
DEVELOPMENT OF A SLEEP MONITORING SYSTEM WITH
WEARABLE VITAL SENSOR FOR HOME USE
Takuji Suzuki
1
, Kazushige Ouchi
1
, Ken-Ichi Kameyama
1
and Masaya Takahashi
1,2
1
Humancentric Laboratory, Corporate Research & Development Center, Toshiba Corporation, Japan
2
National Institute of Occupational Safety and Health, Japan
Keywords: Daily sleep care, Sleep monitoring, Wearable sensor and Data viewer.
Abstract: This paper describes a new sleep monitoring system for home use. The basic system consists of a wearable
physiological sensor and PC software for analyzing sleep quality from user’s wrist motion and heart rate
variability. Different from a conventional sleep monitoring device used in a hospital, the sensor is so small
and easy-to-use that a normal person can use it at home. This means that the system is useful for a sleep
specialist who wants to check a patient's daily sleep pattern. The system can also be used for self-care. We
have developed a wrist-watch-shaped physiological sensor that monitors user’s wrist motion and pulse wave
interval. We have also developed the algorithm for computing the quality of sleep from these physiological
data on PC. Although sleep is a kind of brain activity and our sensor can not directly measure it, the output
of our algorithm is close to medically evaluated sleep quality. We performed dozens of comparison
experiments and found that its accuracy was about 73.5% on average. The value of the accuracy is enough
for assessing a normal person’s sleep quality.
1 INTRODUCTION
In recent years, many people have been suffering
from sleep disorder caused by mental stress,
irregular lifestyle or shift work. However, it is not
easy to determine the quality of sleep because deep
sleep is not always good sleep and shallow sleep is
not always bad sleep. For example, it is natural that
a person cannot sleep well because of jet lag.
However, a person who is always sleepy in the
daytime for a period exceeding one month might
have a health problem. Therefore, it is important for
a doctor to check a patient’s sleep habits for several
days in order to diagnose and cure his/her sleep
disorder properly. Moreover, it is necessary for a
person to check his/her own sleep habits and to
change his/her lifestyle (self-care).
However, there is no good system to record and
analyze daily sleep. For example, most medical
sleep sensors, such as those employed for
polysomnography (PSG), are for recording many
kinds of physiological data (EEG, EMG, EOG and
so on) for only one or two nights, not for recording
sleep habits with natural state in daily life. It is also
too difficult for a normal person to handle PSG at
home because it involves the use of many electrodes
for measuring the physiological data. A doctor can
attempt to learn a patient's sleep habits by
interviewing him or her, but this is an inherently
unreliable approach. A simple and easy-to-use sleep
monitoring system that can be used in the home is
strongly desired in order to get objective data on
sleep habits.
In order to develop such a system, we have
created a wrist-watch-shaped wearable physiological
sensor that monitors user’s wrist motion and pulse
wave intervals (Pulse-to-Pulse Intervals: PPIs). The
sensor can be made small and simple because wrist
motion and pulse wave can be easily measured
compared to the case of using PSG. We have also
developed the algorithm for computing the quality of
sleep from these physiological data. The algorithm
can distinguish sleep stage (wake /REM /NREM
[shallow /deep]) using the relationship between
autonomic nervous activity and sleep stages.
Although sleep is a kind of brain activity and our
sensor cannot directly measure it, the output of our
algorithm is close to medically evaluated sleep
quality.
In the following sections, the way of expressing
sleep data, related works, our system’s hardware and
software, and the validation result of the sleep
estimation are discussed.
326
Suzuki T., Ouchi K., Kameyama K. and Takahashi M. (2009).
DEVELOPMENT OF A SLEEP MONITORING SYSTEM WITH WEARABLE VITAL SENSOR FOR HOME USE.
In Proceedings of the International Conference on Biomedical Electronics and Devices, pages 326-331
DOI: 10.5220/0001784203260331
Copyright
c
SciTePress
2 SLEEP DATA
Generally speaking, sleep is a kind of brain activity
and its purpose is recovery from brain fatigue.
Therefore, sleep state is measured mainly by EEG,
and is classified into several stages. Sleep state is
roughly divided into REM (rapid-eye movement)
sleep and NREM (Non-REM) sleep. NREM sleep is
divided into 4 stages. Stages 3 and 4 of NREM sleep
are so called deep sleep, and stages 1 and 2 are
shallow sleep. These stages are decided by a sleep
specialist using PSG data (Rechtschaffen, 1968), and
their change is shown in a graph called a hypnogram
(shown in Figure 1).
A doctor mainly uses a hypnogram for
evaluating a person’s sleep quality. For example, the
doctor checks the quantity of deep sleep if a patient
complains about oppressive drowsiness in the
daytime. If the patient frequently wakes up in the
night and experiences difficulty in breathing, he/she
might be suffering from sleep apnea syndrome. If
REM sleep always occurs soon after falling asleep,
there might be a problem concerning the patient’s
nervous system. From the viewpoint of healthcare, it
is important to check the balance of deep sleep,
REM sleep or sleep cycle. Therefore, a sleep
monitoring system for home use can also show the
result of one night’s data in a graph similar to a
hypnogram.
Figure 1: Sleep hypnogram.
There are many studies on the relationship between
physiological parameters and sleep stages. For
example, Baharav et al. stated that autonomic
nervous activity level derived from heart rate
variability (HRV) during sleep changes in response
to the sleep stages (Baharav, 1995). A value of
LF/HF shows the activity of the sympathetic nerve.
During a REM sleep, a value of LF/HF and the
variability of that are large, and the value of LF/HF
decreases during a NREM sleep, particularly in the
case of deep sleep (Slow Wave Sleep). Since the
brain stem controls both the cerebrum and the
autonomic nervous system, it may be possible to
estimate the sleep stage using HRV.
3 RELATED WORKS
A number of trials have been conducted with a view
to developing sleep monitors for home use. For
example, body/wrist motion has been used for
wake/sleep identification.
The amount of activity
(number of subtle wrist motions per minute)
measured
from acceleration sensors is often used for
monitoring wake/sleep rhythms (Sadeh, 1989)
although the sleep stages (ex. REM sleep / NREM
sleep) cannot be determined from the data.
More recently, researchers have focused on
measuring heart/pulse rate and analyzing its
variability: HRV (Watanabe, 2004, Michimori, 2003
and Wakuda, 2007). The sleep stages can be
calculated from HRV if the indices of HRV are
properly mapped for the sleep stages.
However, there are two problems in this
approach. One is that body/wrist motion often
disturbs heart/pulse sensing and the HRV value can
not be calculate correctly. The other is that the level
of autonomic nervous activity differs according to
age, sex and body/mental condition. For example,
the autonomic nervous system of the young is
generally more active than that of the old. Sleep
stages cannot be classified using static thresholds.
Our sensor measures both pulse wave interval
and wrist motion. The wrist motion data are used not
only for counting the amount of activity, but also for
detecting errors in HRV data. This solves the first
problem mentioned above.
For the second problem, we employ a statistical
method for deciding sleep stages (Suzuki, 2007). We
assume that there are several stages in a certain
period of sleeping time since the sleep stage
cyclically repeats about every 90 minutes. It means
that the data of autonomic nervous activity can be
classified into several groups if we have any 90-120
minute dataset. In this way, the thresholds for
dividing sleep stages are changed flexibly along with
the dataset.
4 THE OVERVIEW OF THE
SYSTEM
4.1 Wearable Physiological Sensor
Figure 2 shows our wearable physiological sensor.
The size of the sensor is 50mm*60mm*13mm and
the weight is only 35g. A rechargeable battery is
used as an electrical power source. It is possible to
measure physiological data for over 40 hours after
Wake
REM
Stage1
Stage2
Stage3
Stage4
DEVELOPMENT OF A SLEEP MONITORING SYSTEM WITH WEARABLE VITAL SENSOR FOR HOME USE
327

full charge. The sensor incorporates a photoelectric
pulse wave sensor and a 3-axis accelerometer.
Besides, it has an external pulse wave sensor.
Therefore, pulse waves can be measured on the
user’s wrist or on his/her finger, depending on
his/her preference. The front panel serves as a wrist-
watch displaying date and time, and as a sensor
displaying time and the amount of activity. The
sensor has only two buttons; namely, one is a light
switch, and the other is a switch to start/end sensing.
Figure 2: Wearable physiological sensor.
The sensor measures pulse waves and accelerations
on a user’s wrist and stores the computed pulse-to-
pulse intervals (PPIs) and the amount of activity in a
flash memory (4MiB). Both analog and digital filters
are used to remove the fluctuations of the amplitude
and the basal line of pulse waveform, which makes
PPIs more precise. As the size of the data measured
in one night (7 hours) is 256 KiB, the sensor can
store almost 2 weeks’ data in the flash memory.
The sampling rate of the pulse wave and 3-axis
accelerations is 64Hz. However, the resolution of the
PPI is 0.1 ms by using linear interpolation to detect
pulse peak.
The amount of activity is calculated as the
number that the scalar of the 3-axis acceleration is
larger than 0.01 G, which is the same as Actigram
(Cole, 1992).
The stored PPIs and amount of activity data are
sent to PC via USB.
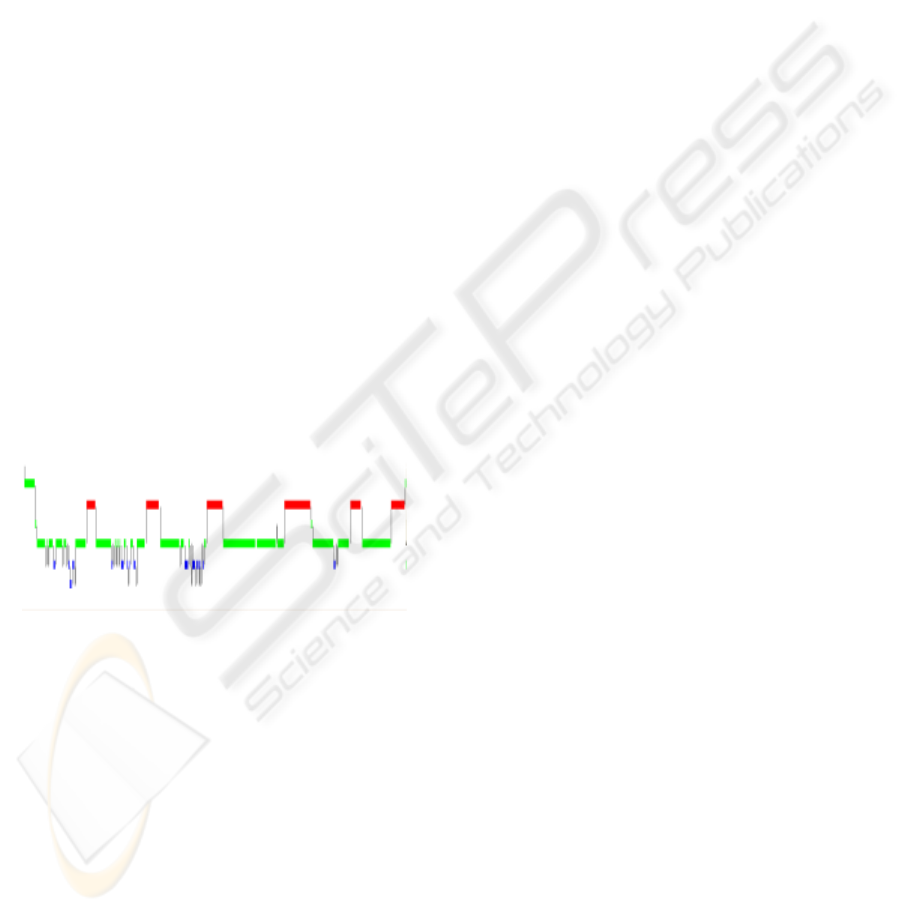
We evaluated the performance. Firstly, the
correlation coefficient between the amount of
activity counted by the sensor worn on the left
forearm and that measured by an actigraph
(Micromini-Motionlogger Actigraph, Ambulatory
Monitoring Inc.) worn on the right forearm during
sleep was 0.95 (average of 3 healthy subjects).
Figure 3 shows an example of the result.
Figure 3: Actigram during sleep. Upper graph shows the
result measured by Actigraph and lower graph shows that
measured by our sensor.
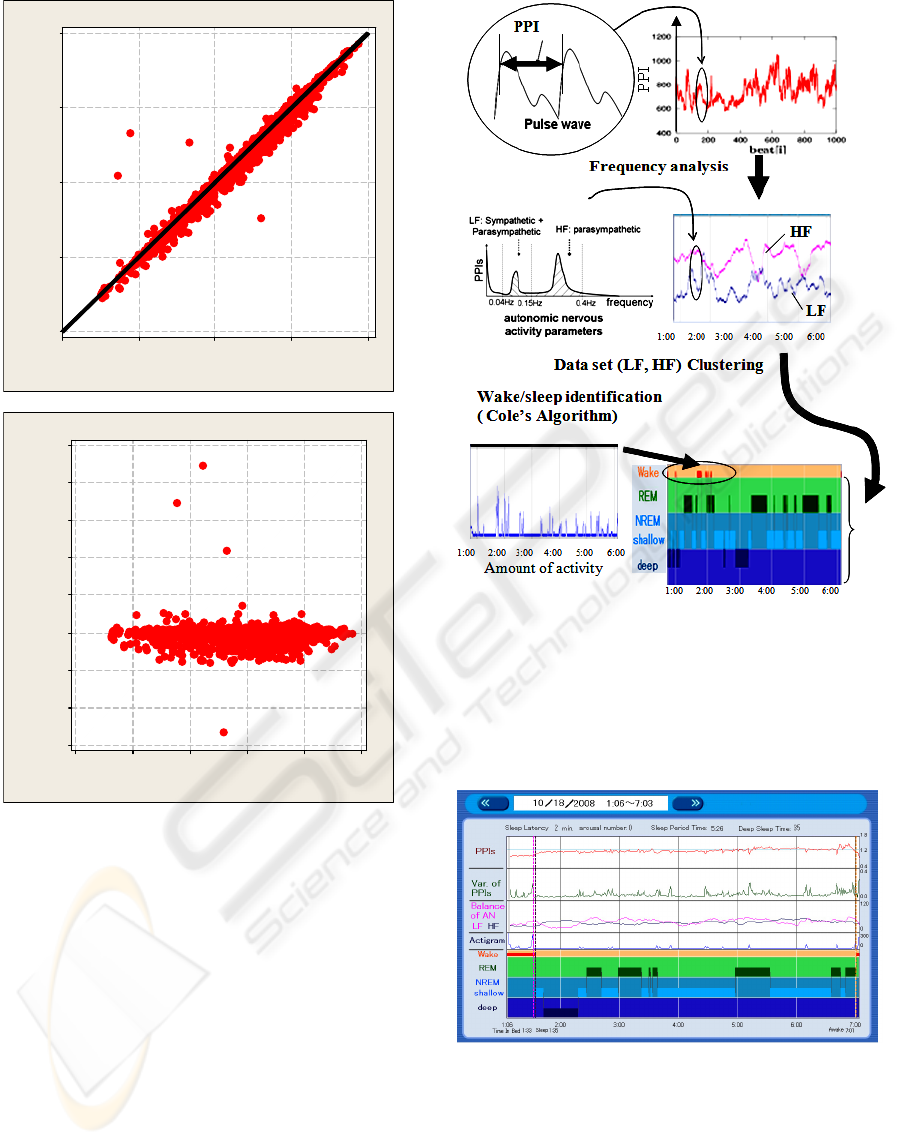
Besides, the correlation coefficient between the PPIs
computed by the pulse wave measured by the sensor
and the R-R intervals computed by a simultaneously
measured electrocardiogram during sleep was
evaluated. Single-channel ECG was measured by
CM5 lead using PSG (Polymate AP1124, TEAC
Corporation, sampling rate: 1 kHz) simultaneously
with the PPI measured by our sensor. R-R intervals
were computed using commercially available R-R
interval analysis software for the PSG (NoruPro
Light Systems, Japan). The correlation coefficient is
0.96 (average of 3 healthy subjects). Figure 4(a)
shows the correlation plot, and (b) shows the Bland
& Altman plot between R-R intervals of ECG and
PPIs.
These values are accurate enough to use the sensor
as a medical device.
4.2 PC Software
Figure 5 shows the flow of the algorithm for
computing sleep stages from the data of PPIs and the
amount of activity.
We employ Cole’s algorithm for wake/sleep
identification from the amount of activity data (Cole,
1992). This algorithm cannot determine wake/sleep
in real time, but its accuracy is about 90%. At the
same time, the indices of autonomic nervous activity
are derived from frequency analysis of the
variability of PPIs. Firstly, sampled PPIs’ dataset in
a minute is interpolated at even intervals by cubic
spline interpolation by the minute. Next, Fast
Fourier Transformation (FFT) is executed for the
even-interval PPIs to get the frequency spectrum. In
the frequency domain, the integral value of the
power from 0.04Hz to 0.15Hz is called LF (low
frequency), which shows both sympathetic and
parasympathetic nervous activities. The integral
value of the power from 0.15Hz to 0.4Hz is called
HF (high frequency), which shows parasympathetic
nervous activity. Therefore, we can get the balance
BIODEVICES 2009 - International Conference on Biomedical Electronics and Devices
328
R - R in te rv a ls o f E C G (s )
Pulse-to-P ulse Intervals (s)
1.501.251.000.750.50
1.50
1.25
1.00
0.75
0.50
(a)
Average of P-P interval and R-R interval of EC G (s)
Difference in P-P interval and R-R interval of EC G (s)
1.51.31.10.90.70.5
0.5
0.4
0.3
0.2
0.1
0.0
-0.1
-0.2
-0.3
(b)
Figure 4: Correlation plot (a) and Bland & Altman plot (b)
between R-R intervals of ECG and PPIs.
of sympathetic and parasympathetic nervous activity,
which is related to sleep stages as we mentioned
above. In order to classify the sleep stages from the
dataset of LF and HF values, the k-means clustering
method is adopted. Firstly, REM/NREM sleep is
divided from 2-hour data set, and then, shallow/deep
sleep is divided from its NREM dataset.
We developed sleep analysis software on
Windows XP/Vista using this algorithm. The
program was coded and compiled by Visual Basic
Ver.6 (Microsoft Corp.).
Figure 5: Algorithm for computing sleep stages.
Figure 6 and 7 are the picture image of the
software. Figure 6 is one night’s data, which shows
pulse rate, variability of pulse rate, LF and HF
trends, amount of activity and the simplified
hypnogram.
Figure 6: Result of one night’s data.
Figure 7 is a summary of a 2-week hypnogram
showing sleep habits. This is the most useful
function for sleep care, which cannot be
implemented in the conventional sleep monitoring
systems.
DEVELOPMENT OF A SLEEP MONITORING SYSTEM WITH WEARABLE VITAL SENSOR FOR HOME USE
329
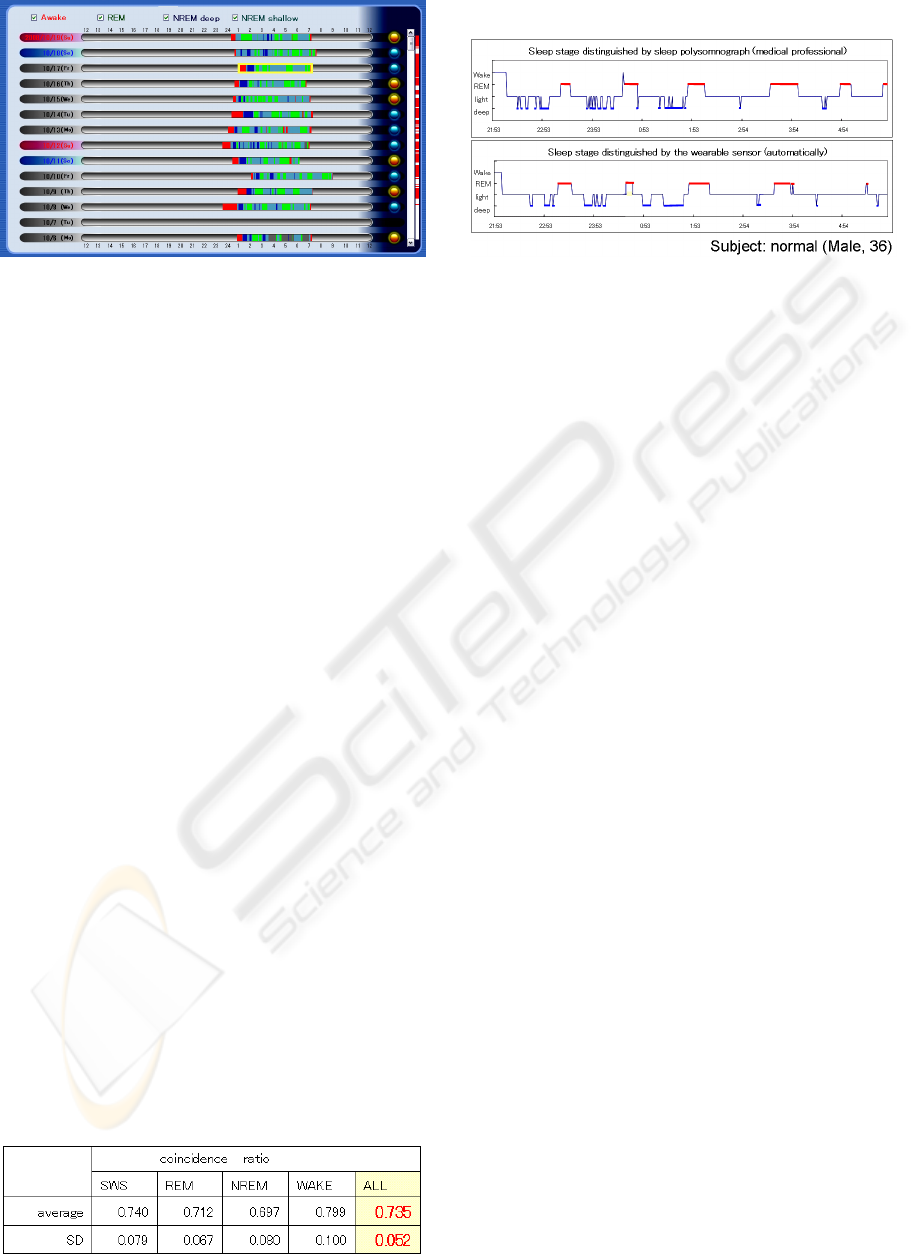
Figure 7: Result of summary of a 2-week hypnogram.
5 VALIDATION OF THE SLEEP
ESTIMATION
Correlation between the sleep stage estimated by the
proposed method using our wearable physiological
sensor and the sleep stage estimated using PSG by
sleep specialists was evaluated. EEG, EOG, chin
EMG, ECG, respiration and SpO2 by PSG
(Polymate AP1000, TEAC Corporation, Sampling
rate: 250Hz), the pulse wave and acceleration by our
sensor was recorded simultaneously in a night (8
hours). The test was held in two cites (Showa
University East Hospital, Tokyo, Japan and The
Institute for Science of Labour, Kawasaki, Japan).
45 normal healthy subjects (30 males and 15
females, 19-72 years old) are measured. All subjects
had informed consent.
The sleep stages of PSG were distinguished
manually by sleep specialists (doctor, clinical
laboratory technologist, or sleep researcher) who
belong to those cite based on Rechtschaffen & Kales
method (Rechtschaffen, 1968) by the minute. Our
sensor also estimated the sleep stages also by the
minute.
We defined coincidence ratio as an evaluation
function to compare the estimation result by our
sensor with the result by PSG.
The coincidence ratio is defined as a correlation
coefficient of moving average of sleep stages (20-
minutes time window) between the stages estimated
by this method and those from PSG. Table 1 shows
the result of the comparison.
Table 1: Result of the comparison.
Figure 8 shows an example of the estimation results.
Figure 8: An example of the estimation results (upper:
sleep stage distinguished by medical professionals using
PSG, lower: sleep stage distinguished automatically by the
sensor).
6 DISCUSSIONS
“Beat-to-beat” pulse interval detection is necessary
to obtain autonomic nervous activity. Our algorithm
has enough ability to get “beat-to-beat” pulse
intervals. However, this algorithm is applicable for
healthy subjects, except for cardiac disease,
peripheral blood circulation disorder.
The coincidence ratio of our sleep stage
estimating algorithm is 0.735. Although it is a rather
low value, PSG results also varied depending on the
examiner (variance is about 20%), and therefore it
seems to be acceptable for home healthcare use.
However, it is also applicable for healthy subjects,
except for autonomic nerve disorder, cardiac disease.
7 CONCLUSIONS
Measurement of sleep habits is a promising new
medical field. However, there are no systems
suitable for it. This is because current sleep
monitoring systems cannot satisfy the needs for
accurate analysis of sleep and convenience in use. In
order to provide a solution, we developed a small
and easy-to-use sensor device. We also developed an
algorithm for analyzing sleep stages. We confirmed
sufficient accuracy in the detection of PPIs by
comparison with R-R Intervals by ECG, and that in
the estimation of the sleep stage by comparison with
the result of PSG. The software can display the sleep
data of one night and the summary of a 2-week
hypnogram. This function is useful not only for a
doctor analyzing a patient's sleep habits, but also for
a user analyzing his/her sleep.
BIODEVICES 2009 - International Conference on Biomedical Electronics and Devices
330

REFERENCES
Rechtschaffen A., and Kales A., A Manual of standardized
terminology, techniques and scoring system for sleep
stages of human subjects. Public Health Service, U.S.
Goverment Priting Office, 1968.
Sadeh A., Alster J., Urbach D. and Lavie P.,
Actigraphically based automatic bedtime sleep-wake
monitor scoring: validity and clinical applications. J
Ambul Monit 2, pp. 209–216, 1989.
Watanabe T. and Watanabe K., Noncontact Method for
Sleep Stage Estimation, IEEE Transactions on
Biomedical Engineering, Vol.51, No.10, pp.1735-
1748, 2004.
Michimori A., Fukushima K. and Hagiwara H., Sleep
Monitoring System by Using Heart Rate Variability
Analysis, Matsusita Technical Report, No.82, 29-33,
2003 (in Japanese).
Wakuda Y., Noda A., Hasegawa Y., Arai F., Fukuda T.,
and Kawaguchi M., Biological Rhythm Based
Wearable Sleep State Observer, Journal of Advanced
Computational Intelligence and Intelligent
Informatics, Vol.11, No.2, pp.232-241, 2007
Baharav A., Kotagal S., Gibbons V., Rubin B.K., Pratt G.,
J. Karin and S. Askelrod, Fluctuations in. autonomic
nervous activity during sleep displayed by. power
spectrum analysis of heart rate variability, Neurology
45:66, 1183-1187, 1995.
Suzuki T, Ouchi K, Moriya A, Kameyama K and
Takahashi M. Development of a Sleep-Stage
Estimation Method using Heart RateVariability and
Actigraphy measured by Wearable Sensor, Sleep and
Biological Rhythms; 5 Suppl.1:A38, 2007.
Cole RJ, et al., Automatic sleep/wake identification from
wrist actigraphy, Sleep, 15(5), 461-469, 1992.
Heart rate variability: standards of measurement,
Physiological interpretation and clinical use. Task
Force of the European Society of Cardiology and the
North American Society of Pacing and
Electrophysiology. Circulation. 93, pp.1043–1065,
1996.
DEVELOPMENT OF A SLEEP MONITORING SYSTEM WITH WEARABLE VITAL SENSOR FOR HOME USE
331
