
Analysis on the Delivery and Formulations of Inhaled Drugs
Jiaying Yan
Department of Chemistry, University of California Davis, U.S.A.
Keywords: Inhaled Drugs, Aerosol Administration, Metered-Dose Inhalers (Mdis), Dry Powder Inhalers (Dpis),
Nebulizers.
Abstract: Inhalation or aerosol administration is an area that deserves more research. It is a helpful way to deliver the
drugs that are hard to administrated by other routes. It is also promising for delivering biomacromolecule
drugs such as insulin and peptide drugs. Aerosol administration is a multidisciplinary topic that includes
physics, chemistry, engineering, and physiology. The relationship between the respiratory system and aerosol
drugs is essential when studying aerosol administration. In order to get desirable effects, inhalation devices,
drug formulations, and the patient are three significant factors to consider. This paper includes three
commonly used delivery devices: metered-dose inhalers (MDIs), dry powder inhalers (DPIs), and nebulizers.
Research has found that most of the aerosol administration devices have low lung depositions, but nebulizers
can reach a relatively higher lung deposition than other devices. All of them have advantages and
disadvantages, but each of them possesses distinct characteristics. These three devices have different
mechanisms and require different formulations.
1 INTRODUCTION
Inhalation or aerosol administration is one of the
common drug administration routes and has been
widely used. It is mainly used to treat respiratory
diseases such as asthma, chronic obstructive
pulmonary disease (COPD), and lung fibrosis.
Research has shown that aerosol administration can
also be used to treat some systemic diseases such as
diabetes, anticoagulation, headache, and osteoporosis
(Groneberg et al., 2003). This paper mainly talks
about the drug delivery to the reparatory system, three
commonly used inhalation devices, and drug
formulations used in different devices.
The delivery pathway of aerosol administrated
drugs is the respiratory tract. There are two types of
respiratory epithelial cells that contribute to the
absorption of inhaled drugs: type I and type II
pneumocytes (Groneberg et al., 2003). In these two
types of cells, type I pneumocytes predominate in the
surface area of the lungs, so they play an important
role in inhaled drug absorption (Ehrhardt et al., 2002).
Through the epithelial cells, drugs can go to the
circulatory systems and exhibit target or systemic
effects. Besides, different devices are used by
different patients to treat various diseases. Three
common inhalation devices are MDIs, DPIs, and
nebulizers. Each of them has different but significant
functions. It is important to consider the patients’
conditions and drug formulations before choosing the
devices. Also, different devices require different drug
formulations to ensure their performances.
Compare to other common drug administration
routes, such as oral and intravenous administration,
aerosol administration shows significant advantages
over other administration routes. When a drug is
delivered by oral and intravenous routes, it circulates
throughout the whole body. In contrast, most aerosol
administrated drugs would directly have effects on
the target organ, the lungs. Only a small
concentration would go to the systemic circulation,
which can reduce the off-target effects (Rau, J. L.,
2005). Aerosol administration is also a promising
way to deliver the macromolecule drugs to human
bodies (Choy & Prausnitz, 2010).
2 CONSIDERATIONS OF
AEROSOL ADMINISTRATION
From the first use of inhalation of epinephrine in 1929
to the present day, problems and challenges of aerosol
administration have shown up (Rau, 2005). The
development of aerosol administrated drugs is a
1314
Yan, J.
Analysis on the Delivery and Formulations of Inhaled Drugs.
DOI: 10.5220/0011509100003443
In Proceedings of the 4th International Conference on Biomedical Engineering and Bioinformatics (ICBEB 2022), pages 1314-1319
ISBN: 978-989-758-595-1
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
multidisciplinary challenge—it needs considerations
from physics, physiology, engineering, and chemistry
aspects. Scientists need to consider the interactions
between the respiratory system and the drug particles.
In order to achieve successful delivery and absorption
of the drugs into our system, it is important for the
manufacturers to consider the drug products' particle
shape and size, humidity, hygroscopy, excipient, and
density (Groneberg et al., 2003). Individual
difference is also a critical factor to consider during
drug delivery. Gender, body weight, age, and tidal
volume might influence the dosing and efficacy of the
drugs.
Compare to oral and intravenous administration,
aerosol administration is more complicated. It needs
devices to help the administration. The three common
aerosol delivery devices are dry powder inhalers
(DPIs), metered-dose inhalers (MDIs), and
nebulizers (Rau, 2005). Not only the drugs, but also
the devices are costly for both the patients and
researchers (Milgrom et al., 2001).
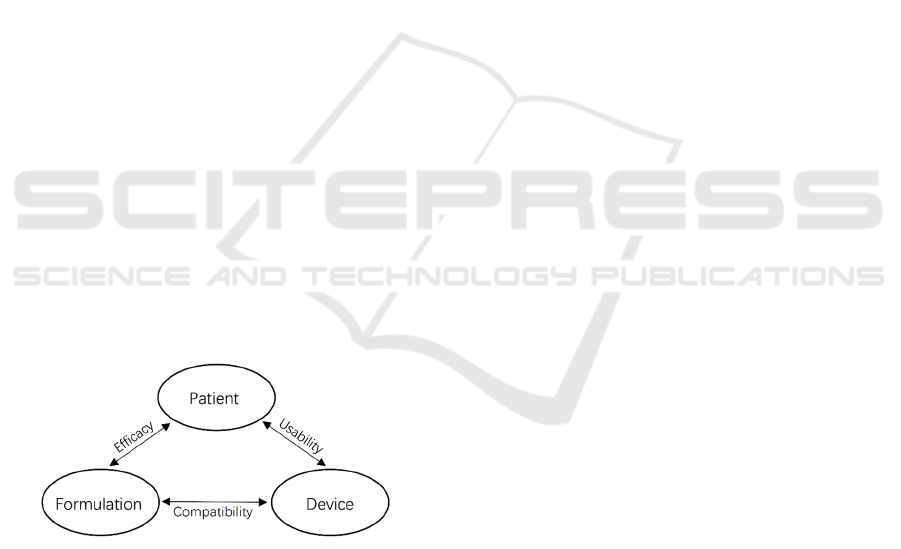
As shown in Figure 1, there are inseparable
relationships among patients, formulation, and
devices of inhaled drugs (Hou et al., 2015). Patients
need to take effective drug formulations by using
available inhalation devices. The drug formulation
needs to fit into the devices and must be delivered by
the devices. Meanwhile, the drug formulation needs
to achieve efficacy in patients. Also, the devices
should be compatible with the drugs and usable by
the patients. The development of pharmaceutical
engineering technologies of inhaled drugs is crucial
to obtain a desirable relationship among these three
considerations.
Figure 1: Considerations of aerosol administered drugs
(Hou et al., 2015).
3 DRUG DELIVERY TO THE
RESPIRATORY SYSTEM
The delivery and absorption of aerosol drugs are
mainly through the lower respiratory tract, including
small bronchioles and alveoli (Groneberg et al.,
2003). Between type I and type II pneumocytes, type
I pneumocytes are in charge of drug absorption
(Groneberg et al., 2003). In addition, type I
pneumocytes epithelial cells are the rate-limiting step
of absorption because they have smaller pore size and
tight junction depth compare to endothelial cells
(Wangensteen et al., 1969). In order to be absorbed
through the epithelial cells, particle size is a
significant characteristic to consider. Aerodynamic
diameter, dA, is used to describe the particle size of
inhaled drugs. Research has shown that particles with
dA smaller than 5 μm can reach small bronchioles
and alveoli to exhibit local effects (Chow et al.,
2007). Meanwhile, particles with dA that are between
1-2 μm can go to the systemic circulation, which
might lead to off-target effects (Chow et al., 2007).
Different excipients are used to manufacture different
drugs, and they might also influence the relationship
between particle size and absorption. For example,
research has shown that the aerodynamic diameter for
solution-based aerosol drugs are usually 2 μm, while
the one for suspension-based aerosol drugs are
usually 4 μm (Chow et al., 2007).
Hygroscopy is also a vital characteristic to
consider. The humidity of the environment might
influence the particle size of drugs. When the
environment reaches a humidity of about 44 μg/cm3,
hygroscopic growth of the drug particles can happen
(Groneberg et al., 2003). Therefore, the administrated
particle size might increase in the respiratory tract.
The hygroscopicity of excipients is one of the major
reasons that lead to an increase in particle size. Water
vapors in the human respiratory tract would bind with
the hygroscopic excipients to increase the size (Worth
Longest & Hindle, 2011). Thus, during drug delivery,
the final particle size after exposure to water vapor is
also an essential factor to consider.
Aerosol administration would have different
effects on different individuals because each patient
has different physiological conditions. Tidal volume,
breath pattern, and flow rates would all affect the
drug efficacy (Groneberg et al., 2003). Age and
gender would lead to individual differences in these
three parameters. Therefore, it is crucial to administer
aerosol drugs differently to different groups of people.
4 DEVICES
4.1
Metered-Dose Inhalers (MDIs)
MDIs are commonly used by asthma and chronic
obstructive pulmonary disease (COPD) patients for
treating bronchospasm (Hou et al., 2015). MDI
containers have three parts, which are metering valve,
Analysis on the Delivery and Formulations of Inhaled Drugs
1315
canister, and actuator. The metering valve controls
the volume of a single dose. The canister contains
pressurized drug formulation. Then the drug
formulation is decompressed and released by the
actuator (Lavorini, 2013). One of the advantages of
MDI devices is that a single device can contain
multiple doses, so that it can be used for a long time.
It has shown that one MDI device contains at least
two hundred doses (Lavorini, 2013). Since every dose
has an equal volume, there is no worry for overdose.
Comparably, MDI devices are also portable and low-
cost. However, MDI devices require good
coordination between patients and the devices.
Patients need to breathe while releasing the drug and
hold breath for a few seconds to increase lungs
deposition (Lavorini, 2013). In addition, the materials
of the metering valve, canister, and actuator would
affect the drug properties. The inner walls of the MDI
container are usually coated with polymers, such as
perfluoroalkoxy (PFA), fluorinated ethylene
propylene–polyether sulphone (FEP–PES), and
polytetrafluoroethylene (PTFE), to prevent changes
in drug properties (Traini et al., 2006).
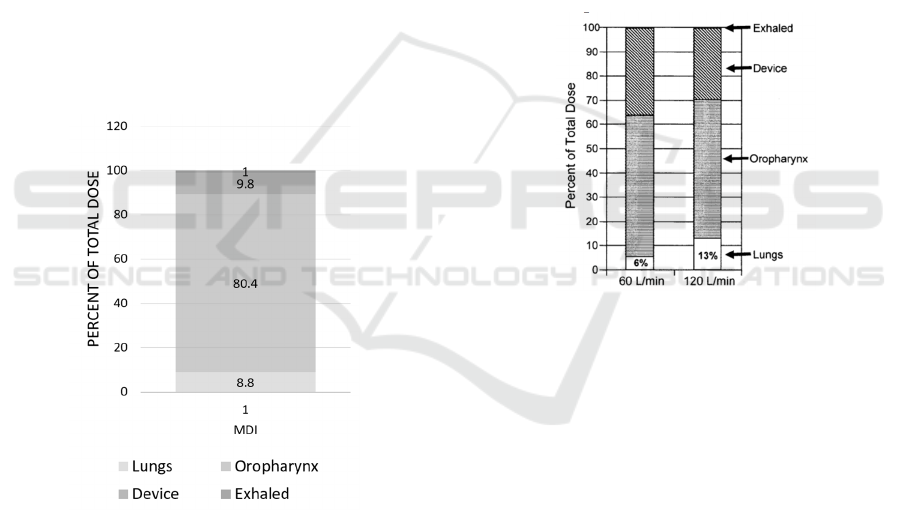
Figure 2: Drug deposition for patients who use MDI
devices (Newman et al., 1981).
Newman et al. has measured drug depositions and
the data is shown in figure 2. When delivered by MDI,
most of the drugs, about 80.4%, would lost in the
oropharynx. Only 8.8% of the total amount would be
delivered to the lungs (Newman et al., 1981). There
are also other studies showed that the lung deposition
when using MDI with good techniques can reach to
11.2% (Newman et al., 1986).
4.2
Dry Powder Inhalers (DPIs)
Similar to MDI devices, DPI devices are portable and
convenient. A DPI device can have only one dose or
multiple doses. If one DPI device is single-dosed, it
is a disposable inhaler (Hou et al., 2015). Recent
research shows that disposable DPIs are suitable for
inhaled COVID-19 vaccines because they can
prevent reuse and contamination (Heida et al., 2021).
Furthermore, unlike MDIs, DPI devices do not
require coordination between patients and devices.
Drug delivery of DPIs only relies on patients’ breath
independently (Hou et al., 2015). However, this leads
to a drawback of this kind of device: DPIs require a
certain amount of inspiratory flow rate to get an
effective dosage (Lavorini, 2013). For example,
Newman et al. conducted an experiment that
measures the drug deposition in patients who use a
DPI device, SpinHaler.
Figure 3: Drug deposition for patients who use SpinHaler
with a high inspiratory flow rate (120 L/min) and with a
low inspiratory flow rate (60 L/min) (Newman et al., 1994).
As shown in Figure 3, the experiment illustrated
that drug deposition in lungs for patients with a higher
inspiratory flow rate and those with a lower
inspiratory flow rate are significantly different
(Newman et al., 1994). The drug deposition in the
lungs is doubled when the inspiratory flow rate is
doubled (Newman et al., 1994). Thus, some patients
might not use them correctly and efficiently.
Research has found that 94% of the patients do not
use the DPI devices correctly (Lavorini et al., 2008).
4.3
Nebulizers
Nebulizers are relatively larger and less portable than
MDIs and DPIs. They can provide continuous drug
delivery, which is especially useful for the delivery of
large-dosed drugs (Hou et al., 2015). This also leads
to a longer time duration of drug delivery, so some
nebulizers require outside energy sources to conduct
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
1316
drug delivery (Lavorini, 2013). However, nebulizers
are easier for patients to use. Nebulizers usually need
mouthpieces or facemasks to delivery drugs to
patients (Lavorini, 2013). Patients only need to
perform their normal breathing pattern to use
nebulizers, which is especially helpful for
incoordinate patients such as infants and elderly
patients (Lavorini, 2013). Tidal volume determines
the amount of drug delivered, so there might be
individual differences in terms of efficacy. On the
other hand, nebulizers are not disposable, so the drugs
need to be loaded into the devices. Thus, compare to
MDIs and DPIs, nebulizers have a higher chance of
causing drug contamination (Lavorini, 2013).
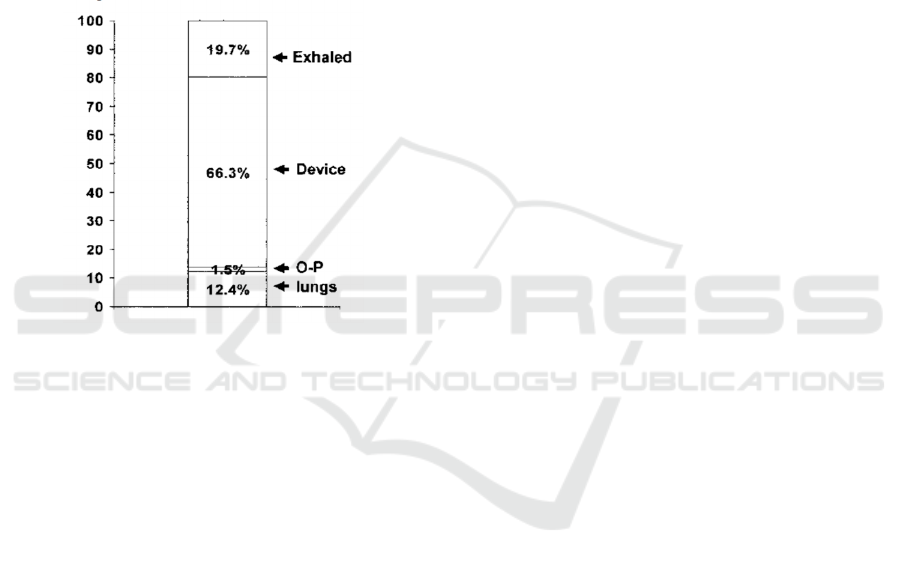
Figure 4: Drug deposition for patients who use Inspiron
Mini-Neb. O-P=oropharyngeal (Lewis & Fleming, 1985).
Experiment has found that nebulizers can reach a
lung deposition of 12.4%, which is higher than the
one for MDIs (Lewis & Fleming, 1985). In addition,
compare to MDIs and DPIs, most of the drugs, about
66.3%, would lost in nebulizer devices (Lewis &
Fleming, 1985).
5 FORMULATIONS
Different devices utilize different drug formulations.
MDIs and nebulizers are usually used to deliver
suspension or aqueous solution formulated drugs,
while DPIs, as the name indicates, are usually used to
deliver drug powders (Hou et al., 2015). Also, MDIs
contain propellants while DPIs and nebulizers do not
(Lavorini, 2013). Since different delivery devices
possess different mechanisms, different excipients
and formulations are needed to ensure the desired
performance of each kind of device.
5.1
Metered-Dose Inhalers (MDIs)
Drug particles are conserved with propellants in
MDIs and delivered together. Different propellants
were used since the first use of MDIs. One of the
previously used propellants is chlorofluorocarbons
(CFC), but it is no longer in use since the Montreal
Protocol in 1987 because of ozone-depleting effects
(Hou et al., 2015). The commonly used propellants
now are hydrofluoroalkane (HFA), which includes
HFA 134a and 227ca, which are less likely to cause
global warming than CFC (Lavorini, 2013). Except
for propellants, MDIs also need surfactants and
cosolvents (Lavorini, 2013). Some common
surfactants are sorbitan trioleate, lecithin, oleic acid,
and polyethylene glycol (PEG), which mainly serve
as valve lubricants and inhibit particle aggregation. It
is also shown that some surfactants contribute to the
taste (Lavorini, 2013). However, the solubility of
some surfactants is not ideal in HFA propellants
(Vervaet & Byron, 1999). Cosolvents such as ethanol
would help to improve the solubility of surfactants
(Hou et al., 2015).
5.2
Dry Powder Inhalers (DPIs)
DPIs are used to deliver solid drug powders into
human body systems. The commonly used excipient
or carrier of DPI drugs is lactose. Micronized drugs
first blend with lactose particles with diameters of 30-
60 μm; they are granulated into micronized particles
using wet or dry granulation (Chow et al., 2007).
Meanwhile, particle size and shape are important
parameters to consider when formulating dry powder
inhaler drugs. Amorphous particles are less efficient
during delivery because of their high-energy surface
(Kawashima et al., 1998). Research has shown that
lung deposition is higher when the particles are
elongated and pollen-shaped (Fults et al., 1997).
Various techniques can be used in DPI drugs
formulation, such as spray drying, freeze-drying, and
roller drying (Hou et al., 2015). Among these
techniques, in vitro research has shown that
anhydrous β-lactose by using roller drying might be
more efficient and doable (Chow et al., 2007).
Trehalose, mannitol, and menthol could be other
excipient carriers that can replace lactose (Chow et
al., 2007).
5.3
Nebulizers
Nebulizers are usually used to deliver drugs with
suspension or aqueous solution formulations. A
common solvent of nebulizer drugs is sterile water,
Analysis on the Delivery and Formulations of Inhaled Drugs
1317

which is the same solvent for intravenous injection
(Hou et al., 2015). Similar to MDI drugs formulation,
ethanol can be the cosolvent for nebulizer drugs as
well (Hou et al., 2015). Furthermore, the physical
properties of drug formulations are essential because
they might lead to change in delivery efficiency and
result in side effects (Labiris & Dolovich, 2003). For
example, a low pH would lead to
bronchoconstriction, which might result in irritation
(Labiris & Dolovich, 2003). In this case, the pH can
be increased by adding sodium hydroxide, while
similarly, hydrochloric acid can be added if the pH is
too high (Hou et al., 2015). Besides, solution
viscosity can influence the size of particles—the
larger the viscosity, the smaller the particle size (Hou
et al., 2015). Therefore, physical properties are
crucial factors to consider in nebulizer drug
formulation. In addition, since nebulizers are not
disposable and have a higher chance of getting
contaminated, preservatives are needed.
Benzalkonium chloride can be an antimicrobial
preservative (Hou et al., 2015).
6 CONCLUSIONS
Aerosol administration is a promising area of drug
delivery and still needs more research. It is not as
common as other administration routes, such as oral
and intravenous administration, but it is a helpful way
of drug delivery. Aerosol administration has benefits
when the local administration is wanted. Meanwhile,
some aerosol drugs also have systemic effects.
Inhaled drugs are mainly absorbed by type I
pneumocytes to exhibit local or systemic effects.
There are three most commonly used inhalation
devices: metered-dose inhalers (MDIs), dry powder
inhalers (DPIs), and nebulizers. Each of them has
advantages and drawbacks compared to others. We
need to put drug formulation and patient conditions
into account when using these devices. Different
drugs and devices need different excipients and
formulations. The inhaled drugs and formulations
need to be compatible with specific devices. There
also might be individual differences in aerosol
administration because each patient has different
physiological conditions. Devices, formulations, and
patients together are three crucial factors to consider
for the development of aerosol administration (Hou
et al., 2015).
There are many approved inhalation devices. This
paper focuses on three common ones with different
formulation requirements. MDIs and nebulizers
require suspension or solution formulations, while
DPIs require drug powder formulations. There are
many aspects to consider in drug formulation, such as
propellant, excipients, and physical properties. More
studies on the specific effects of different
formulations and the optimal devices and
formulations for specific systems are still needed.
ACKNOWLEDGEMENTS
I would like to express my sincere gratitude to
Professor Axel Zeitler for delivering and sharing
knowledge about pharmaceutical engineering, which
laid a foundation of this paper. I was also grateful to
him for providing advice and suggestions. I would
also appreciate my peer tutor, Cheng Wei, for
providing not only advice, but also interesting
discussions.
REFERENCES
Chow, A. H. L., Tong, H. H. Y., Chattopadhyay, P., &
Shekunov, B. Y. (2007). Particle Engineering for
Pulmonary Drug Delivery. Pharmaceutical Research,
24(3), 411–437. https://doi.org/10.1007/s11095-006-
9174-3
Choy, Y. B., & Prausnitz, M. R. (2010). The Rule of Five
for Non-Oral Routes of Drug Delivery: Ophthalmic,
Inhalation and Transdermal. Pharmaceutical Research,
28(5), 943–948. https://doi.org/10.1007/s11095-010-
0292-6
Ehrhardt, C., Fiegel, J., Fuchs, S., Abu-Dahab, R.,
Schaefer, U. F., Hanes, J., & Lehr, C.-M.. (2002). Drug
Absorption by the Respiratory Mucosa: Cell Culture
Models and Particulate Drug Carriers. Journal of
Aerosol Medicine, 15(2), 131–139.
https://doi.org/10.1089/089426802320282257
Fults, K. A., Miller, I. F., & Hickey, A. J. (1997). Effect of
Particle Morphology on Emitted Dose of Fatty Acid-
Treated Disodium Cromoglycate Powder Aerosols.
Pharmaceutical Development and Technology, 2(1),
67–79. https://doi.org/10.3109/10837459709022610
GRONEBERG, D. A., WITT, C., WAGNER, U., CHUNG,
K. F., & FISCHER, A. (2003). Fundamentals of
pulmonary drug delivery. Respiratory Medicine, 97(4),
382–387. https://doi.org/10.1053/rmed.2002.1457
Heida, R., Hinrichs, W. L., & Frijlink, H. W. (2021).
Inhaled vaccine delivery in the combat against
respiratory viruses: a 2021 overview of recent
developments and implications for COVID-19. Expert
Review of Vaccines.
https://doi.org/10.1080/14760584.2021.1903878
Hou, S., Wu, J., Li, X., & Shu, H. (2015). Practical,
regulatory and clinical considerations for development
of inhalation drug products. Asian Journal of
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
1318

Pharmaceutical Sciences, 10(6), 490–500.
https://doi.org/10.1016/j.ajps.2015.08.008
Kawashima, Y., Serigano, T., Hino, T., Yamamoto, H., &
Takeuchi, H. (1998). Effect of surface morphology of
carrier lactose on dry powder inhalation property of
pranlukast hydrate. International Journal of
Pharmaceutics, 172(1-2), 179–188.
https://doi.org/10.1016/s0378-5173(98)00202-6
Labiris, N. R., & Dolovich, M. B. (2003). Pulmonary drug
delivery. Part II: The role of inhalant delivery devices
and drug formulations in therapeutic effectiveness of
aerosolized medications. British Journal of Clinical
Pharmacology, 56(6), 600–612.
https://doi.org/10.1046/j.1365-2125.2003.01893.x
Lavorini, F. (2013). The Challenge of Delivering
Therapeutic Aerosols to Asthma Patients. ISRN
Allergy, 2013, 1–17.
https://doi.org/10.1155/2013/102418
Lavorini, F., Magnan, A., Dubus, J. C., Voshaar, T.,
Corbetta, L., Broeders, M., Dekhuijzen, R., Sanchis, J.,
Viejo, J. L., Barnes, P., Corrigan, C., Levy, M., &
Crompton, G. K. (2008). Effect of incorrect use of dry
powder inhalers on management of patients with
asthma and COPD. Respiratory Medicine, 102(4), 593–
604. https://doi.org/10.1016/j.rmed.2007.11.003
Lewis, R. A., & Fleming, J. S. (1985). Fractional deposition
from a jet nebulizer: How it differs from a metered dose
inhaler. British Journal of Diseases of the Chest, 79(4),
361–367. https://doi.org/10.1016/0007-
0971(85)90069-5
Milgrom, H., Skoner, D. P., Bensch, G., Kim, K. T., Claus,
R., & Baumgartner, R. A. (2001). Low-dose
levalbuterol in children with asthma: Safety and
efficacy in comparison with placebo and racemic
albuterol. Journal of Allergy and Clinical Immunology,
108(6), 938–945.
https://doi.org/10.1067/mai.2001.120134
Newman, S. P., Hollingworth, A., & Clark, A. R. (1994).
Effect of different modes of inhalation on drug delivery
from a dry powder inhaler. International Journal of
Pharmaceutics, 102(1-3), 127–132.
https://doi.org/10.1016/0378-5173(94)90047-7
Newman, S. P., Pavia, D., Moren, F., Sheahan, N. F., &
Clarke, S. W. (1981). Deposition of pressurised
aerosols in the human respiratory tract. Thorax, 36(1),
52–55. https://doi.org/10.1136/thx.36.1.52
Newman, S. P., Woodman, G., Clarke, S. W., & Sackner,
M. A. (1986). Effect of InspirEase on the Deposition of
Metered-Dose Aerosols in the Human Respiratory
Tract. Chest, 89(4), 551–556.
https://doi.org/10.1378/chest.89.4.551
Rau, J. L. (2005). The Inhalation of Drugs: Advantages and
Problems. Respiratory Care, 50(3), 367–382.
Traini, D., Young, P. M., Rogueda, P., & Price, R. (2006).
The Use of AFM and Surface Energy Measurements to
Investigate Drug-Canister Material Interactions in a
Model Pressurized Metered Dose Inhaler Formulation.
Aerosol Science and Technology, 40(4), 227–236.
https://doi.org/10.1080/02786820500543316
Vervaet, C., & Byron, P. R. (1999). Drug–surfactant–
propellant interactions in HFA-formulations.
International Journal of Pharmaceutics, 186(1), 13–30.
https://doi.org/10.1016/s0378-5173(99)00134-9
Wangensteen, O. D., Wittmers, L. E., & Johnson, J. A.
(1969). Permeability of the mammalian blood-gas
barrier and its components. American Journal of
Physiology-Legacy Content, 216(4), 719–727.
https://doi.org/10.1152/ajplegacy.1969.216.4.719
Worth Longest, P., & Hindle, M. (2011). Numerical Model
to Characterize the Size Increase of Combination Drug
and Hygroscopic Excipient Nanoparticle Aerosols.
Aerosol Science and Technology, 45(7), 884–899.
https://doi.org/10.1080/02786826.2011.566592
Analysis on the Delivery and Formulations of Inhaled Drugs
1319
