
N-acetylcysteine (NAC) Inhibits ROS-induced Ferroptosis in CTNS
Knockdown β-cells in Vitro
Hongrui Liang
1,*
, Jingyu Xu
2
, Yanxu Chen
3
, Jingjie Yu
4
and Ruoxun Peng
5
1
China Agricultural University, Haidian District, Beijing 100089, China
2
Shanghai Foreign Language School, Shanghai 200083, China
3
Shanghai Starriver Bilingual School, Shanghai 201108, China
4
The Village School, Houston, TX, U.S.A.
5
International Department, The Second High School Attached to Beijing Normal University, Beijing 100192, China
Keywords: CTNS, Cystine, Ferroptosis, NAC, Insulin Secretion.
Abstract: CTNS silence or mutation in the human body leads to cystinosis, an autosomal recessive lysosomal storage
disease. Ferroptosis is an iron-dependent form of programmed cell death. N-acetylcysteine (NAC), a common
antioxidant, is the acetylated precursor of L-cysteine. There are no studies about the relationship between
cystinosis and ferroptosis yet. Therefore, in this paper, we aimed to 1) find out the relationship between CTNS
knockdown and ferroptosis in β-cells and 2) verify that NAC is a potential agent to protect CTNS knockdown
β-cells and is also a potent ferroptosis inhibitor. Since we do not have the access to do the experiments in a
laboratory, all the following results and conclusions are hypothetical or from existing papers: CTNS-targeting
siRNA inhibit cystinosin expression at mRNA and protein level; CTNS knockdown induces ferroptosis in β-
cells and NAC attenuates ferroptosis; NAC attenuates oxidative stress in CTNS knockdown β-cells; NAC
restores energy level and glucose-stimulated insulin secretion in CTNS knockdown β-cells. We conclude that,
in vitro, the NAC pretreatment can effectively rescue CTNS knockdown β-cells from ferroptosis by elevating
the GPX4 mRNA and its protein level.
1 INTRODUCTION
Cystinosin is a protein transporter that excretes
cystine combined with a proton from the lysosome to
the cytosol, which is encoded by the CTNS gene.
Mutations in the human CTNS gene will hinder the
efflux of lysosomal cystine and cause lysosomal
cystine accumulation, leading to an autosomal
recessive inheritance disease, that is, cystinosis
(Gahl, Thoene, Schneider 2009). Some researchers
discovered that lysosomal cystine accumulation in
cystinosin-deprived cells changes the cytosolic redox
milieu to higher oxidized status by limitation of
glutathione (GSH) synthesis (L. E, de G.-H. A, W. M,
van den H. L, M. L, B. H 2005, S. R, M. B, N. P, M.
T 2016, B. F et al 2010). Furthermore, abundant
research indicated that β-cell is more sensitive to
oxidative stress due to low expression of catalase and
peroxidase and low GSH level compared to other
tissues (N. S, S. H, A. R, T. T, and Y. T 2008, S. K et
al 2003, L. S, D. J, and T. M 1996). Former reports
showed that intracellular cysteine level, reactive
oxygen species (ROS) level, and energy production
correlate with insulin secretion (N. S, S. H, A. R, T.
T, and Y. T 2008, S. K et al 2003), which is
demonstrated in CTNS-knockdown β-cells (M. B, S.
R, S. C, M. T, and N. P 2015). The elevated cystine
and ROS level concomitant with stymied ATP
production in cytosol and mitochondria caused
attenuated insulin secretion. This conclusion at the
cell level keeps aligned with the summary of
cystinosis complication (N. G, G. W 2008).
Ferroptosis is an iron-dependent form of regulated
cell death. It is related to iron-dependent lipid
peroxidation metabolism and regulates cell death
through NADPH/H
+
, polyunsaturated fatty acids,
glutamine catabolism, and other signal pathways.
Ferroptosis demonstrates cellular atrophy and high-
density mitochondria in morphology. The cystine
antiporter system mediates the production of GSH,
which is an important ferroptosis inhibitor (X. Y et al
2015, S. BR et al. 2017). Cysteine is the rate-limiting
metabolite for GSH biosynthesis, so cysteine
depletion leads to the lowering of intracellular GSH
Liang, H., Xu, J., Chen, Y., Yu, J. and Peng, R.
N-acetylcysteine (NAC) Inhibits ROS-Induced Ferroptosis in CTNS Knockdown -Cells in Vitro.
DOI: 10.5220/0011390800003443
In Proceedings of the 4th Inter national Conference on Biomedical Engineering and Bioinformatics (ICBEB 2022), pages 1235-1243
ISBN: 978-989-758-595-1
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
1235
levels. GSH depletion triggers the inactivation of
GPX4 which is an enzyme that specifically reduces
phospholipid hydroperoxides using GSH as a
cofactor (Y. WS et al. 2013). Cystine starvation
impairs GPX4 protein expression by inhibiting
mTORC1/4E-BP1-mediated protein translation (Z. Y
et al. 2021). The inactivation of GPX4 leads to lipid
peroxidation which causes the accumulation of ROS
(Y. WS et al. 2013). ROS can react with
polyunsaturated fatty acids of lipid membranes and
induce lipid peroxidation on membranes. Also,
intracellular cysteine is used for the biosynthesis of
coenzyme A and subsequently CoQ10, a vital
metabolite for preventing membrane lipid
peroxidation and ferroptosis cell death (D. S et al.
2019, B. K et al. 2019, B. MA et al. 2020). Therefore,
the cyst(e)ine level is very essential for regulating
redox status and ferroptosis in cells.
N-acetylcysteine (NAC), the acetylated precursor
of L-cysteine, is a common antioxidant used for
clinical practice and biomedicine research. For
example, with its potent antioxidant ability, NAC
effectively prevents hemin-induced ferroptosis and
ROS/MAPK and p53-mediated ferroptosis and
rescues neuron cells having ferroptosis-like
phenotype (G. G et al. 2020, L. Y et al. 2020, K. SS
et al. 2018). And a clinical trial showed that
additional NAC given to nephropathic cystinosis
patients with routine cysteamine treatment for three
months could reduce oxidative stress and
significantly improve renal function without side-
effects (P. de F. G. L et al. 2014). However, there is
no more cell-level research related to NAC and
cystinosis, leaving the relation between NAC and
lysosomal cystine accumulation unknown.
Currently, the relationship between cystinosis and
ferroptosis has not been studied yet. According to the
results from Bernadette's research (M. B, S. R, S. C,
M. T, and N. P 2015), the CTNS-knockdown β-cells
with attenuated insulin secretion bore the characters
of high ROS, cyst(e)ine, and oxidized GSH (GSSG)
levels. Though the cyst(e)ine level is higher than
normal cells, oxidative stress still occurs. What's
more, β-cells are more sensitive to oxidative stress
than other types of tissues and cells in the human
body, which embodies in terms of insulin secretion
(N. P, R. E, A. F, K. M, C. A, C. R 2012). Combining
the aforementioned information, here we hypothesize
that ROS is a potent factor to cause ferroptosis in β-
cells regardless of the cyst(e)ine. Therefore, CTNS-
knockdown β-cells will undergo ROS-induced
ferroptosis and NAC could inhibit ferroptosis and
reverse the adverse effects caused by lack of
cystinosin.
2 MATERIALS AND METHODS
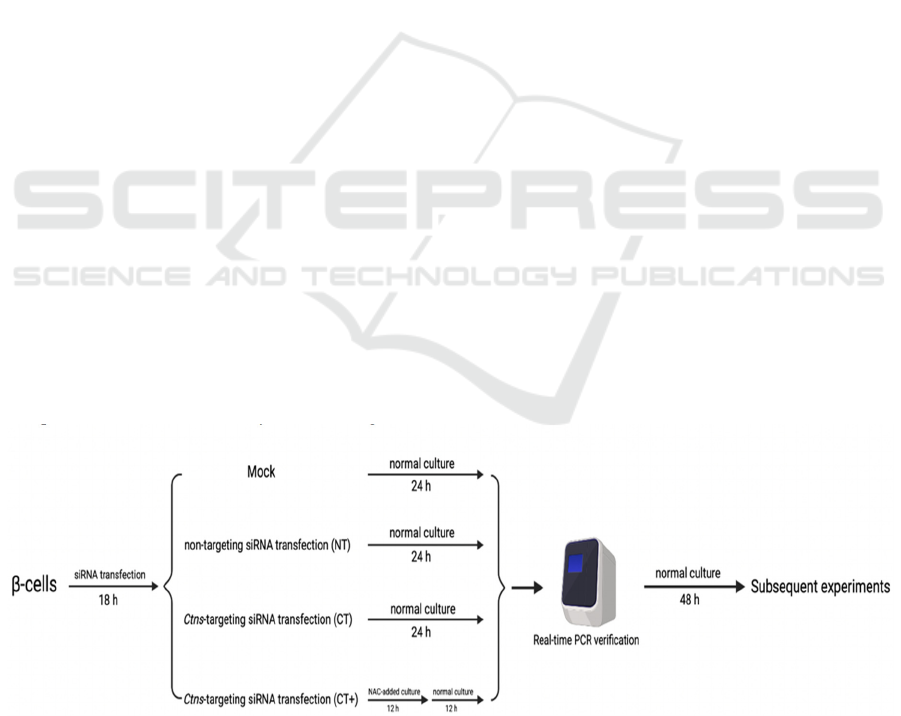
The big picture of our research shows in Figure 1. The
Mock means the β-cells underwent all of the siRNA
transfection steps without siRNA. β-cells were
transfected by non-targeting siRNA (NT) or CTNS-
targeting siRNA (CT) for 18 h. CT+ cells were
cultured with 10 mM NAC for 12 h and transferred to
normal culture for the next 12 h after siRNA
transfection, while other groups lived in normal
culture for 24 h post-transfection. In a word, after 24
h post-transfection, all groups were detected by real-
time PCR to justify whether siRNA interference was
successful or not. Then the subsequent experiments
were practiced for competent groups after 48 h
stabilization in normal culture. The specific
experiment steps are as follows.
Figure 1: Schematic research design.
The BRIN-BD11 β-cells line was processed and
divided into four groups: Mock, non-targeting siRNA
transfection (NT), CTNS-targeting siRNA
transfection (CT), and CTNS-targeting siRNA
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
1236

transfection with NAC treatment (CT+). After
transcription-level verification of real-time PCR, all
groups were cultured in normal culture for 48 h and
then used for subsequent experiments.
2.1 Cell Culture and Process
Here we use the same materials, cell culture protocol,
and gene knockdown protocol of Bernadette's
research (M. B, S. R, S. C, M. T, and N. P 2015).
Briefly, we used ON-TARGETplus SMARTpool
technology by GE Dharmacon (Lafayette, CO, USA).
BRIN-BD11 cells were seeded and allowed to adhere
overnight. CTNS-targeting siRNA pool (80 pmol/μl;
CT) was transiently transfected into the cells for 18 h
using DharmaFECT 1, according to the instructions
of the manufacturer. Mock transfection involved the
transfection in the absence of siRNA, while the
negative control was the non-targeting siRNA pool
(NT). Following the NAC addition of Zhang's paper
(L. Y et al. 2020), half part of the CT cells is
pretreated with NAC (10 mM, CT+) for 12 h and then
cultured in normal culture for 12 h before real-time
PCR.
2.2 sIRNA Gene Knockdown
Verification
The real-time PCR and western blot were practiced
demonstrating CTNS knockdown at transcriptional
and expressional levels. The real-time PCR was
practiced 24 h after siRNA transfection and western
blot was used at 72 h post-transfection. Experiment
steps are strictly repeated according to Bernadette's
research (M. B, S. R, S. C, M. T, and N. P 2015).
2.3 Intracellular Iron Level
Intracellular iron levels were assessed using an
optimized FerroZine™, an iron-based assay
developed by Reimer (R. J, H. HH, C. H, R. SR, and
D. R, 2004). Before testing, each sample tube and the
standard tube is filled with 6.5 mM FerroZine™, 6.5
mM neocuproine, 2.5 M ammonium acetate, and 1 M
ascorbic acid dissolved in water. An iron detection
reagent is then being added to each tube. After that,
incubation will be performed for half an hour at room
temperature, which should allow researchers to
observe color development. Then, a microplate
reader measures the absorbance at 550 nm when 280
μl from both standard and sample tubes was added in
duplicate into wells of a 96-well plate. Finally, the
BCA assay will be carried out to determine
intracellular iron concentration. The concentration is
determined by the amount of dye in the blue ionic
from the measurement of the absorbance of the
solution.
2.4 GPX4 Level
We use the Glutathione peroxidase 4 (GPX4) kit
(Runyu biotechnology co. LTD, Shanghai, China) to
detect the GPX4 level in treated cell lines according
to Zhou's work (Z. Y 2020). The GPX4 gene level can
be detected via Real-Time Quantitative Polymerase
Chain Reaction (RT-qPCR). RNeasy Mini Kit
(Qiagen) was used for the extraction of total RNAs
according to the manufacture’s protocols. M-MLV
Reverse Transcriptase (Promega) was used for DNA
synthesis. GAPDH served as the internal reference.
The 2
−ΔΔCT
method was used for the calculation of the
relative gene transcription level. The GPX4 protein
level is detected using western blot following Li's
method (L. D et al. 2020).
2.5 MDA Level
The measure of MDA levels is performed by TBARS
assay. Glacial acetic acid is used to reconstitute
thiobarbituric acid (TBA) since regular acetic acid
affects TBA stability by its high-water content.
During sample preparation, butylated
hydroxytoluene is used in lysis buffer to prevent
further peroxidation while processing. After that,
TBA solution is added to the sample and then the
sample will be incubated at 95°C for 60 minutes.
Following that, the samples will cool down to room
temperature by using an ice bath for 10 minutes.
Finally, a microplate reader is used to measure the
output.
2.6 ROS Level
The examination of qualitative ROS level is referring
to Li (L. D et al. 2020). The ROS probes solution was
diluted to the required concentration in the
Phosphate-buffered saline (PBS) buffer and then
incubated at room temperature for 45 min. Then the
cells were washed with PBS 3 times and excited with
green light under a fluorescent microscope to observe
and shoot red emission images of the cells, which
represent ROS positive cells.
Referring to Bernadette's method (M. B, S. R, S.
C, M. T, and N. P 2015), the quantitative ROS level
was detected using ROS probe, 5-(and-6)-carboxy-
2’,7’-difluorodihydro fluorescein diacetate (carboxy-
H
2
DFFDA). Cells were incubated with 20μM
carboxy-H
2
DFFDA for 45 min, and the cell
N-acetylcysteine (NAC) Inhibits ROS-Induced Ferroptosis in CTNS Knockdown -Cells in Vitro
1237

suspension was analyzed immediately using the BD
Accuri C6 flow cytometer.
2.7 Intracellular GSH and GSSG
Levels
According to Zhou (Z. Y 2020), the levels of GSH
and GSSG were detected by GSH/GSSG Ratio
Detection Assay II (ab205811, Abcam), which is
used for the measurement of GSH, GSSG, and
GSH/GSSG. The GSH and GSSG levels were
normalized for protein content.
2.8 Cell Viability
Cells incubated in 96-well plates were treated as
indicated and cell proliferation was assessed by CCK-
8 assay (SAB biotech. College Park, MD, USA) at 24
h after CTNS knockdown and at 24 h after NAC
treatment following the manufacturer’s instruction.
Optical density (OD) was recorded at 450 nm.
2.9 Intracellular ATP Level
Following Bernadette's method (M. B, S. R, S. C, M.
T, and N. P 2015), cells were detached using 0.05%
trypsin–EDTA and washed with ice-cold PBS. The
cell pellets were resuspended in 100 μl ice-cold PBS.
The cell suspension was then diluted 25-fold and the
ATP content was assessed using the ATP
Bioluminescence Assay Kit HSII (Roche
Diagnostics, Mannheim, Germany), according to the
instructions of the manufacturer. The protein
concentration of the cell lysates was used to
normalize the ATP content.
2.10 Insulin Secretion
The chronic and acute glucose-stimulated insulin
secretion was repeated following Bernadette's
method(M. B, S. R, S. C, M. T, and N. P 2015). At 72
h post-transfection, the cell supernatant was removed
and used to determine chronic insulin release. The
cells were then washed with PBS and acute insulin
secretion was stimulated after cells were starved for
40 min with Krebs Ringer Buffer (KRB), pH 7.4,
containing 1.1 mM D-glucose. The cells were then
stimulated with KRB containing 16.7 mM glucose
plus 10 mM alanine for 20 min at 37 °C. The KRB
was collected and insulin release was determined
using the Mercodia ultra-sensitive rat insulin ELISA
kit (Uppsala, Sweden), according to the instructions
of the manufacturer.
2.11 Statistic Analysis
This research is based on hypotheses and speculations
instead of real data in the lab. Therefore, the data
presented in the following sections are mock data
from existing research and are modified due to our
prediction. Data are presented as the means of three
independent experiments ± SEM and were analyzed
with the GraphPad Prism 9.2 software (GraphPad
Software Inc., San Diego, CA, USA). Data were
analyzed by Student’s t-test and differences at a P-
value of <0.05 were considered significant. One, two,
three, four asterisks respectively represent P-value of
<0.05, <0.01, <0.001, and <0.0001.
3 HYPOTHESIS AND RESULT
SPECULATION
Here we hypothesize that CTNS knockdown will
induce oxidative stress in β-cells BRIN-BD11 and
express the phenotype of ferroptosis, while NAC can
attenuate ferroptosis by changing redox status in
cells. The mock results are listed as follows.
3.1 CTNS-Targeting sIRNA Inhibit
Cystinosin Expression at mRNA
and Protein Level
Our speculation thoroughly depends on the CTNS
knockdown in β-cells BRIN-BD11. Therefore, we
presume that we successfully silence the CTNS gene.
The CTNS mRNA levels in CT and CT+ are
significantly decreased by more than 80 % compared
to Mock and NT at 24 h post-transfection (Fig. 2A).
Combining mRNA level decrease, cystinosin level in
CT and CT+ is also decreased by approximately 50
% at 72 h post-transfection (Fig. 2B).
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
1238
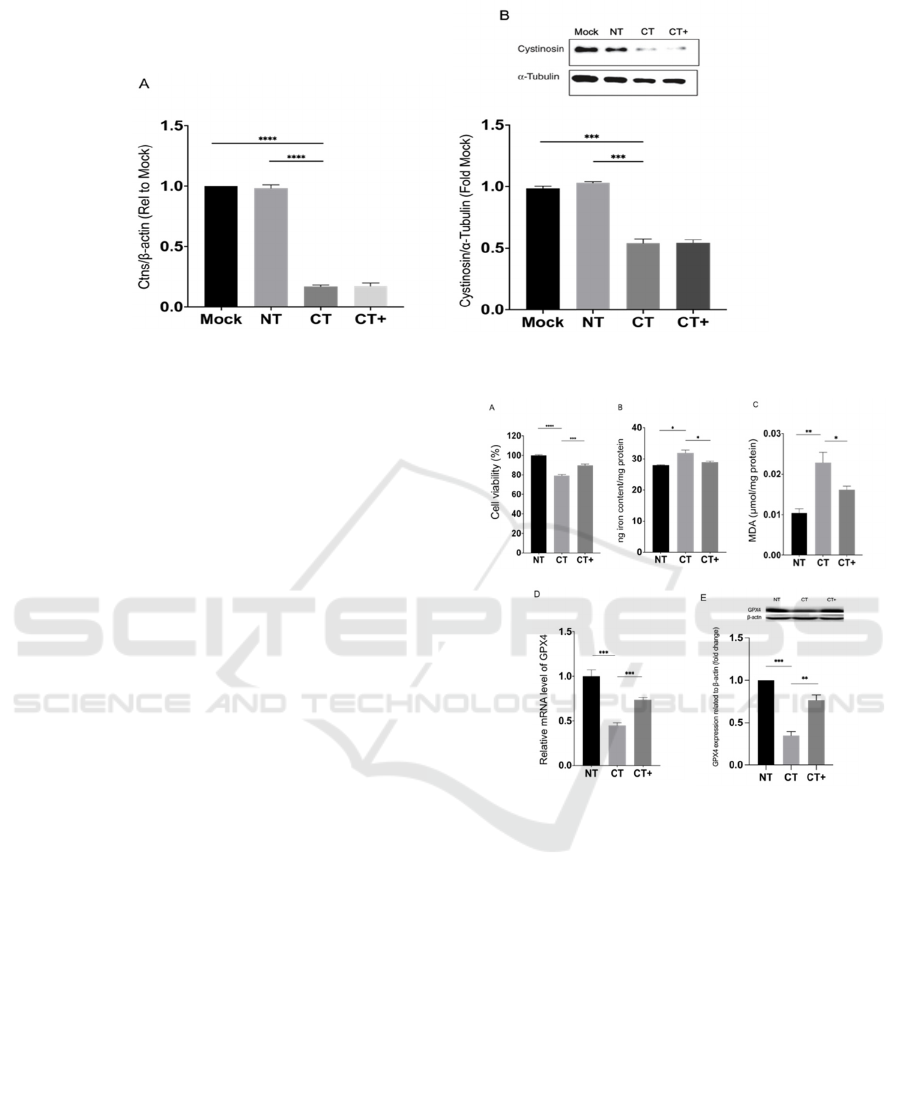
Figure 2: Evaluation of CTNS-targeting siRNA knockdown by real-time PCR and Western blot.
BRIN-BD11 cells were transiently transfected
with a CTNS-targeting siRNA pool (CT) for 18 h
using DharmaFECT 1 transfection reagent, according
to the manufacturer’s instructions. A part of CT
underwent 10 mM NAC pretreatment for 12 h after
transfection (CT+). Mock transfection (Mock) was
performed in the absence of siRNA, while the
negative control contained a scrambled non-targeting
siRNA pool (NT). CTNS mRNA and cystinosin levels
were assessed by real-time PCR (A) and Western blot
(B) at 24 and 72 h post-transfection, respectively.
Lanes from 1 to 4 of the Western blot analysis
correspond to Mock, NT, CT, and CT+, respectively.
Densitometry analysis of Western blots is expressed
as a ratio of cystinosin to α-tubulin expression and is
presented as mean fold change relative to Mock ±
SEM of three or more independent experiments.
***
or
****
show the CT's statistically significant difference
from Mock or NT, as indicated, at P<0.001 and
P<0.0001, respectively. Mock, mock transfection;
NT, non-targeting siRNA; CT, CTNS-targeting
siRNA; CT+, CTNS-targeting siRNA and NAC
treatment.
3.2 CTNS Knockdown Induces
Ferroptosis In β-cells and NAC
Attenuates Ferroptosis
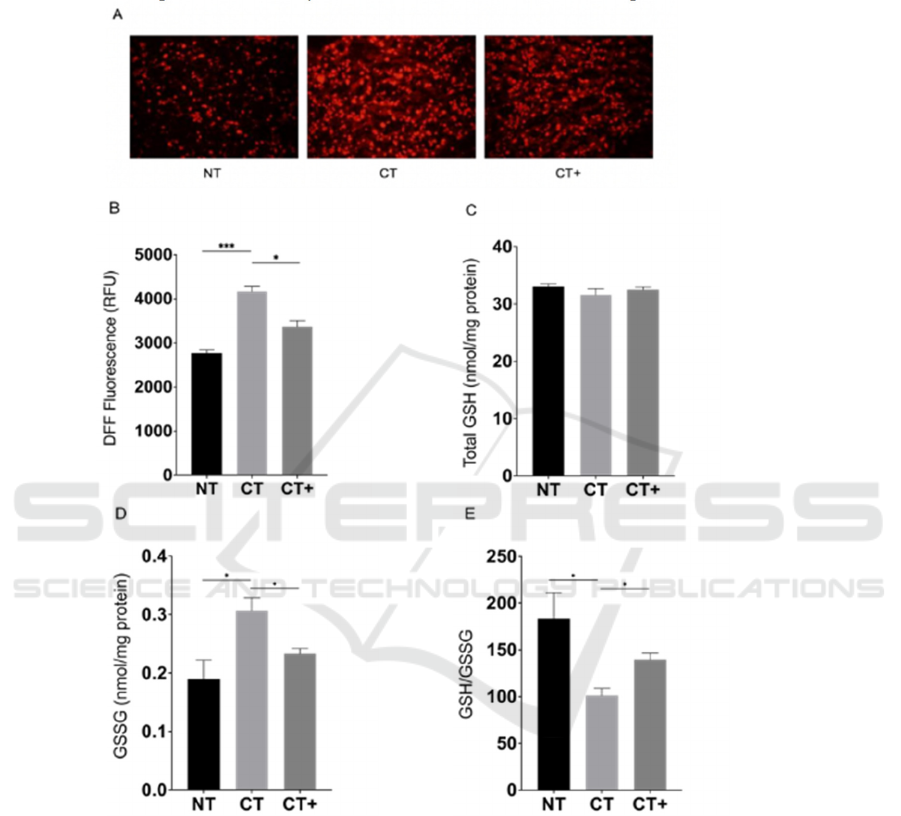
From artificial Figure 3, we could know that the
CTNS knockdown β-cells get apparently damaged
with strong ferroptosis phenomenons: elevated
intracellular iron and MDA level and decreased
GPX4 mRNA and protein level. However, NAC
could potently attenuate negative effects caused by
CTNS knockdown.
Figure 3: Effect of CTNS knockdown and NAC on
ferroptosis in β-cells
At 72 h post-transfection, the BRIN-BD11 cell
viability (A), iron level (B), MDA level (C), GPX4
mRNA level (D), and GPX4 protein level (E) of NT,
CT, and CT+ are determined. Data are presented as
means ± SEM of at least three independent
experiments.
*
,
**
,
***
, and
****
represent significantly
difference at P<0.05, 0.01, 0.001, and 0.0001,
respectively. NT, non-targeting siRNA treatment; CT,
CTNS-targeting siRNA treatment; CT+, CTNS-
targeting siRNA and NAC treatment.
3.3 NAC Attenuates Oxidative Stress
in CTNS Knockdown β-cells
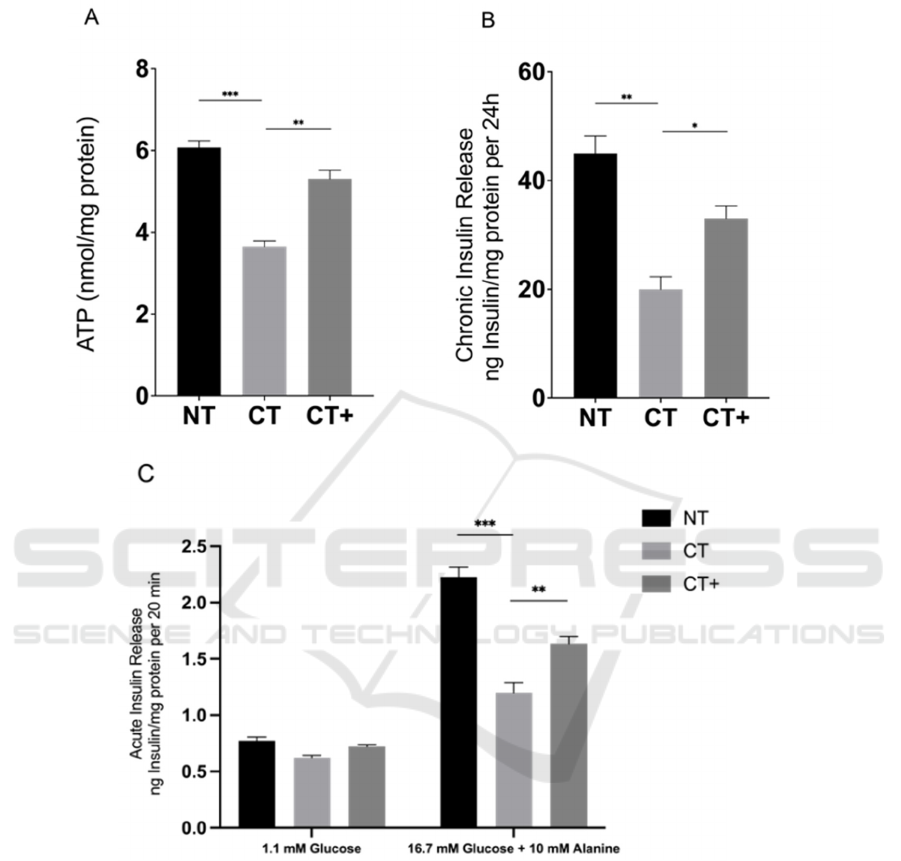
We forecast that BRIN-BD11 cells are affected by
serious oxidative stress after CTNS knockdown (Fig.
4). Compared with NT, the ROS (Fig. 4B) and GSSG
N-acetylcysteine (NAC) Inhibits ROS-Induced Ferroptosis in CTNS Knockdown -Cells in Vitro
1239
(Fig. 4D) levels of CT are extremely elevated, while
the GSH level has not significantly changed (Fig.
4C). The NAC is a competent antioxidant to inhibit
oxidative stress (Fig. 4E). Interestingly, the total GSH
level will not be improved when the cysteine level is
normal (B. JM, V. A, and L. BH 1989). Due to the
known result that total cysteine in CTNS knockdown
cells is a bit more than normal cells (M. B, S. R, S. C,
M. T, and N. P 2015), we speculate that the total GSH
level will not be improved after NAC treatment.
Figure 4: Effect of CTNS knockdown and NAC on redox status in β-cells.
At 72 h post-transfection, the ROS level in BRIN-
BD11 cells was showed in qualitative form (A) by
using Dihydroethidium (DHE) probe and quantitative
form (B) by using ROS probe carboxy-H
2
DFFDA.
The total glutathione (GSH) (C) and oxidized
glutathione (GSSG) (D) levels in BRIN-BD11 cells
were determined. (E) The ratio of GSH and GSSG is
calculated. Data are presented as means ± SEM of at
least three independent experiments.
*
and
***
represent the significant difference at P<0.05 and
<0.001, respectively. NT, non-targeting siRNA
treatment; CT, CTNS-targeting siRNA treatment;
CT+, CTNS-targeting siRNA and NAC treatment.
3.4
NAC Restores Energy Level and
Glucose-Stimulated Insulin
Secretion in CTNS Knockdown
β-cells
The decreased total ATP level (Fig. 5A) and restricted
chronic (Fig. 5B) and acute (Fig. 5C) insulin secretion
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
1240
are demonstrated in CTNS knockdown β-cells. Based
on our hypothesis that β-cells are mainly affected by
oxidative stress, 10 mM NAC treatment can
significantly increase the intracellular ATP level and
improve insulin secretion due to its antioxidant
property.
Figure 5. Effect of CTNS knockdown and NAC on ATP level and insulin secretion
A) total ATP concentration is determined in
BRIN-BD11 cells at 72 h post-transfection. B) the
chronic release of insulin was assessed in cells
between 24–72 h after transfection. Cells are initially
incubated with KRB containing 1.1 mM glucose for
40 min at 37°C before insulin release was stimulated
for 20 min using KRB containing 1.1 mM glucose
(basal) or 16.7 mM glucose and 10 mM alanine. C)
acute stimulated insulin secretion was determined in
BRIN-BD11 cells at 72 h post-transfection. Data are
presented as means ± SEM of at least three
independent experiments.
*
,
**
, and
***
represent
significantly difference at P<0.05, 0.01 and 0.001,
respectively. NT, non-targeting siRNA treatment; CT,
CTNS-targeting siRNA treatment; CT+, CTNS-
targeting siRNA and NAC treatment.
4 DISCUSSIONS
During ferroptosis, lipid peroxidation happens, which
N-acetylcysteine (NAC) Inhibits ROS-Induced Ferroptosis in CTNS Knockdown -Cells in Vitro
1241

is related to the high oxidative level in the cytosol.
Currently, researchers think that N-acetylcysteine can
inhibit ferroptosis due to its potent antioxidant ability
(X. Y et al 2015). However, the specific mechanism
of NAC inhibiting ferroptosis has not received much
attention. We forecast that NAC may not only
scavenge ROS but also restore other substances that
act as ferroptosis inhibitors.
According to current research about ferroptosis
(X. Y et al 2015), cyst(e)ine starvation can lower the
level of GSH, inactivate GPX4, and inhibit the
biosynthesis of coenzyme A and subsequent CoQ10
which can greatly prevent lipid peroxidation and
ferroptosis. GPX4 can prevent normal oxidative
stress and lipid peroxidation by using GSH. No
researchers, however, study whether ROS itself can
inactivate GPX4 at mRNA and protein level under
the condition of normal cyst(e)ine and GSH
concentration.
According to Bernadette's research (M. B, S. R, S.
C, M. T, and N. P 2015), the level of cystine and
cysteine in β-cells both increase after CTNS
knockdown, but the oxidative stress and the
accumulation of ROS still happens, lowering the
secretion of insulin and impairing cell viability. The
cyst(e)ine-mediated ferroptosis is inactivated because
of the sufficient intracellular cyst(e)ine under this
situation. Under the hypothesis we raised before, we
speculate that the GPX4 level will not be affected by
cyst(e)ine but by ROS aggregation. ROS can
independently inactivate GPX4 mRNA and protein
through a certain unknown mechanism and form
positive feedback which leads to more ROS
accumulation and inevitable ferroptosis. Following
the hypothesis, the mRNA and protein level of GPX4
and cell viability will significantly decrease, the
MDA level highly increase. Referring to the
discussion above, the NAC treatment is applied to
reduce ROS, which can restore the GPX4
transcription and expression. Whether NAC could
directly rehabilitate GPX4 in different ways is
valuable to study. By rehabilitation from ferroptosis,
the insulin secretion and intracellular level of β-cells
rise, though we cannot estimate this increase whether
is caused by ferroptosis inhibition or oxidative stress
inhibition.
5 CONCLUSIONS
After CTNS knockdown, the BRIN-BD11 β-cells
undergo ROS-induced ferroptosis with impaired
insulin secretion and energy production. The NAC
pretreatment effectively rescues CTNS knockdown β-
cells from ferroptosis by increasing GPX4 mRNA
and protein level.
Currently, there are no research focusing on the
relationship between ferroptosis and cystinosis. The
intracellular cyst(e)ine level of cystinosis patients is
higher than that of healthy people. Not a few
Researchers study the ferroptosis caused by low
cyst(e)ine in cells, that is, cyst(e)ine starvation. CTNS
knockdown β-cells with high oxidative stress and
intracellular cyst(e)ine level provide a good model to
discover whether ferroptosis occurs with enough
cyst(e)ine. We innovatively exclude the influence of
cyst(e)ine and study the relationship between
ferroptosis and ROS with CTNS knockdown cell
model. Our work to some extant offer a new
perspective of ferroptosis induction.
ACKNOWLEDGEMENT
The authors are grateful to Dr. Shu-bing Qian for his
enlightenment and guide of our research and Dr.
Huijie Han for her instruction of paper writing.
The author's contributions are listed here.
Experimental design, H. LIANG; Materials
organization, H. LIANG, J. XU; Methodology, H.
LIANG, J. YU, R. PENG, Y. CHEN; Hypothesis
speculation, H. LIANG, J. YU, J. XU, Y. CHEN, R.
PENG; Data visualization, H. LIANG; Paper writing,
H. LIANG, J. XU, Y. CHEN; Proofreading, Y.
CHEN. All the authors have read and agreed to the
final version of the report with no conflict of interest.
REFERENCES
B. F et al., “Modulation of CTNS gene expression by
intracellular thiols,” Free radical biology & medicine,
vol. 48, no. 7, pp. 865–872, Apr. 2010, doi:
10.1016/J.FREERADBIOMED.2010.01.011.
B. JM, V. A, and L. BH, “Effect of N-acetylcysteine on
plasma cysteine and glutathione following paracetamol
administration,” European journal of clinical
pharmacology, vol. 36, no. 2, pp. 127–131, Feb. 1989,
doi: 10.1007/BF00609183.
B. K et al., “The CoQ oxidoreductase FSP1 acts parallel to
GPX4 to inhibit ferroptosis,” Nature, vol. 575, no.
7784, pp. 688–692, Nov. 2019, doi: 10.1038/S41586-
019-1705-2.
B. MA et al., “Cysteine depletion induces pancreatic tumor
ferroptosis in mice,” Science (New York, N.Y.), vol.
368, no. 6486, Apr. 2020, doi:
10.1126/SCIENCE.AAW9872.
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
1242

D. S et al., “FSP1 is a glutathione-independent ferroptosis
suppressor,” Nature, vol. 575, no. 7784, pp. 693–698,
Nov. 2019, doi: 10.1038/S41586-019-1707-0.
G. G et al., “Cobalt nanoparticles trigger ferroptosis-like
cell death (oxytosis) in neuronal cells: Potential
implications for neurodegenerative disease,” FASEB
journal : official publication of the Federation of
American Societies for Experimental Biology, vol. 34,
no. 4, pp. 5262–5281, Apr. 2020, doi:
10.1096/FJ.201902191RR.
K. SS et al., “N-acetylcysteine targets 5 lipoxygenase-
derived, toxic lipids and can synergize with
prostaglandin E 2 to inhibit ferroptosis and improve
outcomes following hemorrhagic stroke in mice,”
Annals of neurology, vol. 84, no. 6, pp. 854–872, Dec.
2018, doi: 10.1002/ANA.25356.
L. D et al., “Quercetin Alleviates Ferroptosis of Pancreatic
β Cells in Type 2 Diabetes,” Nutrients, vol. 12, no. 10,
pp. 1–15, Oct. 2020, doi: 10.3390/NU12102954.
L. E, de G.-H. A, W. M, van den H. L, M. L, and B. H,
“Altered status of glutathione and its metabolites in
cystinotic cells,” Nephrology, dialysis,
transplantation : official publication of the European
Dialysis and Transplant Association - European Renal
Association, vol. 20, no. 9, pp. 1828–1832, Sep. 2005,
doi: 10.1093/NDT/GFH932.
L. S, D. J, and T. M, “Low antioxidant enzyme gene
expression in pancreatic islets compared with various
other mouse tissues,” Free radical biology & medicine,
vol. 20, no. 3, pp. 463–466, 1996, doi: 10.1016/0891-
5849(96)02051-5.
L. Y et al., “Disulfiram/Copper Induces Antitumor Activity
against Both Nasopharyngeal Cancer Cells and Cancer-
Associated Fibroblasts through ROS/MAPK and
Ferroptosis Pathways,” Cancers, vol. 12, no. 1, Jan.
2020, doi: 10.3390/CANCERS12010138.
M. B, S. R, S. C, M. T, and N. P, “Cystine accumulation
attenuates insulin release from the pancreatic β-cell due
to elevated oxidative stress and decreased ATP levels,”
The Journal of physiology, vol. 593, no. 23, pp. 5167–
5182, Dec. 2015, doi: 10.1113/JP271237.
N. G and G. W, “Nephropathic cystinosis: late
complications of a multisystemic disease,” Pediatric
nephrology (Berlin, Germany), vol. 23, no. 6, pp. 863–
878, Jun. 2008, doi: 10.1007/S00467-007-0650-8.
N. P, R. E, A. F, K. M, C. A, and C. R, “Reactive oxygen
and nitrogen species generation, antioxidant defenses,
and β-cell function: a critical role for amino acids,” The
Journal of endocrinology, vol. 214, no. 1, pp. 11–20,
Jul. 2012, doi: 10.1530/JOE-12-0072.
N. S, S. H, A. R, T. T, and Y. T, “Regulation of the
susceptibility to oxidative stress by cysteine availability
in pancreatic beta-cells,” American journal of
physiology. Cell physiology, vol. 295, no. 2, Aug. 2008,
doi: 10.1152/AJPCELL.00203.2008.
P. de F. G. L et al., “N-acetyl-cysteine is associated to renal
function improvement in patients with nephropathic
cystinosis,” Pediatric nephrology (Berlin, Germany),
vol. 29, no. 6, pp. 1097–1102, 2014, doi:
10.1007/S00467-013-2705-3.
R. J, H. HH, C. H, R. SR, and D. R, “Colorimetric
ferrozine-based assay for the quantitation of iron in
cultured cells,” Analytical biochemistry, vol. 331, no. 2,
pp. 370–375, Aug. 2004, doi:
10.1016/J.AB.2004.03.049.
S. BR et al., “Ferroptosis: A Regulated Cell Death Nexus
Linking Metabolism, Redox Biology, and Disease,”
Cell, vol. 171, no. 2, pp. 273–285, Oct. 2017, doi:
10.1016/J.CELL.2017.09.021.
S. K et al., “Mitochondrial reactive oxygen species reduce
insulin secretion by pancreatic beta-cells,” Biochemical
and biophysical research communications, vol. 300,
no. 1, pp. 216–222, 2003, doi: 10.1016/S0006-
291X(02)02832-2.
S. R, M. B, N. P, and M. T, “Lysosomal cystine
accumulation promotes mitochondrial depolarization
and induction of redox-sensitive genes in human kidney
proximal tubular cells,” The Journal of physiology, vol.
594, no. 12, pp. 3353–3370, Jun. 2016, doi:
10.1113/JP271858.
W. A. Gahl, J. G. Thoene, and J. A. Schneider,
“Cystinosis,”
http://dx.doi.org/10.1056/NEJMra020552, vol. 347,
no. 2, pp. 111–11121, Oct. 2009, doi:
10.1056/NEJMRA020552.
X. Y et al., “Ferroptosis: process and function,” Cell death
and differentiation, vol. 23, no. 3, pp. 369–379, Mar.
2016, doi: 10.1038/CDD.2015.158.
Y. WS et al., “Regulation of ferroptotic cancer cell death
by GPX4,” Cell, vol. 156, no. 1–2, pp. 317–331, 2014,
doi: 10.1016/J.CELL.2013.12.010.
Z. Y et al., “mTORC1 couples cyst(e)ine availability with
GPX4 protein synthesis and ferroptosis regulation,”
Nature communications, vol. 12, no. 1, Dec. 2021, doi:
10.1038/S41467-021-21841-W.
Z. Y, “The Protective Effects of Cryptochlorogenic Acid on
β-Cells Function in Diabetes in vivo and vitro via
Inhibition of Ferroptosis,” Diabetes, metabolic
syndrome and obesity: targets and therapy, vol. 13, pp.
1921–1931, 2020, doi: 10.2147/DMSO.S249382.
N-acetylcysteine (NAC) Inhibits ROS-Induced Ferroptosis in CTNS Knockdown -Cells in Vitro
1243
