
Recovery of Chitin from Shrimp Waste by Co-fermentation
Qixin Wang
1
, Zhongliang Su
1,*
, Kunmei Huang
1
, Xueying Han
2
and Mingyan He
1
1
College of Chemical Engineering, Qingdao University of Science & Technology, Qingdao, Shangdong, China
2
College of Marine Science and Biological Engineering, Qingdao University of Science & Technology, Qingdao,
Shangdong, China
Keywords:
Chitin, Monomicrobial Fermentation, Two-Step Fermentation, Co-Fermentation.
Abstract:
The large amount of shrimp waste produced every year contains a large amount of natural cellulose material
(chitin), which causes a waste of resources. This paper uses co-fermentation to recover chitin from shrimp
waste and compares it with other common methods (monomicrobial fermentation, two-step fermentation). By
adjusting the inoculation ratio of the Bacillus zanthoxyli strain and Streptococcus thermophilus strain, the
ratio of glucose to sucrose, temperature, and pH, we obtained chitin with DP% and DM% of 68.89% and
83.80%, respectively. Through a comparative analysis, we found that DP% and DM% of chitin extracted by
co-fermentation were more balanced. The chitin improved by the small-size shrimp shells was DP%, DM%
and DA% of 83.76%, 91.48% and 93.47%, respectively, which proved the potential of obtaining the high
quality of chitin using the co-fermentation method. Co-fermentation is a viable alternative biological
fermentation method for extracting chitin from shrimp waste.
1 INTRODUCTION
The annual global amount of biosynthesized chitin
(β(1→4) linked GlcNAc) is approximately 100
billion tons (Ablouh 2020, Zhang 2020). It is the most
abundant natural polysaccharide after cellulose and is
used as a structural component to support cells and
body surfaces (Kumirska 2010, Gbenebor 2017,
Balitaan 2020). The world produces approximately 6
to 8 million tons of waste crab, shrimp, and lobster
shells annually (Yan, Chen 2015). The global market
for chitin and its derivatives include applications in
sewage treatment, food and beverages, cosmetics,
bioplastics, biomedicine, and agriculture (Casadidio
2019, Abdel-Mohsen 2020, Abdel-Mohsen 2020, Liu
2020). The amount of chitin prepared is
approximately 28,000 tons, but its demand exceeds
60,000 tons (Eddya 2020). Therefore, it is urgent to
discover a convenient, fast, environmentally friendly,
and cost-saving alternative for production.
Shrimp shells are composed of three layers (outer
layer, middle layer and inner layer). Chitin is located
in the inner layer of the shell and is wrapped with
protein. The middle layer is composed of chitin and
minerals, and the outer layer contains calcium
carbonate and protein (Balitaan 2020, Xin 2020).
There are two main methods for extracting chitin:
biological extraction and chemical methods. Acids
from microorganisms or HCl solutions in chemical
methods remove minerals from shrimp shells,
whereas proteases or NaOH solutions remove
proteins (Marzieh 2019). Deproteinization rate
(DP%) and demineralization rate (DM%) are often
used as standards of extracted chitin product
(Nidheesh, Suresh 2015). The degree of acetylation
(DA%) is defined as the average number of GlcNAc
units per 100 monomers, expressed as a percentage
(Tolaimate 2003). Chitin can deacetylate partially by
alkali (50% NaOH) in chemical methods or chitinase
in biological extraction (Hamed 2016). When the
DA% of chitin is less than 50%, it will dissolve in an
aqueous acid solution (pH <6.0), which is chitosan
(Kumirska 2010). DA% is the most important factor
affecting the application of chitin and chitosan (e.g.,
biodegradability, chemical modification steps, and
solubility). DA% of chitin depends on the raw
material and the deproteinization process (Tolaimate
2003). The chitin with high DA% and low protein
content is considered as good final products (Marzieh
2019).
Although chemical methods can quickly extract
high-quality chitin, such methods reduce the DA%,
thereby affecting the crystal structure (Gbenebor
2017), and it is impossible to recover value-added by-
1284
Wang, Q., Su, Z., Huang, K., Han, X. and He, M.
Recovery of Chitin from Shrimp Waste by Co-fermentation.
DOI: 10.5220/0011508700003443
In Proceedings of the 4th International Conference on Biomedical Engineering and Bioinformatics (ICBEB 2022), pages 1284-1295
ISBN: 978-989-758-595-1
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

products, such as proteins and pigments (García
2019). In addition, the molecular weight of the
product is low (80–800 kDa), and it cannot be used as
an ideal precursor for high-end functional materials
(Di 2019). Furthermore, these conventional chemical
extraction processes are extremely harmful to the
environment, and the high concentration of inorganic
acid that is consumed requires a large amount of fresh
water for washing after each step (Pachapur 2016,
Zhang 2018). Therefore, extracting chitin by
biological treatment became a research hotspot.
Comparing with chemical methods, chitin obtaining
by biological treatment has some advantages, such as
better antibacterial activity and biocompatibility,
higher molecular weight and crystal index,
environment-friendly, low cost (Tanganini 2020).
The biological method can recover additional
products, such as protein and pigment, and the
fermentation broth can be used as feed for
aquaculture (Younes 2016, Castro 2018). These
values demonstrate that chitin extracted by the
biological method has broader application prospects.
So far, the most studied biological method is
monomicrobial fermentation. In common bacteria,
the DP% and DM% of Bacillus subtilis,
Pseudomonas aeruginosa, Lactobacillus plantarum,
Bacillus cereus, and Lactobacillus rhamnosus were
84% and 72%, 94% and 84%, 95.3% and 99.6%,
78.6% and 73%, and 30.50% and 83.83%,
respectively (Sini 2007, Sorokulova 2009, Sedaghat
2017, Castro 2018, Liu 2020). There are a few strains
that can extract high-quality chitin alone (Nidheesh,
Suresh 2015), and precious few strains that contain
high DP% and high DM% simultaneously. For this
reason, some people combine high DM% strains and
enzymes or high DP% strains for two-step
fermentation. For example, Dun et al. (Dun 2019)
combined high-strain Bacillus coagulans and
proteinase K to ferment shrimp shells in two steps to
obtain high-quality chitin (DP% and DM% are 93%
and 91%, respectively); Yongliang Liu et al. [23]
used a high DM% (83.83%) strain Lactobacillus
rhamnoides and a high DP% strain (83.28%) Bacillus
amyloliquefaciens for joint fermentation and
obtained relatively ideal products (DP% and DM%
are 96.8% and 97.5%, respectively).
Although the fermentation efficiency (DP% and
DM%) of the two-step fermentation is higher, the re-
sterilization and replacement of the fermentation
broth for the second fermentation stage will
complicate the operation and cause a waste of
resources (Zhang 2021). Therefore, in this study, we
tried to explore if the methods of co-fermentation can
be used to extracting chitin from shrimp waste, and
its performance was compared with the
monomicrobial fermentation and two-step
fermentation under the same conditions to determine
whether this method is advantageous for producing
chitin. As far as we know, studies on the obtaining
chitin by co-fermentation have not been reported in
the literature.
2 MATERIALS AND METHODS
2.1 Materials
Fresh shrimp waste was collected from the Licun
Market, and the meat was removed, including head,
tail, and legs. The shrimp shells were dried in an oven
for 24 h and granulated with a crusher. Then, 2.00 - <
0.20 mm was extracted from it and stored in a reagent
bottle at −20℃.
2.2 Bacterial Strains and Culture
Conditions
The target strains were isolated from lactic acid
fermentation powder and soil obtained from Qingdao
University of Science and Technology, and the
culture medium was screened with protease strains
(lysogeny broth [LB] solid medium supplemented
with skimmed milk powder) and an acid-producing
strain selection medium (de Man, Rogosa, and Sharpe
[MRS] solid medium supplemented with CaCO
3
) to
screen out the strains with larger transparent circles
and to select strains, which have the best protease
activity, as well as high-yield acid strain, which can
be identified by cluster analysis on the sequence of
16S rRNA. The primers used were 27F (5′-
AGAGTTTGATCCTGGCTCAG-3′) and 1492R (5′-
ACGGCTACCTTGTTACGACTT-3′).
2.3 Fermentation Conditions and
Program Design
Depending on the literature, we chose the initial pH,
5% glucose, 4% inoculum, 3% shrimp shell, and 120
rpm as the initial fermentation conditions (Zhang
2012, Sedaghat 2017, Liu 2020) and reset the
vaccination plan on this basis: (1) the strains
producing high quality chitin in co-fermentation
method were used to explore the two-step
fermentation method and monomicrobial
fermentation method (fermentation time was 6 days);
(2) the fermentation conditions were optimized for
different fermentation methods (fermentation time
Recovery of Chitin from Shrimp Waste by Co-fermentation
1285

was 3 days); by comparing and analyzing the DP%
and DM% after fermentation, the best biological
fermentation plan was determined (fermentation time
was 6 days); (4) the small-sized shrimp shells were
fermented by the optimal fermentation method
(fermentation time was 6 days), and the products were
characterized by FT-IR.
2.4 The Analysis of Ash Content
Fresh shrimp shells were dried in a drying oven at
105℃ for 48 h, and the moisture content was
calculated by the weight difference before and after
drying ( Bellaaj 2012). The ash content was measured
by heating in a muffle furnace at 550℃ for 4 h
(Sedaghat 2017).
2.5 The Content Measurement of Total
Protein
The total protein, which in shrimp shells was
calculated by subtracting the nitrogen content in
chitin by the total nitrogen content, was measured
using a Kjeldahl Nitrogen Analyzer (JK9830; Jinan
Jingrui Analytical Instruments Ltd., China) (Liu
2020).
2.6 The Determination of Protease
Activity
The protease activity was determined according to the
method described by YaohaoDun et al. (Dun 2019).
In short, casein was used as a substrate, and
fermentation broth and phosphate buffer (pH = 7.5)
were added to induce a reaction at 30℃ for 10 min.
The reaction was terminated immediately with
trichloroacetic acid, and a color reaction with Folin
reagent was induced at 40℃ for 20 min. The
absorbance was measured at 680 nm. The protease
activity unit, which was expressed in U/ml, was
defined as the amount of 1 µg tyrosine produced by 1
ml liquid enzyme hydrolyzing casein in 1 min.
2.7 The Determination of DP% or DM%
The DP% or DM% was calculated by the equation:
Y% = ((C
1
× W
1
) − (C
2
× W
2
))/(C
1
× W
1
)
Y% is DP% or DM%; C
1
is the protein or ash
content before fermentation; W
1
is the protein or ash
dry weight before fermentation; C
2
is the protein or
ash content after fermentation; and W
2
is the protein
or ash dry weight after fermentation.
2.8 Characterization of Chitin
A Fourier transform infrared spectrometer (FT-IR)
was used to study chitin, and the DA% of chitin was
calculated based on the FT-IR (Knidri 2016).
The FT-IR was calculated by the equation:
DA%=
(
A
1655
/A
3450
)
×100/1.33
2.9 Statistical Analysis
All experimental data are observed in triplicate, and
the means ± standard deviations were reported.
Statistical analysis was conducted using SPSS
version 17 software. Statistical significance was
determined at P < 0.05.
3 RESULTS AND DISCUSSION
3.1 Strain Screening
Nine protease-producing strains and three acid-
producing strains with relatively large transparent
circles were selected from the screening medium of
the protease strain and the acid strain.
Protease activity and pH were measured 24 h after
fermentation in LB and MRS broth.
Three strains (2, 4, and 7) with higher protease
activity were selected and named B1, B2, and B3,
respectively (Table 1). Homologous strains were
searched in the NCBI library using 16S rRNA. The
homologies of strains B1, B2, and B3 with Bacillus
mobilis, Bacillus zanthoxyli, and Bacillus proteolytic
strains is as high as 99% or more. The three acid-
producing strains that were identified as the same
strain and have more than 99% homology with
Streptococcus thermophilus strain were named L.
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
1286

Table 1: Protease activity of enzyme-producing strains and pH of acid-producing strains.
numbers Protease activity (U/ml) pH
1 1.01 ± 0.22
2 6.55 ± 0.58
3 1.01 ± 0.63
4 3.22 ± 0.58
5 2.54 ± 0.15
6 2.77 ± 0.1
7 4.15 ± 0.63
8 1.46 ± 0.29
9 2.19 ± 0.19
10 4.97 ± 0.02
11 4.72 ± 0.02
12 4.9 ± 0.01
3.2 Screening of Fermentation Strains
We found that the fermentation efficiency of all
screened strains was relatively lower than previously
reported (Sini 2007, Castro 2018). In our study, the
highest DP% and DM% of the B2 strain were only
39.95% and 58.46%, respectively (Figure 1. A). It
may be that the size of our shrimp shells will not
allow acids and proteases to completely contact the
reactants (Abdelmalek 2017). To verify this
conjecture, we used the B2 strain to ferment shrimp
shells of different sizes. The result shows that the
fermentation efficiency in shrimp shells gradually
increased with the decreasing shrimp shell size and
reached the maximum at 0.45–0.3 mm (Figure 1. E).
The reason for the lower fermentation efficiency of
less than 0.3 mm is that the smaller size of the shrimp
shells facilitates aggregation in the triangular flask
during the fermentation process. In addition, the
smaller the shrimp shell size, the greater the loss
during washing. Therefore, shrimp shells with a
particle size of 2.00-1.43mm were used in the
fermentation exploration stage.
In the process of co-fermentation (Figure 1. B, C),
the DM% of the group with L bacteria was higher
than that of other groups (P < 0.05), but the DP% was
not significantly different (P < 0.05). The DP% and
DM% of B2-L were the best, at 17.04% (P < 0.05)
and 79.89% (P < 0.05), respectively. In the three-
strain and four-strain group, B1-B2-B3-L showed the
highest DM%, which was 81.82%, but the
fermentation conditions were more regulated than
those of B2-L. The other groups (excluding L) had
lower fermentation removal efficiency than had B2,
which did not show the potential to justify further
research. After comprehensive consideration, we
chose B2 and L as the fermentation strains for co-
fermentation.
The performance of B2 and L strains in two-step
fermentation were further explored. Figure 1.D shows
that fermentation efficiency of the replacement broth
group (C) was higher than that of the non-
replacement broth group (NC), and the results of
B2→L were more balanced than those of L→B2. We
found that the fermentation efficiency of during two-
step fermentation tends to depend on the strain used
in the first fermentation stage, which deviated from
the results of Liu, Y et al. (Liu 2020). The reason for
this phenomenon may be that the fermentation broth
of the second fermentation stage cannot provide
sufficient growth for bacteria or that the exposed
chitin in the shrimp shells inhibits the growth of
bacteria. In summary, we chose the B2→L-C group
for the two-step fermentation method for comparison
with the B2-L fermentation method.
Recovery of Chitin from Shrimp Waste by Co-fermentation
1287
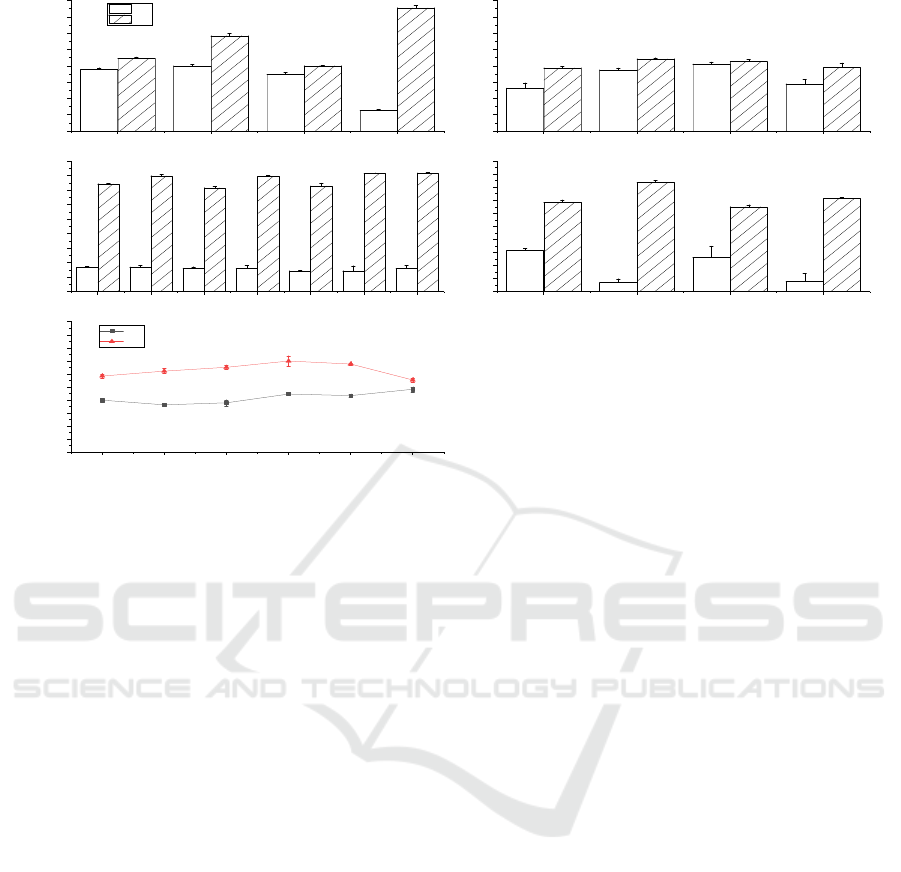
Figure 1: Selection of fermentation strains.
A, B, C, D, E: Residual protein and mineral
content of chitin after fermentation; D: C means
replacing the fermentation broth, NC means using the
fermentation broth of the previous strain for
fermentation
We chose B2 and L strains as the research objects
of co-fermentation, which can be compared with the
fermentation results of B2, L, and B2→L-C. Under
the initial conditions, we determined that the
fermentation of the B2 strain was the most balanced,
and the DM% of B2-L was the best.
3.3 The Optimization of
Monomicrobial Fermentation and
Two-step Fermentation
There are two indicators that the extracted chitin
needs to satisfy during the final evaluation process:
residual protein content and residual mineral content.
In the regulation of fermentation conditions, some
values have only the highest DM% or DP%. As
shows in Figure 3. D, DP% was the highest at 2.5%
sucrose content, but its DM% was the lowest, which
leaded to conflicts when we chose a certain
fermentation condition. We could not guarantee that
DM% and DP% were both at the optimal highest
proportion. Therefore, when selecting DP% and
DM%, it was not only required to have a relatively
small difference, but also a relatively high value, as
shows in Figure 5. B.
To better explored the fermentation of co-
fermentation, we first needed to explore the
fermentation conditions of single bacteria. Figure 2.
shows that carbon (C)/nitrogen (N) sources have a
greater impact on monomicrobial fermentation.
Among them, after sucrose was added, the DP% of
the B2 and L strains increased significantly (P <
0.05), but the decrease of the DM% of the L strain
from 67.73% to 0.63% (P < 0.05) was not suitable for
use as a C source. Tryptone and yeast extract powder
were suitable for B2 and L strains, respectively. In
summary, the B2 strain uses sucrose and tryptone as
C/N sources, whereas the L strain uses glucose and
yeast extract powder.
2.00-1.43 1.43-0.60 0.60-0.45 0.45-0.30 0.30-0.20 <0.20
0.0
0.1
0.2
0.3
0.4
0.5
0.6
0.7
0.8
0.9
1.0
B1 B2 B3 L
0.0
0.1
0.2
0.3
0.4
0.5
0.6
0.7
0.8
()
Removal %
Monomicrobial fermentation
DP
DM
A
B1-B2 B1-B3 B2-B3 B1-B2-B3
0.0
0.1
0.2
0.3
0.4
0.5
0.6
0.7
0.8
()
Removal %
Co-fermentation
B
B1-L B2-L B3-L B1-B2-L B1-B3-L B2-B3-L B1-B2-B3-L
0.0
0.1
0.2
0.3
0.4
0.5
0.6
0.7
0.8
0.9
()
Removal %
Co-fermentation
C
B2→L-C L→B2-C B2→L-NC L→B2-NC
0.0
0.1
0.2
0.3
0.4
0.5
0.6
0.7
0.8
0.9
1.0
()
Removal %
Two-step fermentation
D
()
Remova l %
()
Shrimp shell size mm
DP
DM
E
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
1288
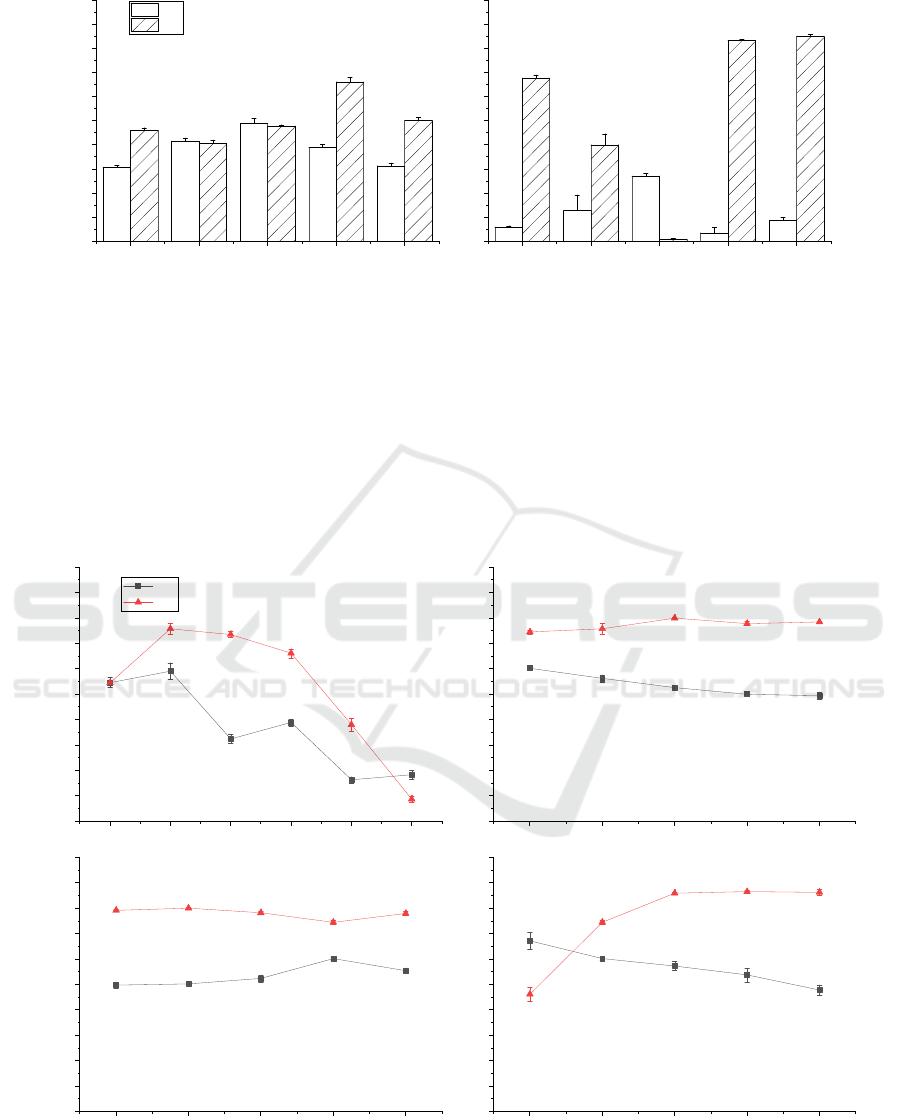
A: B2 strain; B: L strain
Figure 2: Optimization of C/N sources for B2 and L.
After the C/N source was determined, the B2
strain was optimized for temperature, inoculum, pH,
and sucrose content. The results show (Figure 3. A,
D) that the temperature and sucrose content have a
greater impact on the B2 strain. We chose relatively
high and balanced conditions of DP% and DM% for
the fermentation conditions of B2, and the
fermentation conditions of high DP% were used for
the conditions of the B2 strain in two-step
fermentation. Therefore, the optimized result of strain
B2 was 7.5% sucrose, 2% tryptone, 30℃, 2%
inoculum, and pH 7.5, and the optimized result of the
first fermentation as part of a two-step fermentation
process was 2.5% sucrose, 30℃, 2% inoculum, and
pH 7.5.
Figure 3: Optimization of fermentation conditions of B2 strain.
Glucose Maltose Sucrose Tryptone Yeast Extract Powder
0.0
0.1
0.2
0.3
0.4
0.5
0.6
0.7
0.8
0.9
1.0
()
Removal %
C/N Source
DP
DM
A
Glucose Maltose Sucrose Tryptone Yeast Extract Powder
0.0
0.1
0.2
0.3
0.4
0.5
0.6
0.7
0.8
0.9
1.0
()
Removal %
C/N Source
B
25 30 35 37 40 45
0.0
0.1
0.2
0.3
0.4
0.5
0.6
0.7
0.8
0.9
1.0
246810
0.0
0.1
0.2
0.3
0.4
0.5
0.6
0.7
0.8
0.9
1.0
6 6.5 7 7.5 8
0.0
0.1
0.2
0.3
0.4
0.5
0.6
0.7
0.8
0.9
1.0
2.5 5 7.5 10 12.5
0.0
0.1
0.2
0.3
0.4
0.5
0.6
0.7
0.8
0.9
1.0
()
Removal %
)
Temperature(℃
DP
DM
A
()
Removal %
Inoculation Amount(%)
B
()
Removal %
PH
C
Removal(%)
Sucrose Content(%)
D
Recovery of Chitin from Shrimp Waste by Co-fermentation
1289
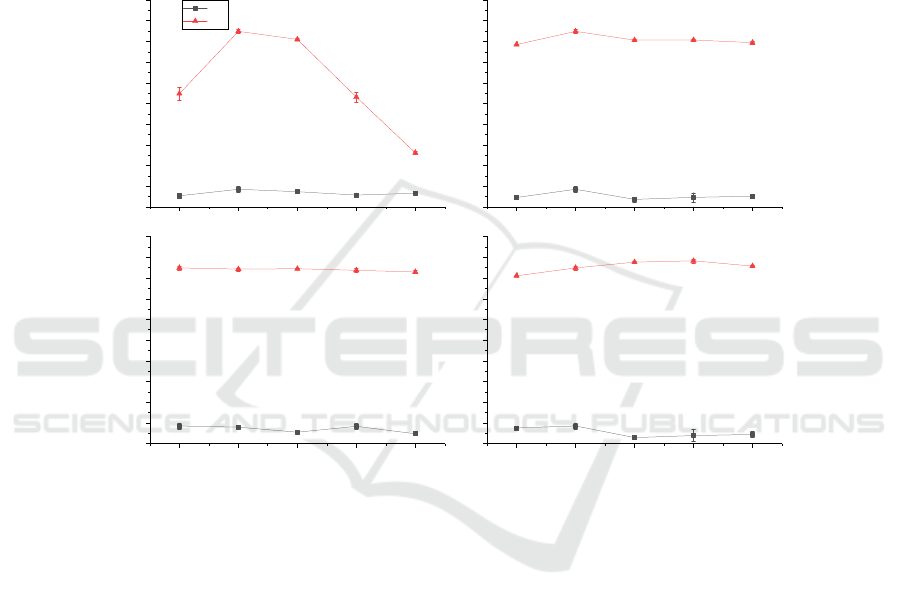
Only the temperature of the L strain had a greater
influence on DM% (Figure 4. A). The selection of
fermentation conditions was the same as that for the
B2 strain. Therefore, the optimized result of the L
strain was 5% glucose, 2% yeast extract, 30°C, 4%
inoculum, and pH 6.0; as the second fermentation
strain in the two-step fermentation process, the
fermentation conditions were 10% glucose, 2% yeast
extract powder, 30℃, 4% inoculum, and pH 6.0.
According to previous reports in literature (Zhang
2021), the two strains in the two-step fermentation
play different roles, one of which is responsible for
DP and the other is responsible for DM. In this
experiment, B2 with high DP% fermentation
conditions was mainly responsible for the
deproteinization of shrimp shells, and the L strain
with high DM% fermentation conditions was used to
demineralization. The final fermentation conditions
of B2→L-C were as follows: The first stage (B2
strain: 2.5% sucrose, 30℃, 2% inoculum, pH 7.5), the
second stage (L strain: 10% glucose, 30℃, 4%
inoculum, pH 6.0, 2% yeast extract powder).
Figure 4: Optimization of fermentation conditions of L strain.
3.4 The Optimization of the
Co-fermentation Conditions
Co-fermentation explores the ratio of each strain and
then considers how different strains may be adapted
to C sources. The DP% of B2-L (1:1) was too low, so
we attempted to increase the DP% of co-fermentation
by adjusting the ratio of B2 strains. Interestingly, as
the proportion of B2 strains increases, DP% gradually
increases and then stabilizes after 1:4. At 1:6, the
fermentation efficiency of chitin was optimal and
most balanced, although it was the same as that of B2
(P < 0.05). We think there is no value in continuing
to explore, so we continued to use 1:1. We mentioned
in Section 3.3 that the B2 strain prefers sucrose,
whereas the L strain prefers glucose. On this basis, we
adjusted the ratio of sucrose and glucose. The results
show (Figure 5. B) that when the ratio of glucose to
sucrose was 1:1, the fermentation efficiency (DP% =
52.49%, DM% = 43.93%) was better than the
fermentation effect of the B2 strain.
Since the optimal temperature of strain B2 and
strain L was the same, and the additional N source
would reduce the DP% (figure 3 and figure 4), we
only discussed changes in the inoculum, pH, and C
source content. Interestingly, the amount of
inoculation also had a huge impact on the results.
After the inoculation amount reached 6%, the
fermentation efficiency was similar to the
unoptimized result (P < 0.05). It is very likely that the
increase of the L strain inhibited enzyme production
of the B2 strain or inhibited the deproteinization of
protease. The final fermentation conditions of B2-L
were B2:L inoculated at a ratio of 1:1, a ratio of
glucose to sucrose of 1:1, 4% inoculation, and pH 7,
temperature 30℃.
25 30 35 40 45
0.0
0.1
0.2
0.3
0.4
0.5
0.6
0.7
0.8
0.9
1.0
246810
0.0
0.1
0.2
0.3
0.4
0.5
0.6
0.7
0.8
0.9
1.0
6 6.5 7 7.5 8
0.0
0.1
0.2
0.3
0.4
0.5
0.6
0.7
0.8
0.9
1.0
2.5 5 7.5 10 12.5
0.0
0.1
0.2
0.3
0.4
0.5
0.6
0.7
0.8
0.9
1.0
()
Removal %
)
Temperature(℃
DP
DM
A
()
Removal %
Inoculation Amount(%)
B
()
Removal %
PH
C
()
Removal %
Glucose Content(%)
D
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
1290
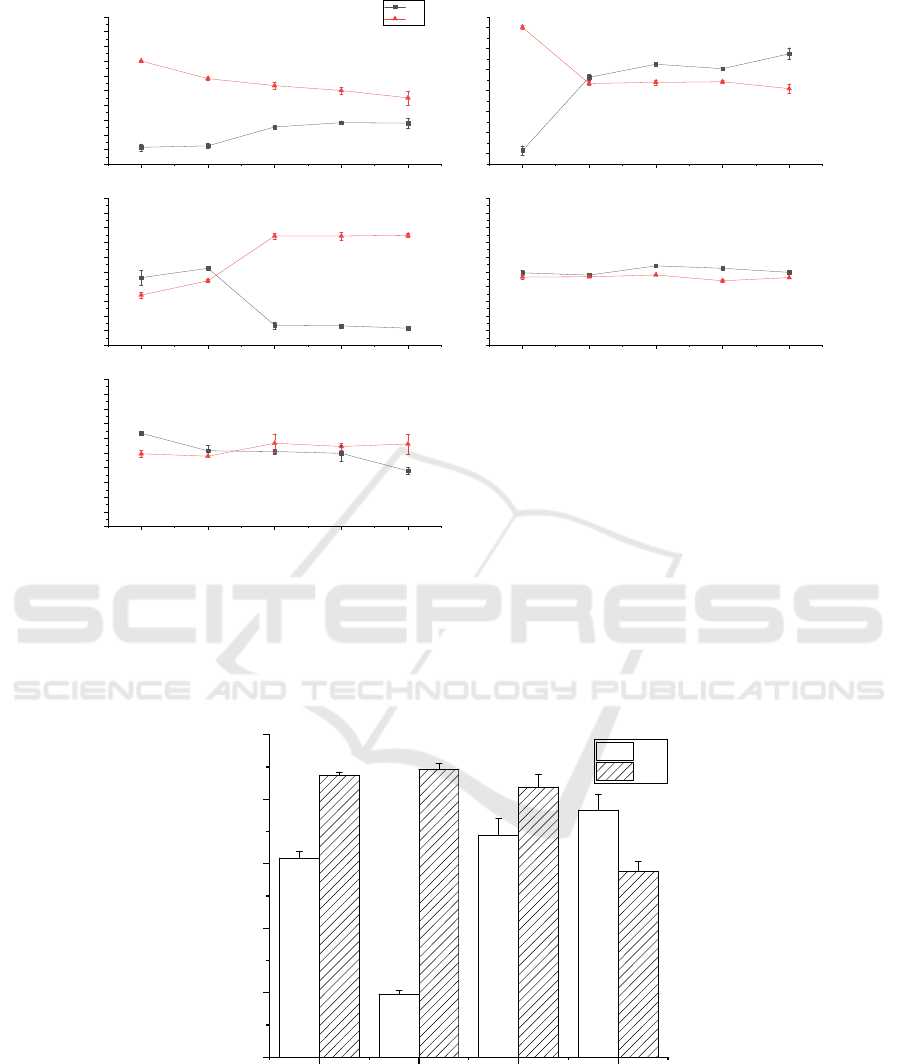
Figure 5: The optimized fermentation conditions of B2-L and the optimized results of three fermentation methods.
3.5 Comparison of Three Fermentation
Methods
Figure 6: The optimized results of the three fermentation methods.
Although the optimized L had the highest DM%
(89.19%, P<0.05, figure 6.), its DP% was only
19.55%. There are too many protein residues in
chitin, so the L strain was not suitable for extracting
chitin from shrimp shells by fermentation alone.
1:1 1:2 1:4 1:6 1:8
0.0
0.1
0.2
0.3
0.4
0.5
0.6
0.7
0.8
0.9
1.0
:10 :10.5 :11 :0.5 1 :01
0.1
0.2
0.3
0.4
0.5
0.6
0.7
246810
0.0
0.1
0.2
0.3
0.4
0.5
0.6
0.7
0.8
0.9
1.0
66.577.58
0.0
0.1
0.2
0.3
0.4
0.5
0.6
0.7
0.8
0.9
1.0
2.5 5 7.5 10 12.5
0.0
0.1
0.2
0.3
0.4
0.5
0.6
0.7
0.8
0.9
1.0
()
Removal %
Inoculation ratio of B2: L
DP
DM
A
()
Removal %
Glucose: Sucrose
B
()
Removal %
()
B2-L inoculation volume %
C
()
Removal %
PH
D
()
Removal %
()
The content of Glucose: Sucrose %
E
B2 L B2-L B2→L-C
0.0
0.2
0.4
0.6
0.8
1.0
()
Removal %
Fermentation methods
DP
DM
Recovery of Chitin from Shrimp Waste by Co-fermentation
1291

The DP% (76.64%) of the B2→L-C group was
the highest (figure 6.), which was different from the
initial fermentation results (figure 1. D). The reasons
for this phenomenon were that we purposely
increased the deproteinization efficiency of B2 strains
by optimization of fermentation conditions. Although
additional nutrients (C/N sources) could be added to
make up for the second stage fermentation of the
strain, this would also increased the cost of chitin
extraction. In addition, wastewater discharge and
energy consumption would also occur when the
fermentation broth was replaced (Zhang 2021).
Therefore, the B2 and L strains in this experiment
were not suitable for the production of chitin from
shrimp shells using the two-step fermentation
method.
The DP% and DM% of the optimized B2 strain
were 61.78% and 87.41% (figure 6.), respectively,
and there was a big difference between the DP% and
DM% (25.63%). Nevertheless the difference between
the DP% and DM% of the B2-L group (the DP% and
DM% is 68.89% and 83.80%) was the smallest
(14.91%), so its quality of fermentation product was
the highest when comparing the monomicrobial
fermentation and the two-step fermentation.
Although the regulation process of co-
fermentation (B2-L) is complicated, there are more
fermentation conditions that can be regulated than
monomicrobial fermentation. For example, co-
fermentation can optimize the ratio of different strains
and the ratio of different carbon sources. Therefore,
there are more opportunities to find suitable
fermentation conditions, and its potential to produce
high-quality chitin is higher. Compared with the two-
step fermentation method, the co-fermentation
operation was simple, and it only needed to complete
the whole fermentation in one step (Zhang 2012).
Therefore, the co-fermentation method for preparing
chitin is a potential fermentation method.
3.6 The Optimal Fermentation Results
for B2-L
Co-fermentation (B2-L), compared with the other two
fermentation methods, was considered to be the best
fermentation method. In order to further improve the
quality of its product, the effect of shrimp shell size
was studied (figure 7). The fermentation bottle was
vigorously shaken once a day in view of the tendency
of small-size shrimp shells to aggregate. The results
shows that the DP% and DM% could reach to 83.76%
and 91.48%, respectively when reduced the size of
shrimp shell (<0.2mm, figure 7), which indicated the
great potential of co-fermentation.
Figure 7: The fermentation efficiency of different sizes of shrimp shells in B2-L.
Sini T K et al. (Sini 2007) showed that the DP%
and DM% of chitin obtained from the fermentation of
shrimp shells by Bacillus subtilis were 84% and 72%.
Compared with it, the DM% increased by 19.48%
after co-fermentation of B2 and L (DP% = 83.76%,
DM% = 91.48%). The latest report showed that the
two-step fermentation of Bacillus subtilis and
Acetobacter pasteurianus can produce chitin with
DP% and DM% of 94.5% and 92.0% (Zhang 2021).
The DP% in this trial was 10.5% lower than the
2-1.43 1.43-0.6 0.6-0.45 0.45-0.3 0.3-0.2 <0.2
0.5
0.6
0.7
0.8
0.9
1.0
()
Removal %
DP
DM
()
Shrimp shell size mm
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
1292
former report. The DP% and DM% of Bacillus
subtilis, Bacillus cereus, and Bacillus mojavensis
reported by Hajji, S et al. (Hajji 2015) were 81.6%
and 76.6%, 83.1% and 81.8%, 80.4% and 73.2%
respectively, which were lower than the DP% and
DM% of chitin in this experiment. This showed that
the co-fermentation is a potential and effective
method for preparing chitin. The reason why their
fermentation result is lower than the former report
may be the weak DP ability of L strain. Therefore,
when selecting dominant strains, we must pay
attention to selecting strains with better acid-
producing ability and protease-producing ability.
3.7 Fourier Transform Infrared (FT-IR)
Spectra Analysis and Determination
of the Degree of Acetylation
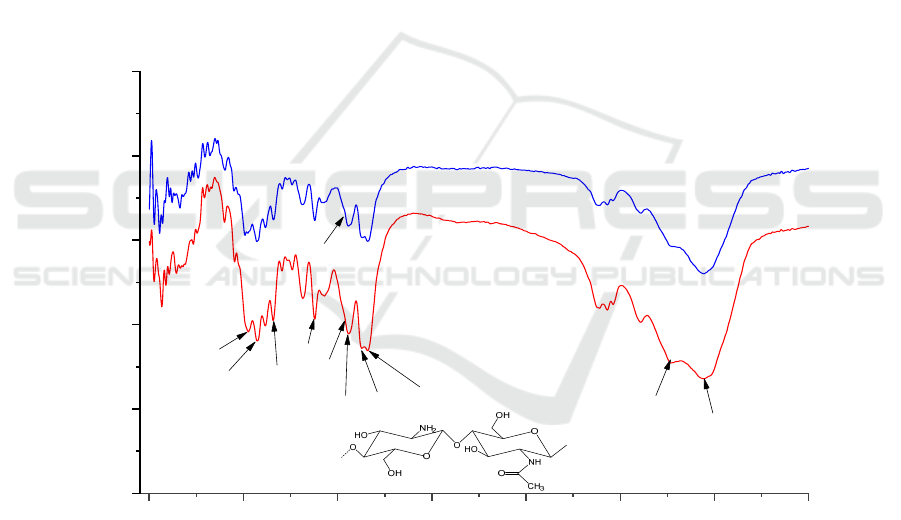
Figure 8. showed that the FT-IR of chitin prepared
from shrimp shells (<0.2mm) by co-fermentation of
B2 and L was similar to that of commercially
available chitin. The prepared chitin had the typical
characteristic peaks of α-chitin, including amide I
bands about 1659 cm
-1
and 1627 cm
-1
(C=O stretching
vibration), amide II bands at 1556 cm
-1
(N-H bending
vibration), and amide III at bands 1378 cm
-1
(C-N
stretching vibration). The stretching vibrations of -
OH and -NH appeared at 3441 cm
-1
and 3265 cm
-1
.
The other peaks of the chitin structure were 1157 cm
-
1
(C-O-C asymmetric vibration), 1025 cm
-1
and 1072
cm
-1
(C-O stretching vibration), and 1378 cm
-1
(C-H
shear vibration). This was the same as described by
El Knidri, H. et al (Knidri 2016). The spectrum of
chitin prepared by the co-fermentation method and
commercial chitin was lacking the absorbance peak at
1540 cm
−1
, where proteins would normally give rise
to absorption (Liu 2020).
The DA% of chitin prepared by B2-Lwere
93.47%, which is higher than that of chitin prepared
by commercial (86.37%) and Manni, L et al.
(89.50%) (Manni 2010).
A: Commercial chitin.
B: Chitin prepared by mixed-bacteria simultaneous fermentation method (B2-L).
Figure 8: FT-IR spectra of chitin.
4 CONCLUSIONS
The DP%, DM% and DA% with 83.76%, 91.48% and
93.47% of chitin were co-fermentation of B2 and L,
respectively. Comparing to the monomicrbial
fermentation and the two-step fermentation, the co-
fermentation can be used to extract chitin from
shrimp waste, which provides a feasible fermentation
method for the large-scale production of chitin in the
future.
500 1000 1500 2000 2500 3000 3500 4000
0
20
40
60
80
100
()
Transmittance %
Wavenumber (cm
-1
)
3441
3265
1659
1627
1556
1540
1025
1072
1157
1378
1540
A
B
Recovery of Chitin from Shrimp Waste by Co-fermentation
1293

ACKNOWLEDGMENTS
The authors would like to thank numerous individuals
who participated in this study. This work was
supported by the National Natural Science
Foundation of China under Grant 41471279.
REFERENCES
Abdel-Mohsen, A. M., R. M. Abdel-Rahman, I. Kubena, L.
Kobera, Z. Spotz, M. Zboncak, R. Prikryl, J. Brus and
J. Jancar (2020). "Chitosan-glucan complex hollow
fibers reinforced collagen wound dressing embedded
with aloe vera. Part I: Preparation and characterization."
Carbohydrate Polymers 230: 115708.
Abdel-Mohsen, A. M., J. Frankova, R. M. Abdel-Rahman,
A. A. Salem, N. M. Sahffie, I. Kubena and J. Jancar
(2020). "Chitosan-glucan complex hollow fibers
reinforced collagen wound dressing embedded with
aloe vera. II. Multifunctional properties to promote
cutaneous wound healing." International Journal of
Pharmaceutics 582: 119349.
Abdelmalek, B. E., A. Sila, A. Haddar, A. Bougatef and M.
A. Ayadi (2017). "β-Chitin and chitosan from squid
gladius: Biological activities of chitosan and its
application as clarifying agent for apple juice."
International Journal of Biological Macromolecules
104: 953-962.
Ablouh, E. H., R. Jalal, M. Rhazi and M. Taourirte (2020).
"Surface modification of alpha-chitin using an acidic
treatment followed by ultrasonication: Measurements
of their sorption properties." International Journal of
Biological Macromolecules 151: 492-498.
Aranday-García, R., H. Saimoto, K. Shirai and S. Ifuku
(2019). "Chitin biological extraction from shrimp
wastes and its fibrillation for elastic nanofiber sheets
preparation." Carbohydrate Polymers 213: 112-120.
Balitaan, J. N. I., J. M. Yeh and K. S. Santiago (2020).
"Marine waste to a functional biomaterial: Green facile
synthesis of modified-β-chitin from Uroteuthis
duvauceli pens (gladius)." International Journal of
Biological Macromolecules 154: 1565-1575.
Casadidio, C., D. V. Peregrina, M. R. Gigliobianco, S.
Deng, R. Censi and P. Di Martino (2019). "Chitin and
chitosans: Characteristics, eco-friendly processes, and
applications in cosmetic science." Marine drugs 17(6):
369.
Castro, R., I. Guerrero Legarreta and R. Bórquez (2018).
"Chitin extraction from Allopetrolisthes punctatus crab
using lactic fermentation." Biotechnology Reports 20:
e00287.
Di Nardo, T., C. Hadad, A. N. Van Nhien and A. Moores
(2019). "Synthesis of high molecular weight chitosan
from chitin by mechanochemistry and aging." Green
Chemistry 21(12): 3276-3285.
Dun, Y., Y. Li, J. Xu, Y. Hu, C. Zhang, Y. Liang and S.
Zhao (2019). "Simultaneous fermentation and
hydrolysis to extract chitin from crayfish shell waste."
International Journal of Biological Macromolecules
123: 420-426.
Eddya, M., B. Tbib and K. El-Hami (2020). "A comparison
of chitosan properties after extraction from shrimp
shells by diluted and concentrated acids." Heliyon 6(2):
e03486.
Gbenebor, O. P., S. O. Adeosun, G. I. Lawal, S. Jun and S.
A. Olaleye (2017). "Acetylation, crystalline and
morphological properties of structural polysaccharide
from shrimp exoskeleton." Engineering Science and
Technology-an International Journal-Jestech 20(3):
1155-1165.
Ghorbel-Bellaaj, O., I. Younes, H. Maâlej, S. Hajji and M.
Nasri (2012). "Chitin extraction from shrimp shell
waste using Bacillus bacteria." International Journal of
Biological Macromolecules 51(5): 1196-1201.
Hajji, S., O. Ghorbel-Bellaaj, I. Younes, K. Jellouli and M.
Nasri (2015). "Chitin extraction from crab shells by
Bacillus bacteria. Biological activities of fermented
crab supernatants." International Journal of Biological
Macromolecules 79: 167-173.
Hamed, I., F. Ozogul and J. M. Regenstein (2016).
"Industrial applications of crustacean by-products
(chitin, chitosan, and chitooligosaccharides): A
review." Trends in Food Science & Technology 48: 40-
50.
Knidri, H. E., R. E. Khalfaouy, A. Laajeb, A. Addaou and
A. Lahsini (2016). "Eco-friendly extraction and
characterization of chitin and chitosan from the shrimp
shell waste via microwave irradiation." Process Safety
and Environmental Protection 104: 395-405.
Kumirska, J., M. Czerwicka, Z. Kaczynski, A. Bychowska,
K. Brzozowski, J. Thoming and P. Stepnowski (2010).
"Application of Spectroscopic Methods for Structural
Analysis of Chitin and Chitosan." Marine Drugs 8(5):
1567-1636.
Liu, T., H. Y. Han, D. Wang, X. G. Guo, Y. Zhou, T.
Fukamizo and Q. Yang (2020). "Potent Fungal
Chitinase for the Bioconversion of Mycelial Waste."
Journal of Agricultural and Food Chemistry 68(19):
5384-5390.
Liu, Y., R. Xing, H. Yang, S. Liu, Y. Qin, K. Li, H. Yu and
P. Li (2020). "Chitin extraction from shrimp
(Litopenaeus vannamei) shells by successive two-step
fermentation with Lactobacillus rhamnoides and
Bacillus amyloliquefaciens." International Journal of
Biological Macromolecules 148: 424-433.
Manni, L., O. Ghorbel-Bellaaj, K. Jellouli, I. Younes and
M. Nasri (2010). "Extraction and Characterization of
Chitin, Chitosan, and Protein Hydrolysates Prepared
from Shrimp Waste by Treatment with Crude Protease
from Bacillus cereus SV1." Applied Biochemistry and
Biotechnology 162(2): 345-357.
Marzieh, M. N., F. Zahra, E. Tahereh and K. N. Sara (2019).
"Comparison of the physicochemical and structural
characteristics of enzymatic produced chitin and
commercial chitin." International Journal of Biological
Macromolecules 139: 270-276.
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
1294

Nidheesh, T. and P. V. Suresh (2015). "Optimization of
conditions for isolation of high quality chitin from
shrimp processing raw byproducts using response
surface methodology and its characterization." Journal
of Food Science and Technology-Mysore 52(6): 3812-
3823.
Pachapur, V. L., K. Guemiza, T. Rouissi, S. J. Sarma and S.
K. Brar (2016). "Novel biological and chemical
methods of chitin extraction from crustacean waste
using saline water." Journal of Chemical Technology
and Biotechnology 91(8): 2331-2339.
Sedaghat, F., M. Yousefzadi, H. Toiserkani and S.
Najafipour (2017). "Bioconversion of shrimp waste
Penaeus merguiensis using lactic acid fermentation: An
alternative procedure for chemical extraction of chitin
and chitosan." International Journal of Biological
Macromolecules 104: 883-888.
Sini, T. K., S. Santhosh and P. T. Mathew (2007). "Study
on the production of chitin and chitosan from shrimp
shell by using Bacillus subtilis fermentation."
Carbohydrate Research 342(16): 2423-2429.
Sorokulova, I., A. Krumnow, L. Globa and V. Vodyanoy
(2009). "Efficient decomposition of shrimp shell waste
using Bacillus cereus and Exiguobacterium
acetylicum." Journal of Industrial Microbiology &
Biotechnology 36(8): 1123-1126.
Tanganini, I. C., L. D. Shirahigue, M. A. da Silva, K. R.
Francisco and S. R. Ceccato-Antonini (2020).
"Bioprocessing of shrimp wastes to obtain chitosan and
its antimicrobial potential in the context of ethanolic
fermentation against bacterial contamination." 3
Biotech 10(3): 135.
Tolaimate, A., J. Desbrieres, M. Rhazi and A. Alagui
(2003). "Contribution to the preparation of chitins and
chitosans with controlled physico-chemical properties."
Polymer 44(26): 7939-7952.
Xin, R., W. Xie, Z. Xu, H. Che, Z. Zheng and X. Yang
(2020). "Efficient extraction of chitin from shrimp
waste by mutagenized strain fermentation using
atmospheric and room-temperature plasma."
International Journal of Biological Macromolecules
155: 1561-1568.
Yan, N. and X. Chen (2015). "Sustainability: Don't waste
seafood waste." Nature 524(7564): 155-157.
Younes, I., S. Hajji, M. Rinaudo, M. Chaabouni, K. Jellouli
and M. Nasri (2016). "Optimization of proteins and
minerals removal from shrimp shells to produce highly
acetylated chitin." International Journal of Biological
Macromolecules 84: 246-253.
Zhang, A., G. G. Wei, X. F. Mo, N. Zhou, K. Q. Chen and
P. K. Ouyang (2018). "Enzymatic hydrolysis of chitin
pretreated by bacterial fermentation to obtain pure N-
acetyl-D-glucosamine." Green Chemistry 20(10):
2320-2327.
Zhang, H., Y. Jin, Y. Deng, D. Wang and Y. Zhao (2012).
"Production of chitin from shrimp shell powders using
Serratia marcescens B742 and Lactobacillus plantarum
ATCC 8014 successive two-step fermentation."
Carbohydrate Research 362: 13-20.
Zhang, Q., L. Wang, S. Liu and Y. Li (2021).
"Establishment of successive co-fermentation by
Bacillus subtilis and Acetobacter pasteurianus for
extracting chitin from shrimp shells." Carbohydrate
Polymers 258: 117720.
Zhang, W. C., Y. Zhao, L. Xu, X. Q. Song, X. X. Yuan, J.
Y. Sun and J. S. Zhang (2020). "Superfine grinding
induced amorphization and increased solubility of
alpha-chitin." Carbohydrate Polymers 237: 116145.
Recovery of Chitin from Shrimp Waste by Co-fermentation
1295
