
Granger Causality Changes during Rt-fMRI Neurofeedback Training
of Emotion Regulation for Insomnia Patients
Zhiyuan Feng
1
, Hui Gao
1a
, Zhonglin Li
2b
, Linyuan Wang
1c
, Chi Zhang
1d
, Yan Bin
1e
,
Yongli Li
2f
and Li Tong
1g
1
Henan Key Laboratory of Imaging and Intelligent Processing, PLA Strategic Support, Force Information Engineering
University, Zhengzhou, Henan, China
2
Department of Radiology, Henan Provincial People’s Hospital & People’s Hospital of Zhengzhou University, Zhengzhou,
Henan, China
Keywords: Rt-Fmri, Granger Causality Model, Emotion Regulation.
Abstract: The amygdala is a key brain region in the emotional network. Studies have shown that emotion regulation
neurofeedback training targeting amygdala based on real-time functional magnetic resonance can effectively
improve the symptoms of insomnia patients. However, the brain mechanism for this improvement remains
unclear. In this paper, Granger causality model was constructed to analyze the difference of causality
between different brain regions before and after neurofeedback training. Firstly, the brain regions related to
emotion regulation with significant differences in ReHo before and after neurofeedback training were
selected as regions of interest. Secondly, the time series of the regions of interest were extracted to establish
the Granger causality model. Finally, through group level analysis, the difference of effective connection
before and after neurofeedback training was used as a biomarker to evaluate the effect of emotion
regulation. The results have shown that rt-fMRI neurofeedback training targeting the amygdala significantly
regulated the activity of brain regions related to emotion regulation in insomnia patients. And the effective
connections from the right triangle inferior frontal gyrus to the left amygdala, the left precuneus to the left
middle frontal gyrus, and the right middle cingulate gyrus to the left middle frontal gyrus were significantly
enhanced. While the effective connection from the left middle frontal gyrus to the left precuneus was
significantly reduced. Moreover, these changes were consistent with the scale evaluation results and clinical
psychiatric studies, which further demonstrated that real-time fMRI neurofeedback training can change the
effective connectivity of brain regions related to emotion regulation, and these changes could be used as a
potential biomarker to evaluate the effect of neurofeedback training.
1 INTRODUCTION
1
Insomnia is a common clinical disorder
characterized by difficulty falling asleep or
maintaining sleep for more than three months. With
the development of brain science, non-invasive
methods have become a hot topic in the field of
insomnia research (Christopher and Decharms
a
https://orcid.org/0000-0001-7829-7244
b
https://orcid.org/0000-0001-5023-3222
c
https://orcid.org/0000-0001-5072-0354
d
https://orcid.org/0000-0001-6751-3087
e
https://orcid.org/0000-0003-4559-4925
f
https://orcid.org/0000-0001-5934-8181
g
https://orcid.org/0000-0002-4654-456X
2008). Among them, the neurofeedback technology
based on real-time functional magnetic resonance
imaging(rt-fMRI) detects the changes in blood
oxygen caused by the enhancement of neuronal
activity in a specific area of the brain to measure the
neuronal activity of the brain indirectly, and
feedback relevant information to the subjects in real
time. It has been used in the diagnosis and
intervention of various psychiatric diseases because
it can target the brain regions related to patients'
neurological defects and has high spatial and
temporal resolution (Brühl 2015). Recent studies
have shown that rt-fMRI neurofeedback emotion
regulation training can effectively improve the
symptoms of insomnia patients (CHEN 2021, Zhang
and Gao 2021).
Feng, Z., Gao, H., Li, Z., Wang, L., Zhang, C., Bin, Y., Li, Y. and Tong, L.
Granger Causality Changes during Rt-fMRI Neurofeedback Training of Emotion Regulation for Insomnia Patients.
DOI: 10.5220/0011387300003438
In Proceedings of the 1st International Conference on Health Big Data and Intelligent Healthcare (ICHIH 2022), pages 577-583
ISBN: 978-989-758-596-8
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
577

At present, neurofeedback emotion regulation
technology based on rt-fMRI has been successfully
applied as an adjunctive treatment for major
depression (Bodurka and Jerzy 2017; Mehler D and
Sokunbi 2018, Bruce and Doré 2018), anxiety
disorder (Abraham and Kaufmann 2013),
schizophrenia (Zweerings and Hummel 2019) and
other diseases related to emotion regulation
disorders. A number of studies have also shown that
sleep supports continuous changes in neuronal
representation of emotional experiences, and
insomnia disorder may be related to the inability of
patients to eliminate emotional distress (Bonnet and
Arand 2010). There are also data suggesting that
insomnia and depression may share a common
pathology, that is, the diagnosis of insomnia and the
severity of sleep disorders are both related to high
cortisol secretion (Young and Korszun 2010). These
studies have proved pathologically that insomnia
patients can be treated by emotion regulation
training.
How to analysis the effectiveness of rt-fMRI
neurofeedback is focus of attention. In addition to
the evaluation method of clinical scale, the
evaluation of training effect by using brain image
features has become one of the focuses of scientific
research and clinical attention. The brain
connectivity analysis of fMRI functional data is
mainly divided into two types: functional
connectivity and effective connectivity. From the
direction of connection, functional connection only
emphasizes whether there is a connection between
brain regions, and does not study the direction of the
connection, that is, undirected connection. In order
to explain the coupling relationship between neural
activities in different brain regions more accurately,
scholars put forward the concept of effective
connection, which refers to the influence exerted by
the activity of one brain region on the activity of
another brain region, revealing the causal effect of
non-adjacent brain regions in finger spac2 (Friston
and Frith 2010). For the analysis of effective
connections, model-driven methods are generally
adopted to reflect the changes of neural activities by
establishing the causal relationship between neurons.
The commonly used methods are as follows:
psychophysiological interaction (PPI), structural
equation model (SEM), dynamical causal model
(DCM) and Granger causality analysis (GCA)
(Liang X and Wang J H, 2010). GCA is one of the
most widely used effective connection analysis
methods in current research.
Many researchers have analyzed brain
connectivity abnormalities in insomniacs using
different functional connectivity methods. For
example, in 2018, Li C et al. (Li and Dong 2018)
used Granger causality model to analyze the
effective brain connectivity between patients with
primary insomnia and healthy subjects, and found
that patients with insomnia had reduced effective
connectivity from the right anterior insula to
bilateral precuneus, left posterior central gyrus and
bilateral posterior cerebellum, and decreased
effective connectivity from bilateral orbitofrontal
cortex to the right anterior insula. Dai X J et al. (Dai
and Wang 2021) used Granger causality analysis and
mediating causality analysis to study the relationship
between chronic insomnia and the seeking system
and value-driven attention network, and found that
the value-driven attention network reduced the
mediating effect of sleep regulation and the seeking
system reduced the mediating effect of negative
emotions after insomnia. However, the interaction
between these brain regions with abnormal
functional connectivity and the change of the
interaction relationship after treatment remains
unclear. In this study, a GCA model based on resting
state fMRI data of insomnia patients was established
to compare and analyze the differences of effective
brain connections of insomnia patients before and
after neurofeedback training. This study provides a
new perspective for understanding the pathological
mechanism of insomnia patients, thus promoting the
development of brain network imaging markers for
early diagnosis and treatment evaluation of
insomnia.
2 MATERIALS AND METHODS
2.1 Participants
This experimental design is described in detail in
Zhang's research (Zhang and Gao 2021). The
original study recruited 32 healthy subjects for the
experimental group and 36 healthy subjects for the
control group. Among them, the experimental group
received neurofeedback signals from the amygdala
during training, while the healthy control group
received only one baseline scan but didn’t
participate in neurofeedback training. The two
studies have been approved by the Ethics Committee
of Henan Provincial People's Hospital, and all
volunteers signed the informed consent to participate
in this study.
ICHIH 2022 - International Conference on Health Big Data and Intelligent Healthcare
578

2.2 Experimental Paradigm
The experimental design is described in detail in
Zhang's research paper (Zhang and Gao 2021). In
this experiment, the experimental group completed 6
stages of experiment, while the control group
completed 2 stages of experiment. The experiment
was conducted once a week. During stage 1, general
demographic characteristics of the experimental
group were collected. During stage 2, baseline scans
were performed for both experimental and control
subjects. Stage 3 to 5 were three neurofeedback
sessions for insomnia patients, with each session
lasting 50 minutes. Stage 6 was the follow-up
period.
The specific steps of the three neurofeedback
training sessions are shown in Figure 1. Each run
consisted of 7 "rest" blocks and 6 "happy" blocks
alternately, lasting for 6min30s. In the "happy"
block, participants upregulated the height of the
thermometer on the screen by recalling the specific
positive autobiographical memory they had written
down, and the feedback signal was updated every
repetition time (TR=2s). In the "resting" block, the
"+" appears on the screen and the subject looks at
the "+" calmly to return their brain activity to
baseline levels.
Figure 1: Experimental paradigm of rt-fMRI
neurofeedback training for amygdala.
2.3 Data Acquisition
Behavioral data and related experimental results
from the same sample have been published in an
earlier study. The data analyzed in this paper are
resting state functional images of insomnia patients
collected before Session3 and after Session5 during
neurofeedback training.
The restplus toolbox based on MATLAB was
used to preprocess data (Jia and Wang 2019). The
data preprocessing mainly includes: dicom to nifti,
remove first 10 time points, slice timing,
realign/head motion correction, normalization and
smooth (FWHM: 6mm). Subjects with head
movements greater than 2.5mm and 2.5° were
excluded in this experiment.
2.4 Analysis of fMRI Data
2.4.1 Choice of ROI
Firstly, Regional Homogeneity (ReHo) analysis
were performed on resting state data before and after
neurofeedback training to study the difference in
effective connection of emotion-related brain
regions in insomnia patients before and after
neurofeedback training. Through the paired T-test of
ReHo results, we found that 10 brain regions related
to emotion regulation were significantly changed in
insomnia patients after neurofeedback training. The
results are shown in Table 1, where: Right precuneus
(10, -47, 18), right posterior cingulate gyrus (12, -47,
30), left precuneus (-5, -47, 15), right middle
cingulate gyrus (-4, -12, 32), left insula (-42, 15, 9),
right triangle inferior frontal gyrus (52, 25, 19),
opercular part of inferior frontal gyrus (48, 18, 9),
left middle frontal gyrus (-51, 24, 33), right middle
frontal gyrus (36,39,39), right dorsolateral prefrontal
cortex (24, 6, 57). Therefore, these brain regions
were selected as ROI with a radius of 7 mm for the
next GCA analysis. In addition, since BOLD signal
of the patient's left amygdala was provided to the
subjects during the experiment, the left and right
amygdala were used as ROI, where: left amygdala (-
24, -1, -17) and right amygdala (26, 1, -18), and the
radius was 7 mm.
Table 1: Significant differences in brain regions analyzed
by Reho before and after neurofeedback training.
Brain regions Coordinates
Cluster
size
Peak
intensity
1
Right
precuneus
(10, -47, 18) 115 4.795
2
Right
posterior
cingulate
gyrus
(12, -47, 30) 32 4.394
3 Left precuneus (-5, -47, 15) 24 4.916
4
Right middle
cingulate
gyrus
(-4, -12, 32) 56 3.165
5 Left insula (-42, 15, 9) 36 -5.1656
6
Right triangle
inferior frontal
gyrus
(52, 25, 19) 51 -3.184
Granger Causality Changes during Rt-fMRI Neurofeedback Training of Emotion Regulation for Insomnia Patients
579

7
Opercular part
of inferior
frontal gyrus
(48, 18, 9) 45 -4.4361
8
Left middle
frontal gyrus
(-51, 24, 33) 28 -4.4819
9
Right middle
frontal gyrus
(36, 39, 39) 21 -3.8115
10
Right
dorsolateral
prefrontal
cortex
(24, 6, 57) 27 -4.0626
2.4.2 GCA Analysis
The GCA method was first applied to fMRI data for
effective connection analysis by Goebel et al. in
2003(Goebel and Roebroeck 2013). Granger
causality tends to be multi-variable, that is, the
causal relationship between two time series under
the influence of multiple additional time series.
GCA tests whether the current and past values of the
first sequence can better predict the results of the
second sequence by building a linear model. If so,
then these two sequences constitute a causal
relationship.
Specifically, In the Granger causality model, we
take and as generalized prediction errors. Then we
predict two time series and by linear projections of
their respective past values respectively. According
to Geweke's feedback model (Jiao and Zou 2014),
the time dynamics of two time series and with length
T can be described as,
()
11,1 1
1
() ()
p
k
k
Xt aX t k te
=
=-+
å
(1)
()
22,2 2
1
() ()
p
k
k
Xt aX t k te
=
=-+
å
(2)
Where p is the maximum number of lagged
observations included in the model (model orderp<
k ). a
,
and a
,
are autoregressive coefficients.
ε
(t) and ε
(t) are the autoregressive residuals of
each time series respectively.
That is, if the inclusion of X
's past observations
can reduce the prediction error in the linear
regression model of X
andX
compared to a model
containing only previous observations of X
,
then X
cause toX
. For time series X
(t) and X
(t),
we assume that both X
(t) and can be described as
binary autoregressive models (Jiao Z-Q and Zou L,
2014),
() ()()
111,1 12,21
11
()
pp
kk
kk
Xt AXtk AXtk et
==
=-+-+
åå
(3)
() ()()
221,1 22,22
11
()
pp
kk
kk
X
tAXtkAXtket
==
=-+-+
åå
(4)
Where p is the model order. A
,
and A
,
are
signed path coefficients. and are autoregressive
coefficients respectively. e
(t) and e
(t) are
residuals of and X
(t), respectively.
GCA is a data-driven method, which overcomes
the limitations of SEM and DCM. The GCA method
does not require a prior hypothesis model, and the
model is relatively simple with low computational
complexity, which can be used to analyze the
effective connection of large brain networks
(Roebroeck and Formisano 2005).
In this study, the time series of the above 12
ROIs related to emotion regulation was extracted to
establish a GCA model based on fMRI. Then the
GCA results of subjects before and after
neurofeedback training were statistically analyzed at
group level, so as to detect the effect of
neurofeedback training on the effective connection
between emotion-related brain regions of subjects.
3 RESULT
The results of GCA-paired T test before and after
neurofeedback training for insomnia patients were
shown in Table 2 and Figure 2. It was found that
after neurofeedback training, the effective
connections from the right triangle inferior frontal
gyrus to the left amygdala, the left precuneus to the
left middle frontal gyrus, and the right middle
cingulate gyrus to the left middle frontal gyrus were
significantly enhanced. The effective connection
from the left middle frontal gyrus to the left
precuneus was significantly reduced. In figure2, Red
‘→’ represents a significant increase of the
effective connection, and blue ‘→’ represents a
significant decrease of the effective connection.
Render visualized using BrainNet Viewer (Xia and
Wang 2013).
Table 2: Significant differences in brain effective
connections before and after neurofeedbacktraining
through Granger causality analysis.
ROI A→ROI B
t
p
1
Right triangle inferior
frontal gyrus→Left
amygdala
2.470863 0.019598
2
Left precuneus→Left
middle frontal gyrus
2.587577 0.014944
3
Left middle frontal
gyrus→Left precuneus
-2.685914 0.011842
4
Right middle cingulate
gyrus→Left middle frontal
gyrus
2.413076 0.022365
ICHIH 2022 - International Conference on Health Big Data and Intelligent Healthcare
580
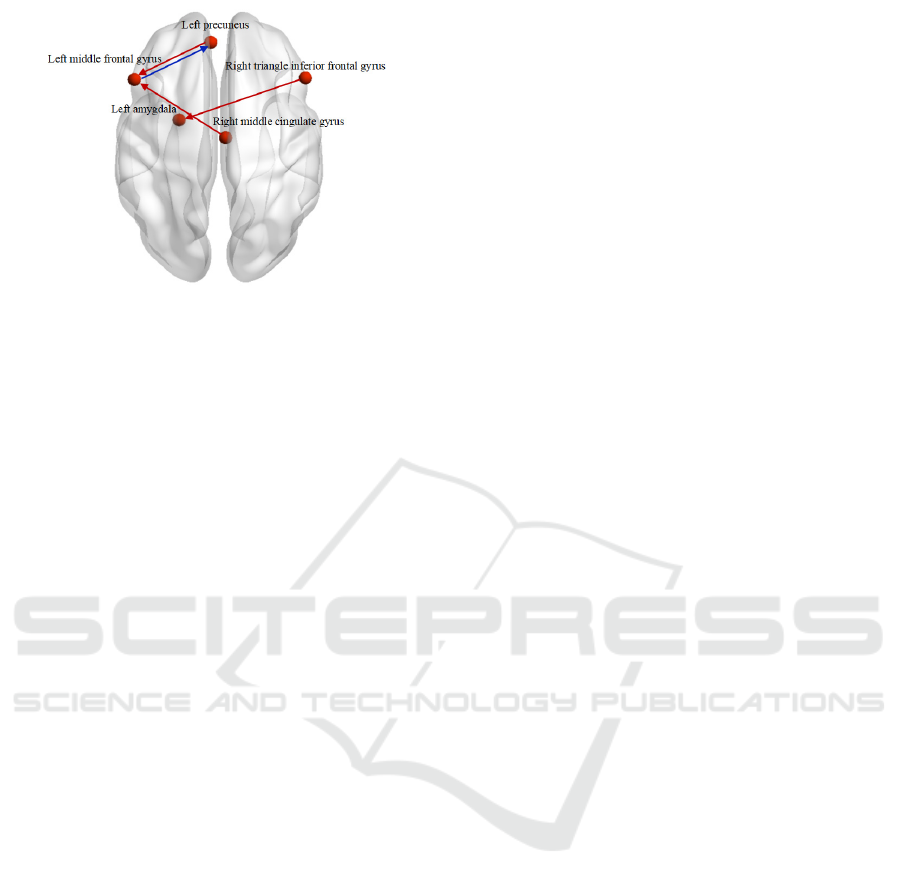
Figure 2: The changes of effective connections between
emotional brain regions in insomnia patients before and
after neurofeedback training.
4 DISCUSSION
The prefrontal cortex can respond to positive
emotional faces, pleasant stimulating images and
happy memories, which is an atypical reward
mechanism (Vijayakumari and Menon 2020). These
responses can be used to guide emotion and may
play an important role in emotion regulation. The
results of GCA showed that after neurofeedback
training, the effective connection of the right triangle
inferior frontal gyrus to the left amygdala was
enhanced, suggesting that the activity of the
prefrontal lobe directly affects and controlls the
amygdala. This is similar to the previous results of
rt-fMRI neurofeedback emotion regulation by Paret
et al. (Paret and Ruf 2016) in healthy subjects in
2016. Similar results have been obtained in the drug
treatment for patients with depression and other
psychiatric diseases, that is, antidepressants enhance
the activities of the prefrontal lobe in cognitive
control and other tasks, thus promoting the top-down
control of emotions. In 2018, Guo et al. (Guo and
Liu 2018) also found that functional connectivity
between amygdala, medial prefrontal cortex and
inferior frontal gyrus decreased in Alzheimer's
disease patients with depression compared with
healthy controls, proving that the functional
connectivity between amygdala and prefrontal lobe
may be an important feature of AD patients with
depression.
The precuneus, located in the posterior medial
parietal lobe, is an important structure of the
posterior default mode network and is associated
with cognitive functions (self-related information
processing, consciousness, episodic memory
extraction and visuo-spatial imagination) (Cavanna
and Trimble 2006). Research by Quinten Van Geest
et al. (Van Geest and Westerik 2017) has shown that
people with insomnia have reduced FC in the
thalamus, anterior cingulate cortex and precuneus. A
study of 18 healthy women by K Helmbold et al.
(Helmbold and Zvyagintsev 2016) showed that acute
consumption of tryptophan (a precursor of 5-HT
synthesis) led to reduced FC in precuneus and DMN.
Therefore, the decrease of FC in precuneus and
DMN was related to the decrease of 5-HT level. The
results of this study showed that after neurofeedback
training, the sending ability of left precuneus to left
middle frontal gyrus was enhanced while the
receiving ability was decreased, suggesting that the
improvement of effective connection between left
precuneus and left middle frontal gyrus was related
to the improvement of insomnia symptoms.
The cingulate gyrus is located between the
corpus callosum sulcus and cingulate sulcus, and is
an important part of the limbic system. It was first
defined and named by Professor Broca (Broca
1878), who divided the cingulate gyrus into three
parts by making vertical lines for the center. The
anterior cingulate gyrus (ACC), the middle cingulate
gyrus (MCC) and the posterior cingulate gyrus
(PCC), among which the anterior cingulate gyrus
and the middle cingulate gyrus have similar
functions, so most experiments have studied them as
a functional region. Moreover, studies have found
that the cumulative and negative memories of
objective objects and experiences can activate the
anterior cingulate gyrus and the middle cingulate
gyrus. Li et al. (Li and Yan 2018) used Dartel-VBM
technology to evaluate the changes of gray matter in
53 healthy subjects and 60 patients with primary
insomnia, and found that the gray matter volume in
the lateral prefrontal cortex and anterior cingulate
cortex in patients with insomnia was lower than that
in healthy subjects, and was negatively correlated
with the scores of anxiety scales such as SAS and
SDS, which leads to higher levels of negative
emotions such as anxiety and depression. Wang et
al. (Shi and Wang 2021) observed under fMRI plain
scanning that brain activities in bilateral brainstem,
left parahippocampal gyrus, frontal lobe and right
anterior cingulate gyrus of 15 insomnia patients
were significantly more active than before
acupuncture at Shenmen, Sanyinjiajie and Baihui
points 5 weeks after acupuncture. The results of this
study showed that the effective connection between
the left middle cingulate gyrus and the left middle
frontal gyrus was enhanced after neurofeedback
training in insomnia patients, suggesting that the
enhancement of the effective connection between
Granger Causality Changes during Rt-fMRI Neurofeedback Training of Emotion Regulation for Insomnia Patients
581

the left middle cingulate gyrus and the left middle
frontal gyrus was correlated with the improvement
of anxiety, depression and other symptoms, and thus
improved their sleep quality.
5 CONCLUSIONS
In this paper, Granger causality model was
constructed to analyze the difference of causality
between different brain regions before and after
neurofeedback training. The results showed that rt-
fMRI neurofeedback training significantly regulated
the activity of brain regions related to emotion
regulation in insomnia patients. The effective
connections between the prefrontal lobe to the
amygdala, the prefrontal lobe and the precuneus, and
the cingulate gyrus to the prefrontal lobe also
changed. These conclusions were consistent with the
results of the scale before and after the experiment
and were consistent with the results of clinical
psychiatric studies. These results further indicated
that rt-fMRI neurofeedback training can alter the
effective connectivity of brain regions related to
emotion regulation, and this change could be used as
a potential biological marker to evaluate the effect of
neurofeedback training.
Although rt-fMRI neurofeedback training of
amygdala emotion regulation has been studied from
the perspective of effective connection of brain
regions related to emotion regulation, and
preliminary results have been achieved, there are
still some limitations. Due to time constraints, the
control group experiment of neurofeedback training
for insomnia patients is still in progress. The data of
the experimental group and the control group can be
compared and analyzed in the future to further
explore the feasibility of this method in disease
treatment.
ACKNOWLEDGEMENTS
This research was supported by the National Natural
Science Foundation of China under grant 82071884
and the National Natural Youth Foundation of China
under grant 62106285.
REFERENCES
A research on resting-state functional network
connectivity after rt-fMRI neurofeedback in
insomnia[J]. Journal of Physics: Conference Series,
2021, 1907(1): 012013 (7pp).
Abraham A, Kaufmann C, Redlich R, et al. Self-referential
and anxiety-relevant information processing in
subclinical social anxiety: an fMRI study[J]. Brain
Imaging & Behavior, 2013, 7(1): 35-48.
Bodurka, Jerzy, Siegle, et al. Randomized Clinical Trial of
Real-Time fMRI Amygdala Neurofeedback for Major
Depressive Disorder: Effectson Symptoms and
Autobiographical Memory Recall[J]. American
journal of psychiatry, 2017, 174(8): 748-755.
Bonnet M H, Arand D L. Hyperarousal and insomnia:
State of the science[J]. Sleep Medicine Reviews, 2010,
14(1): 9-15.
Broca P. Le grand lobe limbique et la scissure limbique
dans la série des mammifères[J]. Revue
d'Anthropologie, 1878, 2: 385-498.
Bruce P, Doré, Odile, et al. Negative Autobiographical
Memory in Depression Reflects Elevated Amygdala-
Hippocampal Reactivity and Hippocampally
Associated Emotion Regulation[J]. Biological
psychiatry. Cognitive neuroscience and neuroimaging,
2018.
Brühl A. Making Sense of Real-Time Functional Magnetic
Resonance Imaging (rtfMRI) and rtfMRI
Neurofeedback[J]. International Journal of
Neuropsychopharmacology, 2015, 18(6).
Cavanna A E, Trimble M R. The precuneus: a review of
its functional anatomy and behavioural correlates[J].
Brain, 2006, 129(3): 564-583.
CHEN Bai-ru.Real-time functional magnetic resonance
neurofeedback technique in intervention of insomnia
patients disorder with depression
state.2021,35(04):401-404.
Christopher, Decharms. Applications of real-time fMRI[J].
Nature Reviews Neuroscience, 2008.
Dai X J, Wang N, Ai S Z, et al. Decreased modulation of
segregated SEEKING and selective attention systems
in chronic insomnia[J]. Brain Imaging and Behavior,
2021, 15(1): 430-443.
Friston K J, Frith C D, Frackowiak R S J. Time ‐
dependent changes in effective connectivity measured
with PET[J]. Human Brain Mapping, 2010, 1(1): 69-
79.
Goebel R, Roebroeck A, Kim D S, et al. Investigating
directed cortical interactions in time-resolved fMRI
data using vector autoregressive modeling and
Granger causality mapping[J]. Magnetic Resonance
Imaging, 2003, 21(10): 1251-1261.
Guo Z, Liu X, Xu S, et al. Abnormal changes in functional
connectivity between the amygdala and frontal regions
are associated with depression in Alzheimer’s
disease[J]. Neuroradiology, 2018, 60(12): 1315-1322.
Helmbold K, Zvyagintsev M, Dahmen B, et al.
Serotonergic modulation of resting state default mode
network connectivity in healthy women[J]. Amino
Acids, 2016, 48(4): 1109-1120.
Jia X Z, Wang J, Sun H Y, et al. RESTplus: an improved
toolkit for resting-state functional magnetic resonance
imaging data processing[J]. Science Bulletin, 2019.
ICHIH 2022 - International Conference on Health Big Data and Intelligent Healthcare
582

Jiao Z-Q, Zou L, Cao Y, et al. Effective connectivity
analysis of fMRI data based on network motifs[J]. The
Journal of Supercomputing, 2014, 67(3): 806-819.
Li C, Dong M, Yi Y, et al. Aberrant Effective
Connectivity of the Right Anterior Insula in Primary
Insomnia[J]. Frontiers in Neurology, 2018, 9: 317-.
Li M, Yan J, Li S, et al. Altered gray matter volume in
primary insomnia patients: a DARTEL-VBM study[J].
Brain imaging and behavior, 2018, 12(6): 1759-1767.
Liang X, Wang J H, He Y. Human connectome: Structural
and functional brain networks (in Chinese). Chinese
Sci Bull (Chinese Ver), 2010, 55:
Mehler D, Sokunbi M O, Isabelle H, et al. Targeting the
affective brain—a randomized controlled trial of real-
time fMRI neurofeedback in patients with
depression[J]. Neuropsychopharmacology, 2018.
Paret C, Ruf M, Gerchen M F, et al. FMRI neurofeedback
of amygdala response to aversive stimuli enhances
prefrontal-limbic brain connectivity[J]. Neuroimage,
2016, 125: 182-188.
Roebroeck A, Formisano E, Goebel R. Mapping directed
influence over the brain using Granger causality and
fMRI[J]. Neuroimage, 2005, 25(1): 230-242.
Shi X H, Wang Y K, Li T, et al. Gender ‐ related
difference in altered fractional amplitude of low ‐
frequency fluctuations after electroacupuncture on
primary insomnia patients: A resting ‐ state fMRI
study[J]. Brain and Behavior, 2021, 11(1): e01927.
Van Geest Q, Westerik B, Van Der Werf Y, et al. The role
of sleep on cognition and functional connectivity in
patients with multiple sclerosis[J]. Journal of
neurology, 2017, 264(1): 72-80.
Vijayakumari A A, Menon R N, Thomas B, et al.
Glutamatergic response to a low load working
memory paradigm in the left dorsolateral prefrontal
cortex in patients with mild cognitive impairment: a
functional magnetic resonance spectroscopy study[J].
Brain imaging and behavior, 2020, 14(2): 451-459.
Xia M, Wang J, He Y. BrainNet Viewer: a network
visualization tool for human brain connectomics[J].
PloS one, 2013, 8(7): e68910.
Young E, Korszun A. Sex, trauma, stress hormones and
depression[J]. Molecular psychiatry, 2010, 15(1): 23-
28.
Zweerings J, Hummel B, Keller M, et al. Neurofeedback
of core language network nodes modulates
connectivity with the default-mode network: a double-
blind fMRI neurofeedback study on auditory verbal
hallucinations[J]. NeuroImage, 2019, 189: 533-542.
Granger Causality Changes during Rt-fMRI Neurofeedback Training of Emotion Regulation for Insomnia Patients
583
