
Intelligent Multi-sensor Arrays for Next Generation Diagnostic
Biodevices
Hadar Ben-Yoav
Department of Biomedical Engineering, Ben-Gurion University of the Negev, Beer Sheva, Israel
Keywords: Electrochemical Sensors, Lab-on-a-Chip, Intelligent Sensors, Chemometrics, Redox, Biomedical
Diagnostics.
Abstract: Redox molecules play an essential role in many biological pathways, including energy metabolism,
biosynthesis, and cell respiration. Moreover, a specific spectrum of redox molecules (comprising their type,
concentration, and redox state) is involved in the development of diseases, including cancer. Thus, profiling
the redox spectrum in the body is highly beneficial for a wide range of biomedical applications, from in vivo
diagnostics to in situ monitoring of cell metabolism. Yet, the fundamental challenge lies within the ability to
efficiently extract and analyze physiologically and medically relevant information from redox molecules in
biofluids. This paper will discuss the advantageous approach to rapidly and continuously monitor redox
molecules in biofluids using electrochemical lab-on-a-chip biodevices. The current approaches will be
discussed to selectively measure redox molecules from biofluids without diminishing the rapid and
continuous monitoring capabilities of these translational biodevices, i.e., by simultaneously analyzing
multiple diagnostic redox biomarkers in a stand-alone operation, without any sample pretreatment or
elimination of other interfering molecules. Then, the promising approach of using intelligent multi-sensor
arrays will be highlighted for the rapid detection of a spectrum of redox molecules and the current
challenges impeding their important utilization for next generation biomedical diagnostic devices.
1 INTRODUCTION
‘Redox’ molecules—i.e., molecules that transfer
electrons through a Reduction-Oxidation reaction—
play an essential role in many biological pathways,
including energy metabolism, biosynthesis, and cell
respiration (Jones and Sies, 2015). Moreover, the
specific ‘redox landscape’ in the body—i.e., the
type, concentration, and redox state of various redox
molecules—plays an important role in regulation
and communication between different physiological
pathways (Griffiths, Gao et al., 2017; Sies, 2017),
and it is even involved in the development of
diseases (Swomley and Butterfield, 2015;
Chandrasekaran, Idelchik et al., 2017), including
cancer (Yuan, Liu et al., 2015). Importantly, in
2017, the European Cooperation in Science and
Technology [COST] Action ‘EU-ROS’ expressed
the significance and need to “providing new insights
and tools for better understanding redox biology and
medicine and, in the long run, to finding new
therapeutic strategies to target dysregulated redox
processes in various diseases” (Egea, Fabregat et al.,
2017). Thus, profiling the ‘redox landscape’ in the
body is highly beneficial for a wide range of
biomedical applications, from in vivo diagnostics to
in situ monitoring of cell metabolism. Yet, the
fundamental challenge lies within the ability to
efficiently extract and analyze physiologically and
medically relevant information from redox
molecules in biofluids.
Electrochemical lab-on-a-chip are well suited for
the analytical task of measuring redox molecules
(Bandodkar and Wang, 2014; Katz, Fernandez et al.,
2015; Katz, 2016; Sekretaryova, Eriksson et al.,
2016). These translational, cost-effective, and
portable miniaturized biodevices enable a rapid and
continuous monitoring with superior sensitivity of
multiple redox molecules without externally-added
labels [‘unlabeled’], by directly recording
electrochemical reactions happening between the
molecules and an electrode, generating a unique
electronic signal according to the redox potential of
a molecule of interest. Thus, these ‘portable
laboratories’ provide the means in which the sensor
continuously measures the in situ levels of unlabeled
236
Ben-Yoav, H.
Intelligent Multi-sensor Arrays for Next Generation Diagnostic Biodevices.
DOI: 10.5220/0006653602360240
In Proceedings of the 11th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2018) - Volume 1: BIODEVICES, pages 236-240
ISBN: 978-989-758-277-6
Copyright © 2018 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

redox-active diagnostic markers in the sample
(Bandodkar, Jia et al., 2015; Topkaya, Azimzadeh et
al., 2016). However, the selectivity of
electrochemical lab-on-a-chip towards several
diagnostic redox biomarkers dramatically decreases
in the presence of multicomponent samples [e.g.,
biofluids] due to the presence of other redox
molecules generating overlapping electrochemical
signals, impeding the ability to distinguish between
the various unlabeled, redox biomarkers in the
sample (Corrie, Coffey et al., 2015; Rocchitta,
Spanu et al., 2016).
This paper will focus on current approaches to
overcome the key challenge of selectivity in
biofluids without diminishing the rapid and
continuous monitoring capabilities of
electrochemical lab-on-a-chip biodevices, i.e., by
simultaneously analyzing multiple diagnostic redox
biomarkers from biofluids in a stand-alone
operation, without any sample pretreatment or
elimination of other interfering molecules. First, the
commonly used analytical approaches will be
reviewed that enable the selective detection of
specific redox biomarkers. Then, the promising
approach of using intelligent multi-sensor arrays will
be highlighted for the detection of a spectrum of
redox molecules and the current challenges
impeding unleashing the potential utilization for
biomedical diagnostics.
2 ELECTROCHEMICAL
LAB-ON-A-CHIP BIODEVICES
FOR REDOX BIOMARKERS
DETECTION
2.1 Detection of Specific Diagnostic
Redox Biomarkers
The signal in electrochemical sensors – namely, the
electronic current generated at the surface of the
electrode – is determined by three main factors: (a)
the properties of the molecules, e.g., their standard
redox potentials, molecular weights, particle
charges, diffusion rates, and concentrations; (b) the
properties of the electrode used, e.g., electrode
material type and electrode geometry; and (c) the
properties of the redox reaction at the solid–
electrolyte interface, e.g., the rate of electron transfer
between the molecules and the electrode and the
thickness of the electric double layer (Bard and
Faulkner, 2001). For samples containing more than
one redox molecule [such as biofluids], the resulting
electrochemical signal is complex and must undergo
digital deconvolution through direct and simple
signal processing methods (Jakubowska, 2011; Xia
and Behnamian, 2015). Naturally, however, the level
of complexity increases as a function of the number
of molecules in the solution, making such a
separation impractical for solutions with multiple
redox molecules – and practically impossible when
these molecules have overlapping electrochemical
signals.
Another approach to discriminate between
molecules in multicomponent mixtures is to use a
pre-separation step [e.g., liquid chromatography],
which increases the selectivity of the sensor by
physically separating the molecule of interest
(Huang, Lin et al., 2011; Kutter, 2012; Sikanen,
Aura et al., 2012; Chan, Pasikanti et al., 2015;
Fekete, Guillarme et al., 2016; Hong, Yang et al.,
2016; Songa and Okonkwo, 2016). This solution
indeed increases separation efficiency but it is
laborious, time-consuming, and increases the
number of analytic steps and the overall cost of the
discrimination step, thus preventing its effective
utilization for in situ recording of the numerous
molecules continuously generated in the body.
An alternative approach involves modifying the
surface of the electrode with a film that recognizes a
specific type of molecule in the sample, thus
increasing the selectivity of the sensor to the specific
electrochemical signal generated by this molecule
(Xie, Liu et al., 2015; Bala and Gorski, 2016;
Weltin, Kieninger et al., 2016). Both artificial and
biological receptors [e.g., enzymes] can be used to
produce such highly selective films (Palchetti, 2016;
Weltin, Kieninger et al., 2016), and, indeed, these
modified electrodes can be integrated into an array
of specific sensors [Figure 1] that can analyze the
types of the redox molecules at suitable limit-of-
detection values [usually between nano- and femto-
molars (Bougrini, Florea et al., 2016)]. However, the
development of such films is costly and is multiplied
by the number of the detected molecules and
required electrodes, thereby limiting the multi-
molecule detection capabilities of such modified
electrodes (Bunyakul and Baeumner, 2015; Zaffino,
Galan et al., 2015; Heikenfeld, 2016). Moreover,
with the growing evidence that a disease is no more
governed by a specific biomarker but profiles of
molecules, a single or combination of highly
selective measurements may be inadequate as
appropriate biomarkers are seldom known for
complex diseases.
Intelligent Multi-sensor Arrays for Next Generation Diagnostic Biodevices
237
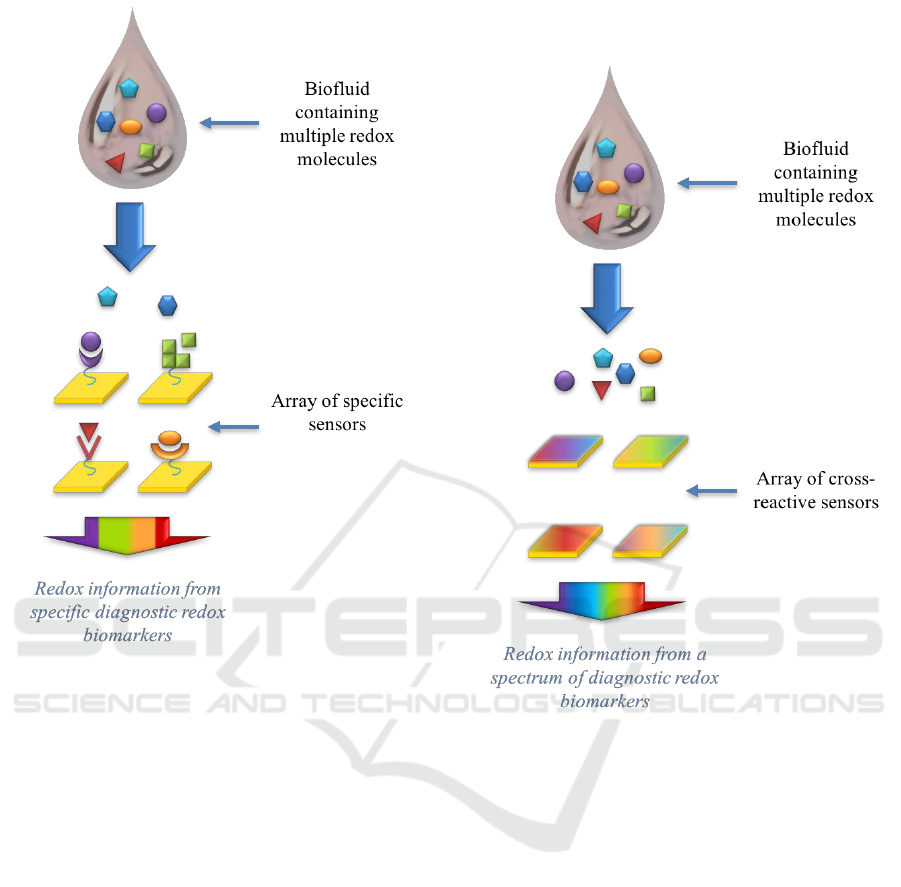
Figure 1: Array of electrodes modified with films that are
specific to multiple diagnostic redox biomarkers.
2.2 Detection of a Spectrum of
Diagnostic Redox Biomarkers
Recently, a promising trend has emerged based on
arrays of partially-selective electrodes that
simultaneously cross-react with multiple redox
molecules in the mixture [Figure 2]. Such an array
of electrodes thus generates a set of complex
electrochemical signals that are analyzed using
intelligent chemometric algorithms [e.g., pattern
recognition algorithms] to facilitate the in situ
analysis of these molecules (Cipri, Schulz et al.,
2016; Kilmartin, 2016; Wadehra and Patil, 2016)).
Inspired by the sensory system of taste in
mammals, wherein several taste receptors on the
tongue can respond to a large variety of flavor-
inducing substances, these intelligent
electrochemical sensors enable fast response, low-
cost, portability, ease-of-use, and simultaneous
detection of a large spectrum of redox molecules in
one step without preforming any pretreatment, as
data processing stage may offset any matrix or
interference effect from the sample itself, drifts or
nonlinearity obtained with the sensors (Cetó,
Voelcker et al., 2016).
Figure 2: Array of electrodes modified with partially-
selective films that cross-react with multiple diagnostic
redox biomarkers.
The cross-reactivity is achieved by using
electrodes consisting of materials that possess
different electron transfer rates, thereby generating
slightly diverse electrochemical signals from the
analyzed redox mixture. To extract information
about the redox molecule of interest [concentration,
type, etc.], machine learning algorithms—including
multivariate analysis models, such as principal
component analysis, artificial neural networks, and
others—are used together with training sets of
calibration mixtures, thus shifting the complexity of
the analysis from the physical domain to the digital
processing domain (Górski, Kubiak et al., 2016).
However, despite the vast contribution of such
‘intelligent sensors’ to differentiate between groups
of food types or environmental conditions (Wei,
Yang et al., 2018), utilizing them for biomedical
applications is more challenging, mainly because
biofluids contain an abundance of redox molecules,
BIODEVICES 2018 - 11th International Conference on Biomedical Electronics and Devices
238

which generate overlapping electrochemical signals
that are hard to distinguish.
To solve this issue and provide an accurate and
meaningful profile of the redox molecules in
biofluids, previous studies suggested modifying
electrodes with films that provide various
reactivities between the electrode and the redox
molecules based on the films’ properties, i.e., their
electrocatalytic activity [e.g., noble metals as
electrode material or conductive polymers to coat
the electrodes (Wei, Yang et al., 2018)] or their
biofunctionality [e.g., enzymes immobilized on the
electrode (Cetó, Voelcker et al., 2016)]. Indeed, such
approaches have enabled, for the first time, profiling
a predetermined set of redox molecules—e.g., for
identifying the redox state of specific molecules
involved in a specific type of cancer [namely,
prostate cancer (Pascual, Campos et al., 2016)].
However, despite this marked advancement in the
ability to differentiate between groups of specific,
disease-oriented ‘redox landscapes’, this approach is
currently lacking the ability to extract redox
information from all redox molecules present in a
biofluid at a given time, hence limiting the diagnosis
to a specific biomedical condition.
3 CONCLUSIONS
This paper highlights the potential contribution of
intelligent multi-sensor arrays for the important need
to profile the ‘redox landscape’ in the body. By
overcoming the fundamental challenge of selectivity
in biofluids, these intelligent sensors will enable the
rapid and continuous quantitative analyses of redox
information in biofluids. Such an achievement is
highly beneficial for a wide range of biomedical
applications, from in vivo diagnostics to in situ
monitoring of cell metabolism, and will offer the
next generation of diagnostic biodevices that can be
used to study and monitor disease initiation and
development. Ultimately, integrating these
intelligent sensors in electrochemical lab-on-a-chip
biodevices will facilitate the development of highly
sensitive monitoring tools for the continuous in vivo
monitoring of various biomedical conditions.
REFERENCES
Bala, A. and Gorski, L., 2016. Application of nucleic acid
analogues as receptor layers for biosensors. Analytical
Methods 8(2): 236-244.
Bandodkar, A. J., Jia, W. Z. and Wang, J., 2015. Tattoo-
based wearable electrochemical devices: A review.
Electroanalysis 27(3): 562-572.
Bandodkar, A. J. and Wang, J., 2014. Non-invasive
wearable electrochemical sensors: A review. Trends in
Biotechnology 32(7): 363-371.
Bard, A. J. and Faulkner, L. R., 2001. Electrochemical
Methods: Fundamentals and Applications, Wiley.
Bougrini, M., Florea, A., Cristea, C., et al., 2016.
Development of a novel sensitive molecularly
imprinted polymer sensor based on
electropolymerization of a microporous-metal-organic
framework for tetracycline detection in honey. Food
Control 59: 424-429.
Bunyakul, N. and Baeumner, A. J., 2015. Combining
electrochemical sensors with miniaturized sample
preparation for rapid detection in clinical samples.
Sensors 15(1): 547-564.
Cetó, X., Voelcker, N. H. and Prieto-Simón, B., 2016.
Bioelectronic tongues: New trends and applications in
water and food analysis. Biosensors and
Bioelectronics 79: 608-626.
Chan, E. C. Y., Pasikanti, K. K., Hong, Y. J., et al., 2015.
Metabonomic Profiling of Bladder Cancer. Journal of
Proteome Research 14(2): 587-602.
Chandrasekaran, A., Idelchik, M. D. S. and Melendez, J.
A., 2017. Redox control of senescence and age-related
disease. Redox Biology 11: 91-102.
Cipri, A., Schulz, C., Ludwig, R., et al., 2016. A novel
bio-electronic tongue using different cellobiose
dehydrogenases to resolve mixtures of various sugars
and interfering analytes. Biosensors and
Bioelectronics 79: 515-521.
Corrie, S. R., Coffey, J. W., Islam, J., et al., 2015. Blood,
sweat, and tears: Developing clinically relevant
protein biosensors for integrated body fluid analysis.
Analyst 140(13): 4350-4364.
Egea, J., Fabregat, I., Frapart, Y. M., et al., 2017.
European contribution to the study of ROS: A
summary of the findings and prospects for the future
from the COST action BM1203 (EU-ROS). Redox
Biology 13: 94-162.
Fekete, S., Guillarme, D., Sandra, P., et al., 2016.
Chromatographic, electrophoretic, and mass
spectrometric methods for the analytical
characterization of protein biopharmaceuticals.
Analytical Chemistry 88(1): 480-507.
Górski, Ł., Kubiak, W. W. and Jakubowska, M., 2016.
Independent components analysis of the overlapping
voltammetric signals. Electroanalysis 28(7): 1470-
1477.
Griffiths, H. R., Gao, D. and Pararasa, C., 2017. Redox
regulation in metabolic programming and
inflammation. Redox Biology 12: 50-57.
Heikenfeld, J., 2016. Non-invasive analyte access and
sensing through eccrine sweat: Challenges and outlook
circa 2016. Electroanalysis 28(6): 1242-1249.
Hong, T. T., Yang, X., Xu, Y. J., et al., 2016. Recent
advances in the preparation and application of
Intelligent Multi-sensor Arrays for Next Generation Diagnostic Biodevices
239

monolithic capillary columns in separation science.
Analytica Chimica Acta 931: 1-24.
Huang, Z. Z., Lin, L., Gao, Y., et al., 2011. Bladder cancer
determination via two urinary metabolites: A
biomarker pattern approach. Molecular and Cellular
Proteomics 10(10): M111 007922.
Jakubowska, M., 2011. Signal processing in
electrochemistry. Electroanalysis 23(3): 553-572.
Jones, D. P. and Sies, H., 2015. The redox code.
Antioxidants and Redox Signaling 23(9): 734-746.
Katz, E., 2016. Magneto-switchable electrodes and
electrochemical systems. Electroanalysis 28(5): 904-
919.
Katz, E., Fernandez, V. M. and Pita, M., 2015. Switchable
bioelectrocatalysis controlled by pH changes.
Electroanalysis 27(9): 2063-2073.
Kilmartin, P. A., 2016. Electrochemistry applied to the
analysis of wine: A mini-review. Electrochemistry
Communications 67: 39-42.
Kutter, J. P., 2012. Liquid phase chromatography on
microchips. Journal of chromatography A 1221: 72-
82.
Palchetti, I., 2016. New trends in the design of enzyme-
based biosensors for medical applications. Mini-
Reviews in Medicinal Chemistry 16(14): 1125-1133.
Pascual, L., Campos, I., Vivancos, J.-L., et al., 2016.
Detection of prostate cancer using a voltammetric
electronic tongue. Analyst 141(15): 4562-4567.
Rocchitta, G., Spanu, A., Babudieri, S., et al., 2016.
Enzyme biosensors for biomedical applications:
Strategies for safeguarding analytical performances in
biological fluids. Sensors (Basel) 16(6).
Sekretaryova, A. N., Eriksson, M. and Turner, A. P. F.,
2016. Bioelectrocatalytic systems for health
applications. Biotechnology Advances 34(3): 177-197.
Sies, H., 2017. Hydrogen peroxide as a central redox
signaling molecule in physiological oxidative stress:
Oxidative eustress. Redox Biology 11: 613-619.
Sikanen, T., Aura, S., Franssila, S., et al., 2012. Microchip
capillary electrophoresis-electrospray ionization-mass
spectrometry of intact proteins using uncoated
Ormocomp microchips. Analytica Chimica Acta 711:
69-76.
Songa, E. A. and Okonkwo, J. O., 2016. Recent
approaches to improving selectivity and sensitivity of
enzyme-based biosensors for organophosphorus
pesticides: A review. Talanta 155: 289-304.
Swomley, A. M. and Butterfield, D. A., 2015. Oxidative
stress in Alzheimer disease and mild cognitive
impairment: evidence from human data provided by
redox proteomics. Archives of Toxicology 89(10):
1669-1680.
Topkaya, S. N., Azimzadeh, M. and Ozsoz, M., 2016.
Electrochemical biosensors for cancer biomarkers
detection: Recent advances and challenges.
Electroanalysis 28(7): 1402-1419.
Wadehra, A. and Patil, P. S., 2016. Application of
electronic tongues in food processing. Analytical
Methods 8(3): 474-480.
Wei, Z., Yang, Y., Wang, J., et al., 2018. The
measurement principles, working parameters and
configurations of voltammetric electronic tongues and
its applications for foodstuff analysis. Journal of Food
Engineering 217: 75-92.
Weltin, A., Kieninger, J. and Urban, G. A., 2016.
Microfabricated, amperometric, enzyme-based
biosensors for in vivo applications. Analytical and
Bioanalytical Chemistry 408(17): 4503-4521.
Xia, D.-H. and Behnamian, Y., 2015. Electrochemical
noise: A review of experimental setup, instrumentation
and DC removal. Russian Journal of Electrochemistry
51(7): 593-601.
Xie, S. D., Liu, Y., Wu, Z. Y., et al., 2015. Application of
inorganic layered materials in electrochemical sensors.
Chinese Journal of Analytical Chemistry 43(11):
1648-1655.
Yuan, K. F., Liu, Y., Chen, H. N., et al., 2015. Thiol-based
redox proteomics in cancer research. Proteomics 15(2-
3): 287-299.
Zaffino, R. L., Galan, T., Pardo, W. A., et al., 2015.
Nanoprobes for enhanced electrochemical DNA
sensors. Wiley Interdisciplinary Reviews-
Nanomedicine and Nanobiotechnology 7(6): 817-827.
BIODEVICES 2018 - 11th International Conference on Biomedical Electronics and Devices
240
