTech Europe.
Somers, S. M., Spector, A. A., DiGirolamo, D. J.,
Grayson, W. L., 2017. “Biophysical stimulation for
Engineering functional skeletal muscle,” Tissue Eng
Part B Rev. 23 (4), 362-372.
Monitoring Muscle Stem Cell Cultures with Impedance Spectroscopy
99
