
Optimization of Cr(Ⅵ) Adsorption by Bacillus amyloliquefaciens and
Its Mechanism Study
Hedong Lu
1,2,* a
, Chengyuan Gu
1b
, Panping Yang
1c
, Hai Xu
1d
, Muhanmmad Bilal
1e
and Yan Ding
1f
1
School of Life Science and Food Engineering, Huaiyin Institute of Technology, Huaian, China
2
School of Food Science and Technology, Jiangnan University, Wuxi, China
1492726453@qq.com
Keywords: Bacillus amyloliquefaciens, Chrome(Ⅵ), Adsorption, Optimization.
Abstract: In recent decades, with the rapid development of social economy, heavy metal pollution has become
increasingly serious. With laboratory preservation a strong resistant Bacillus amyloliquefaciense was used
as experiment strains to verify whether Ca
2+
could improve the tolerance of the experimental strains to
metal and explore the adsorption characteristics of the experimental strains to Cr
6+
as well as optimize the
adsorption conditions. This experiment used the single factor experiment combined with dibenzoyl
hydrazine method to optimize Bacillus amyloliquefaciens of Cr
6+
adsorption conditions. The experimental
results showed that when calcium chloride (0.1 g/L) was in the medium, the tolerance was increased by
21.26%, 76.21% and 269.66% at Cr
6+
concentrations of 20, 40 and 60 mg/L, respectively. When the carbon
source was maltose (25 g/L) and the nitrogen source was trypsin (25 g/L), the best adsorption temperature
and pH value weree 35 ℃ and 7.5, respectively. When the concentration of Cr
6+
was 20mg/L, the
adsorption rate was as high as 89.20%, which was 24.34% higher than that before optimization. Bacillus
amyloliquefaciens has good adsorption potential for Cr
6+
, which can provide excellent microbial resources
for bioremediation or environmental pollution.
1 INTRODUCTION
1
With the rapid development of economic and
industrial technology, heavy metal pollution
gradually poses a serious threat to the natural
environment and human health. As an essential raw
material, chromium is widely used in metalworking,
metallurgy, electroplating, leather processing,
printing and dyeing industries, in which processes
produce wastewater and waste containing hexavalent
chromium (Ma, 2018. Brasili, 2020. Kazakis, 2017.
Jones, 2019. Sukumar, 2014). Chromium in
ecological environment mainly exists in hexavalent
and trivalent forms, among which hexavalent
chromium has high toxicity and mobility (Pellerin,
a
https://orcid.org/0000-0002-1440-9902
b
https://orcid.org/0000-0002-6723-9572
c
https://orcid.org/0000-0003-3147-1123
d
https://orcid.org/0000-0002-6553-0243
e
https://orcid.org/0000-0001-5388-3183
f
https://orcid.org/0000-0002-2092-4115
2000), which can cause skin allergy, dermatitis and
chromium sores (Tumolo, 2020). Furthermore, it can
cause nasal septum hemorrhage, erosion and even
perforation (Lu, 2018); as well as diarrhea,
decreased gastrointestinal function, gastrointestinal
ulcer and even bronchial cancer (Sethuraman, 2010.
Yuling, 2021).
Hexavalent chromium is considered one of the
eight most harmful chemicals and one of the three
metals most likely to cause cancer (Sethuraman,
2010). Therefore, it is very important and
meaningful to treat hexavalent chromium in waste.
Traditional treatment methods include electric
repair, activated carbon adsorption, chemical
precipitation, ion exchange method, membrane
separation and other methods (Sukumar, 2014), but
these methods have high cost, complex operation,
easy to lead to secondary pollution and high
treatment requirements. Biological method is
characterized by wide source of adsorbent, high
selectivity, no pollution and low cost, which can
solve various problems existing in traditional
1206
Lu, H., Gu, C., Yang, P., Xu, H., Bilal, M. and Ding, Y.
Optimization of Cr(VI) Adsorption by Bacillus amyloliquefaciens and Its Mechanism Study.
DOI: 10.5220/0011382700003443
In Proceedings of the 4th International Conference on Biomedical Engineering and Bioinformatics (ICBEB 2022), pages 1206-1212
ISBN: 978-989-758-595-1
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

physical and chemical methods and gradually
become a hot spot in the field of environmental
protection and heavy metal treatment (Tumolo,
2020). Biological methods are relative
environmentally friendly methods, and they use
biological cells and extracellular metabolites with
heavy metals, through oxidation - reduction reaction,
electrostatic adsorption, surface complexation and
gravity of heavy metals and does not cause
secondary pollution. Biological methods mainly
include phytoremediation, biological flocculation
method and microbial adsorption method (Xue-Nai,
2019. Valeria, 2020. Sasmita, 2014).
In this study, Bacillus amyloliquefaciens stored
in the laboratory was mainly used as the research
object to carry out the research on its adsorption
characteristics and optimization of adsorption
conditions for Cr
6+
, so as to develop the potential of
the bacteria as a microbial resource for
bioremediation or environmental pollution control.
Be advised that papers in a technically unsuitable
form will be returned for retyping. After returned the
manuscript must be appropriately modified.
2 MATERIALS AND METHODS
2.1 Microbial Strains and Medium
Bacillus amyloliquefaciens is a strain with strong
stress resistance which was screened and preserved
in laboratory (CGMCC 18719). The strain was
stored on the LB solid ramp at 4℃ and activated
before use. In the Cr(VI) chromium adsorption test,
two kinds of media were used. The seed medium
contained (per liter): 6 g beef extract, 12 g Glucose,
8 g NaCl,12 g peptone. The medium was adjusted to
pH 6.8~7.2. 18 g agar powder was added to the solid
medium on the above basis. The fermentation
medium contained (per liter): 10 g Glucose, 8 g
NaCl, 10 g peptone, 1.5 g K
2
HPO
4
, 1 g KH
2
PO
4
.
The medium was adjusted to pH 6.8~7.2.
The Bacillus amyloliquefaciens stored on a solid
inclined plane was inoculated into the seed culture
medium by inoculation loop and incubated overnight
in a constant temperature oscillating incubator at
37℃ and 150 rpm for 12 hours as the initial seed
culture liquid.
2.2 Cr (VI) Resistance Test and
Establish Standard Curve
Two groups of fermentation medium containing
hexavalent chromium ion of 0, 20, 40, 60, 80 and
100 mg/L were prepared, respectively. One group
was treated normally and the other group was added
CaCl
2
. Then, 3% (V/V) seed culture solution was
inoculated into 100 mL seed medium and incubated
overnight for 12 hours in a constant temperature
oscillating incubator at 35℃ and 150 rpm. Finally,
the absorbance of the bacterial solution was
measured at the wavelength of 600 nm, and the
tolerance curve of Bacillus amyloliquefaciens to
hexavalent chromium ions was plotted.
Appropriate amount of 1 g / L chromium ion
mother liquor (Precisely weigh 0.282 9 g K2Cr2O7
into a 100ml volumetric flask and fix the volume)
and fermentation culture were added to 50 ml
volumetric flasks to make the concentration of heavy
metal chromium ion in the culture medium 0, 0.5,
1.0, 1.5, 2.0, 2.5, 3.0 and 4.0 mg / L respectively.
Then 0.6mL (1+1) hydrochloric acid solution and
1.0 mL diphenylcarbazide solution were added
successively, immediately mixed and the volume
was fixed to the scale line with deionized water.
After standing reaction for 9 min, the absorbance
was measured at 540 nm wavelength with the
solution without chromium ion mother liquor as the
control. The standard curve reflecting the
relationship between chromium ion concentration
and absorbance value was drawn (Hadia-E, 2018).
2.3 Effect of the Adsorption Conditions
on the Adsorption Effect
Batch experiments were carried out in 250mL
conical flasks containing 100 mL medium with
appropriate volume of chromium ion mother liquor.
Then, the bacterial solution was inoculated into the
medium with 3%(V/V) of the inoculation amount
and placed in a constant temperature oscillating
incubator culture at 150 rpm. After overnight culture
for 12 h, the samples were extracted and centrifuged
at 5 000 rpm for 10 min. After the reaction with
dibenzoyl hydrazine to form purplish red complex,
spectrophotometer was used to determine the
absorbance value at 540nm, and the residual
concentration of Cr (VI) in the supernatant was
analyzed. The adsorption rate (β) of Cr (VI) is
calculated according to the following formula:
β = (C
0
-C
e
)÷C
0
(1)
where C
0
and C
e
are the initial and residual Cr (VI)
concentrations, respectively.
In order to optimize the Cr(VI) adsorption
efficiency of selected strains, the effects of Carbon
sources (sucrose, fructose, lactose, maltose,
glucose), nitrogen sources (ammonium citrate,
soybean peptone, tryptone, peptone), temperature
Optimization of Cr(VI) Adsorption by Bacillus amyloliquefaciens and Its Mechanism Study
1207
(30, 32, 35, 38, 40℃) were studied, pH (6.0, 6.5,
7.0, 7.5, 8.0) and initial Cr(VI) concentration (10,
20, 30, 40, 50mg/L) were investigated.. Each set of
experiments was in triplicate, and the average value
was taken for further analysis.
3 RESULTS AND ANALYSIS
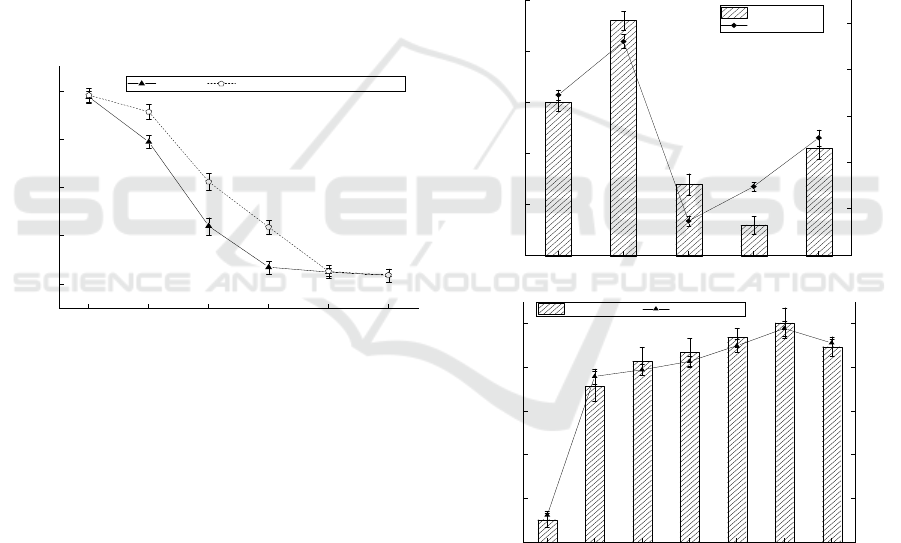
3.1 Effects of Chromium and Calcium
on the Growth of the Strain
This study verified whether Ca
2+
could improve the
tolerance of Bacillus amyloliquefaciens to Cr
6+
. As
can be seen from Figure 1, with the increase of Cr
6+
concentration, the tolerance of the strain to Cr
6+
increased significantly with the addition of calcium
chloride. When calcium chloride was present, the
tolerance to Cr
6+
increased by 21.26%, 76.21% and
239.66% at 20, 40 and 60mg/L of Cr
6+
, respectively.
0 20406080100
0.0
0.5
1.0
1.5
2.0
normal 0.1g/L Calcium chloride
K
2
Cr
2
O
7
concentration(mg/L)
OD
600
Figure 1: Tolerance curve of Bacillus amyloliquefaciens to
Cr
6+
(conditions: temperature = 35℃, pH=7, agitation rate
= 150 rpm, contact time = 12 h).
In acidic environment, Cr
6+
reacts with
dibenzodiazide solution to form a purplish red
complex with a maximum absorption wavelength of
540 nm. Therefore, the absorbance value at 540 nm
was used as the abscissa and the concentration of
chromium ions as the ordinate to draw the working
curve. The linear regression equation y = 2.562 1x-
0.053 and the correlation coefficient R
2
= 0.994
were obtained. The linear relationship was good as
well as the experimental stability.
3.2 Effect of Carbon Source on
Adsorption Effect
It can be seen from Figure 2 that when the carbon
source was maltose, the adsorption effect was better.
The concentration of residual Cr
6+
in the medium
was 4.78 mg/L, the bacteria weight was 0.56 g and
the adsorption rate was as high as 76.08%. When the
carbon source was fructose, it had the worst
adsorption effect. The concentration of residual Cr
6+
was 12.52 mg/L, the bacteria weight was 0.24 g and
the adsorption rate was only 37.40%. With the
increase of carbon source concentration, the
adsorption rate reached 88.94% when maltose
concentration was 25 g/L, which increased by
9.48%. It can be clearly seen from Figure 2 that,
with the increase of concentration and adsorption
rate, the bacterial weight also increased. Therefore,
it can be inferred that the increase of carbon source
concentration provides sufficient carbon source for
the growth of bacterial strains, leading to the
increase of the number of bacterial strains in the
medium, thus enhancing the adsorption effect.
glucose maltose fructose sucrose lactose
0.1
0.2
0.3
0.4
0.5
0.6
bacteria weight
adsorption rate
(
bacteria weight g
)
30
40
50
60
70
80
(
adsorption rate %
)
0 5 10 15 20 25 30
0.10
0.15
0.20
0.25
0.30
0.35
0.40
0.45
0.50
bacteria weight adsorption rate
concentration
()
mg/L
bacteria weight
()
g
40
50
60
70
80
90
adsorption rate(%)
Figure 2: Effect of carbon sources on the adsorption effect
(conditions: temperature = 35℃, pH=7, agitation rate =
150 rpm, contact time = 12 h).
3.3 Effect of Nitrogen Source on
Adsorption Effect
As can be seen from Figure 3, when the nitrogen
source was peptone, the adsorption effect was the
best, the concentration of residual Cr
6+
in the
medium was 3.48 mg/L, the bacteria weight was
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
1208
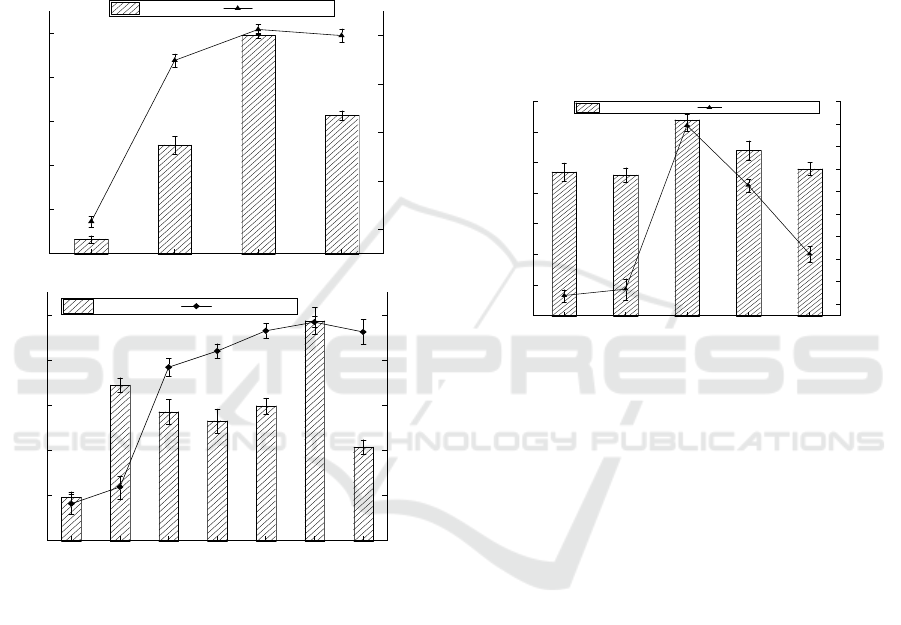
0.99 g and the adsorption rate was as high as
82.67%. When the nitrogen source was ammonium
citrate, the concentration of residual Cr
6+
was 19.35
mg/L, the bacteria weight was 0.07 g, and the
adsorption rate was only 3.24%. The cells may have
died and the dead cells still had certain adsorption
capacity for heavy metals. With the increase of
nitrogen source concentration, the adsorption rate
reached 88.43% when the concentration of peptone
was 25 g/L, which increased by 18.57%.
ammonium citrate peptone tryptone soybean peptone
0.0
0.2
0.4
0.6
0.8
1.0
bacteria weight adsorption rate
bacteria weight
()
g
0
20
40
60
80
adsorption rate
(
%
)
0 5 10 15 20 25 30
0.0
0.1
0.2
0.3
0.4
0.5
bacteria weight adsorption rate
concentration
()
g/L
bacteria weight(g)
40
50
60
70
80
90
adsorption rate
(
%
)
Figure 3: Effect of the nitrogen source on the adsorption
effect (conditions: temperature = 35℃, pH=7, agitation
rate = 150 rpm, contact time = 12 h, carbon source = 25
g/L maltose).
3.4 Effect of Temperature on
Adsorption Effect
The life activities of microorganisms cannot be
separated from the help of enzymes and enzyme
activity is closely related to temperature18. The
adsorption of Cr(VI) was studied at 20 mg/L initial
Cr(VI) concentration, pH=7 and 150 rpm and 5
different temperatures (30, 32, 35, 38, 40℃). As can
be seen from Figure 4, when the temperature was
35℃, the activity of enzymes related to adsorption
was strong and the effect was the best. The
concentration of residual Cr
6+
in the medium was
only 4.06 mg/L, the bacteria weight was 0.64 g and
the adsorption rate was as high as 79.72%. At 30 ℃,
the enzyme activity was weak and the effect was the
worst. The concentration of residual Cr
6+
was 11.61
mg/L, the bacteria weight was 0.47 g and the
adsorption rate was only 41.93%.
Extreme temperature will have adverse effects on
the growth of bacteria and chrome reductase, and the
growth and development of bacteria will be inhibited
at low temperature. At higher temperature, the
conformation of ribosome will change to some
extent, which will lead to the change of membrane
structure and decrease or even inactivation of
chromium reductase.
30℃ 32℃ 35℃ 38℃ 40℃
0.0
0.1
0.2
0.3
0.4
0.5
0.6
0.7
bacteria weight adsorption rate
bacteria weight
()
g
40
45
50
55
60
65
70
75
80
85
adsorption rate
(
%
)
Figure 4: Effect of the temperature on the adsorption
effect (conditions: agitation rate = 150 rpm, pH=7, contact
time = 12 h, carbon source = 25 g/L maltose, nitrogen
source =25 g/L tryptone).
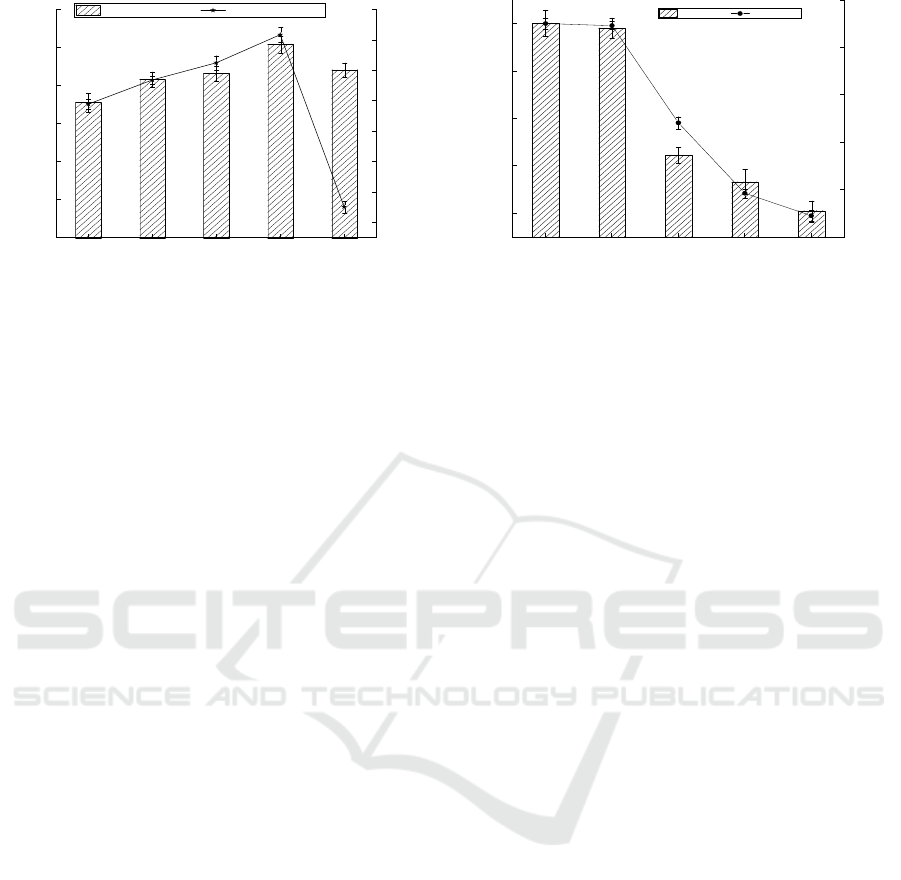
3.5 Effect of pH on Adsorption Effect
In the adsorption process, the pH of the medium can
affect the ionized state of the major functional
groups responsible for metal ion binding, such as
carboxyl, amino, and phosphorylation. At low pH,
these groups retain their protons, which reduces the
possibility of binding with other positively charged
ions. On the other hand, at higher pH, the carboxyl
groups become deprotonated and negatively
charged, which helps to attract positively charged
metal ions.As can be seen from Figure 5, when
pH=7.5, the effect was the best, the concentration of
residual Cr
6+
in the medium was 3.83 mg/L, the
bacterial weight was 0.51 g and the adsorption rate
was 80.87%. When pH=8, the effect was the worst,
the bacteria weight was 0.44 g, the residual Cr
6+
concentration was 9.49 mg/L and the adsorption rate
was only 52.56%.
Optimization of Cr(VI) Adsorption by Bacillus amyloliquefaciens and Its Mechanism Study
1209
6.0 6.5 7.0 7.5 8.0
0.0
0.1
0.2
0.3
0.4
0.5
0.6
bacteria weight adsorption rate
pH
bacteria weight
()
g
50
55
60
65
70
75
80
85
adsorption rate
(
%
)
Figure 5: Effect of the pH on the adsorption effect
(conditions: temperature = 35℃, agitation rate = 150 rpm,
contact time = 12 h, carbon source = 25 g/L maltose,
nitrogen source =25 g/L tryptone).
3.6 Effect of Initial Concentration of
Cr
6+
on Adsorption Effect
Five different initial metal ion concentrations (10,
20, 30, 40 and 50 mg/L) and 3% inoculation were
used to determine the biosorption of chromium by
the strain. The effect of the initial concentration of
chromium on the adsorption is shown in Figure 6. It
can be clearly seen that, with the increase of
concentration, the adsorption effect decreased
gradually, which may be related to the saturation
degree of the adsorption site of the bacteria4. When
the initial concentration was 10 mg/L and 20 mg/L,
the adsorption sites on the cell wall of the bacteria
had basically reached the saturation state, so the
effect was good. The bacteria weight was 0.501 g
and 0.491 g, respectively and the adsorption rate was
90.10% and 89.20%. When the concentration of Cr
6+
was 20 mg/L, the adsorption rate of the optimized
medium increased from 64.56% to 89.20%, which
was 24.64% higher than that before the optimization.
When the initial concentration was 50 mg/L, metal
ions had a great toxic effect on cells18 and inhibited
the adsorption effect and the adsorption rate was
only 9.01%.
4 DISCUSSION
The mechanisms of chromium ion removal by
microorganisms mainly include membrane surface
adsorption, transformation, intracellular absorption,
intracellular transformation and extracellular
transformation. Chromium ions can be fixed on the
binding sites provided by polysaccharides, proteins
and lipids on the surface of the cell membrane by the
10 20 30 40 50
0.1
0.2
0.3
0.4
0.5
bacteria weight adsorption rate
concentration
()
mg/L
bacteria weight (g)
0
20
40
60
80
100
adsorption rate (%)
Figure 6: Effect of the initial Cr6+ concentration on the
adsorption effect (conditions: temperature = 35℃,
pH=7.5, agitation rate = 150 rpm, contact time = 12 h,
carbon source = 25 g/L maltose, nitrogen source =25 g/L
tryptone).
chemical binding ability of functional groups on the
surface of the cell membrane, electrostatic force and
the electrostatic attraction of cations on the surface
of the cell membrane(Hadia-E, 2018. Sasmita, 2014.
Ramírez-Díaz, 2008.Gunnar, 2018). Most of these
chromium ions enter the cell through a unique
mechanism and a small part may be reduced by
membrane-bound reductase mediated action and
extracellular polysaccharide complexation with the
involvement of membrane surface functional
groups(Tang, 2020).
Since chromium ions exist in the form of CrO
4
2-
,
a regular tetrahedral structure similar to SO
4
2-
in
spatial conformation, they can enter the cell channel
through SO
4
2-
. Then, soluble reductase (NADH,
NemA, NAPH, etc.) and reducing agents (ascorbic
acid, glutathione, etc.) in the cytoplasm were
reduced to Cr
3+
with lower toxicity(Karthik, 2017).
In the environment of ferric reducing bacteria and
sulfate reducing bacteria, the extracellular products
(ferrous ions, hydrogen sulfide, etc.) that can be
anaerobic metabolized by bacteria are reduced
without ATP consumption(Long, 2021).
The adsorption tests in this study were all carried
out in an environment containing only chromium
ions, while the actual chromium pollution treatment
often contains metal ions such as Cu
2+
, Mg
2+,
Pb
2+
,
Mn
2+
, Fe
2+
, Ca
2+
and some oxygen anions (sulfate
ion, nitrate ion, etc.), which may affect the
adsorption. HANG found that 5 mg/L Cu
2+
promoted the adsorption of Cr6+ to Bacillus sp.
CrB-B1, and the adsorption rate increased to
92.21%. When Cd
2+
concentration was 5 mg/L, the
adsorption rate decreased by 25.03%(Tang, 2020).
Oxygen anions such as SO
4
2-
and NO
3
-
have little
effect on the adsorption of Cr6+. LUO showed that
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
1210

Ca
2+
and SO
4
2-
promoted the reduction of chromium
by 52.5% and 55.9%, respectively(Luo, 2020). In
addition, studies have shown that some
microorganisms can also remove other ions (Pb
2+
,
NO
3-
, etc.) in the environment in the process of
chromium ion adsorption(Zhong, 2017. Yu, 2016.
An, 2020). XU isolated a strain of Serseria
marcescens from tannery wastewater, which can
remove carcinogenic o-dichlorobenzene while
absorbing hexavalent chromium(Xu, 2018). The co-
removal of chromium ions and other ions as well as
the co-removal of chromium ions and organic matter
further provides a theoretical basis for the
application of microorganisms in the practical heavy
metal pollution treatment, which is of great
significance to the remediation of chromium
pollution by microorganisms. In practical
application, the influence of the external
environment on the growth of microorganisms and
the adsorption effect should be considered
comprehensively. Therefore, while excavating the
adsorption potential of microorganisms for heavy
metal ions, we should also pay attention to their
adaptability to the complex and changing
environment.
5 CONCLUSIONS
In this experiment, the adsorption capacity of Cr
6+
of
a strain of Bacillus amyloliquefaciens with strong
stress resistance preserved in the laboratory was
studied. At the same time, it was also verified that
Ca
2+
improved the tolerance of the strain to metals.
The results showed that when the concentration of
Cr
6+
was 20 mg/L, the temperature was 35℃, the pH
value was 7.5, the carbon source was maltose (25
g/L) and the nitrogen source was tryptone (25 g/L),
the adsorption rate of Cr
6+
was 89.20%, which was
24.34% higher than that before optimization. When
calcium chloride (0.1 g/L) was added to the culture
medium, the tolerance was increased by 21.26%,
76.21% and 239.66% when the concentration of Cr
6+
was 20, 40 and 60 mg/L, respectively. In the Cr
6+
adsorption test, it was found that the cell content
may have a certain relationship with the adsorption
effect. Within the limited concentration range, the
cell content has a negative correlation with the
residual Cr
6+
concentration, that is, the cell content
has a positive correlation with the adsorption effect,
and the more cells, the better the adsorption effect.
ACKNOWLEDGEMENTS
This work was supported by the National Natural
Science Foundation of China (31801524), and
Natural Science Foundation of Jiangsu Province
(BK20170461, BK20181063).
REFERENCES
An, Q., Deng, S., Xu, J., Nan, H., Li, Z. and Song, J.-
L(2020). Simultaneous reduction of nitrate and Cr(VI)
by Pseudomonas aeruginosa strain G12 in wastewater
%J Ecotoxicology and Environmental Safety, 191 .
Brasili, E., Bavasso, I., Petruccelli, V., Vilardi, G.,
Valletta, A., Dal Bosco, C., Gentili, A., Pasqua, G. and
Di Palma, L(2020). Remediation of hexavalent
chromium contaminated water through zero-valent
iron nanoparticles and effects on tomato plant growth
performance. Scientific reports, 10, 1 , 1920.
Gunnar, S., Stefanie, B., Elena, S., Felix, D., Johannes, R.,
Peter, G., Rizlan, B.-L., Juraj, M. and Johannes,
G(2018). Chromate Resistance Mechanisms in
Leucobacter chromiiresistens. %J Applied and
environmental microbiology, 84, 23 .
Hadia-E, F. and Ambreen, A((2018)). Micro-remediation
of chromium contaminated soils. J PeerJ, 6 .
Jones, A. S., Marini, J., Solo-Gabriele, H. M., Robey, N.
M. and Townsend, T. G (2019). Arsenic, copper, and
chromium from treated wood products in the U.S.
disposal sector. Waste management (New York,
N.Y.), 87 , 731-740.
Karthik, C., Ramkumar, V. S., Pugazhendhi, A.,
Gopalakrishnan, K. and Arulselvi, P. I(2017).
Biosorption and biotransformation of Cr(VI) by novel
Cellulosimicrobium funkei strain AR6 J Journal of
the Taiwan Institute of Chemical Engineers, 70 .
Kazakis, N. Kantiranis, N. Kalaitzidou, K(2017). Origin of
hexavalent chromium in groundwater: The example of
Sarigkiol Basin, Northern Greece. J The Science of the
total environment, 593-594 .
Long, B., Ye, J., Ye, Z., He, J., Luo, Y., Zhao, Y. and Shi,
J(2020). Cr(VI) removal by Penicillium oxalicum
SL2: Reduction with acidic metabolites and form
transformation in the mycelium %J Chemosphere, 253
.
Lu, J., Fu, F., Zhang, L. and Tang, B(2018). Insight into
efficient co-removal of Se(IV) and Cr(VI) by magnetic
mesoporous carbon microspheres: Performance and
mechanism %J Chemical Engineering Journal, 346 .
Luo, Y., Ye, B., Ye, J., Pang, J., Xu, Q., Shi, J., Long, B.
and Shi, J(2020). Ca 2+ and SO 4 2− accelerate the
reduction of Cr(VI) by Penicillium oxalicum SL2 %J
Journal of Hazardous Materials, 382 .
Ma, S., Song, C.-S., Chen, Y., Wang, F. and Chen, H.-
L(2018). Hematite enhances the removal of Cr(VI) by
Bacillus subtilis BSn5 from aquatic environment %J
Chemosphere, 208.
Optimization of Cr(VI) Adsorption by Bacillus amyloliquefaciens and Its Mechanism Study
1211

Pellerin, C. and Booker, S. M (2000). Reflections on
hexavalent chromium: health hazards of an industrial
heavyweight. Environmental health perspectives, 108,
9, A402-407.
Ramírez-Díaz, M. I., Díaz-Pérez, C., Vargas, E., Riveros-
Rosas, H., Campos-García, J. and Cervantes, C(2008).
Mechanisms of bacterial resistance to chromium
compounds %J BioMetals, 21, 3 .
Sasmita, D, Mishra, J., Das, S. K., Pandey, S., Rao, D. S.,
Chakraborty, A., Sudarshan, M., Das, N. and Thatoi,
H. Investigation on mechanism of Cr(VI) reduction
and removal by Bacillus amyloliquefaciens , a novel
chromate tolerant bacterium isolated from chromite
mine soil %J Chemosphere, 96 (2014).
Sethuraman, P. and Balasubramanian, N(2010). Removal
of Cr(VI) from aqueous solution using Bacillus
subtilis, Pseudomonas aeruginosa and Enterobacter
cloacae %J International Journal of Engineering
Science and Technology, 2, 6 .
Sukumar, C., Janaki, V., Kamala-Kannan, S. and Shanthi,
K(2014). Biosorption of chromium(VI) using Bacillus
subtilis SS-1 isolated from soil samples of
electroplating industry %J Clean Technologies and
Environmental Policy, 16, 2 .
Tang, H., Can, W., Guoquan, Z., Yao, L., Hao, L. and
Heng, X (2020). Bioreduction and biosorption of
Cr(VI) by a novel Bacillus sp. CRB-B1 strain. %J
Journal of hazardous materials, 386.
Tumolo, M., Ancona, V., De Paola, D., Losacco, D.,
Campanale, C., Massarelli, C. and Uricchio, V.
F(2020). Chromium Pollution in European Water,
Sources, Health Risk, and Remediation Strategies: An
Overview. International journal of environmental
research and public health, 17, 15.
Valeria, A., Claudia, C., Marina, T., Domenico, D. P.,
Claudio, A., Angela, V., Uricchio and Felice, V(2020).
Enhancement of Chromium (VI) Reduction in
Microcosms Amended with Lactate or Yeast Extract:
A Laboratory-Scale Study. %J International journal of
environmental research and public health, 17, 3.
Xu, W., Duan, G., Liu, Y., Zeng, G., Li, X., Liang, J. and
Zhang, W(2018). Simultaneous removal of hexavalent
chromium and o-dichlorobenzene by isolated Serratia
marcescens ZD-9. Biodegradation, 29, 6 , 605-616.
Xue-Na, H., Di, M., Dong-Feng, L., Lei, C., Chen, Q.,
Wen-Wei, L. and Han-Qing, Y (2019). Formation
mechanism of organo-chromium (III) complexes from
bioreduction of chromium (VI) by Aeromonas
hydrophila. %J Environment international, 129.
Yuling, Z., Xiaoyun, H., Jiali, X., Zheng, F., Siying, W.,
Jian, N. and Baowei, H(2021). Insight into efficient
removal of Cr(VI) by magnetite immobilized with
Lysinibacillus sp. JLT12: Mechanism and
performance J Chemosphere, 262 .
Yu, X., Jiang, Y., Huang, H., Shi, J., Wu, K., Zhang, P.,
Lv, J., Li, H., He, H., Liu, P. and Li, X (2016).
Simultaneous aerobic denitrification and Cr(VI)
reduction by Pseudomonas brassicacearum LZ-4 in
wastewater J Bioresource Technology, 221 .
Zhong, L., Lai, C.-Y., Shi, L.-D., Wang, K.-D., Dai, Y.-J.,
Liu, Y.-W., Ma, F., Rittmann, B. E., Zheng, P. and
Zhao, H.-P (2017). Nitrate effects on chromate
reduction in a methane-based biofilm %J Water
Research, 115.
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
1212
