
NKG2A: A Novel Immune Checkpoint Protein for Cancer Treatment
Qiwen Huang
Ulink College Guangzhou, Guangzhou, Guangdong, China
Keywords:
Cancer Immunotherapy, Immune Checkpoint Inhibitor (ICI), Monoclonal Bodies (mAbs), CD94/NKG2A,
Monalizumab.
Abstract:
Immune checkpoint inhibitor (ICI) drugs have been figured prominently in various of cancer immunotherapy.
Immune checkpoint inhibitor monoclonal antibodies (mAbs) targeting cytotoxic T-lymphocyte antigen 4
(CTLA-4), programmed cell death protein 1 (PD-1) or its ligand (PD-L1), have achieved unprecedented
improvements. Nonetheless, due to some issues such as tumor resistance, genetic heterogeneity, and its
intricacy of immune regulatory pathways, immunotherapy remains a major challenge. As a result, it's critical
to discover further about immunological checkpoints and the application of their inhibitors in clinical practice.
The potential inhibitory CD94/NKG2A receptor has been explored in recently, which involve in the
stimulation of both natural killer (NK) and CD8+ T cell immunological activity, as well as predominantly
link to immune cell-tumor interaction. However, the specific mechanisms for immune regulation, NKG2A-
targetd inhibitors and related clinical trials are still underestimated. Therefore, we'll look at the basic structure
and function of CD94/NKG2A, and its immune regulatory mechanisms, as well as the current NKG2A-
targeted clinical results in the review.
1 INTRODUCTION
Immune checkpoint protein originally acts as
mediators for protection of normal tissues from
carcinogenesis. However, it is found that tumor cells
utilize some of the immune checkpoints as a
significant channel for evasion of immune
surveillance and immune resistance. To date, immune
checkpoint inhibitor (ICI) drugs that efficiently block
or modulate the ligand-receptor interaction have been
developed to improve the treatment for cancers. The
ICI targeting PD-1/PD-L1 and CTLA-4 were showed
therapeutically potent and improved the clinical
benefits for patients. Seven of them therefore were
approved by the US Food and Drug Administration
(FDA), involving one for CTLA-4 (ipilimumab), three
for PD-1 (nivolumab, pembrolizumab, cemiplimab)
and another three for PD-L1 (atezolizumab,
avelumab, duralumab) (Verma, Sprave, Haque, et al.
2018). Although ICIs displayed remarkable outcomes
in clinical trials for cancers, e,g non-small cell lung
carcinoma (NSCLC), melanoma, and relapsed or
refractory Hodgkin’s lymphoma, it can only benefit a
small number of patients due to the complicate
mechanisms of different types of tumor micro-
environment (TME) for protection of tumor against
immune responses (Darvin, Toor, Sasidharan Nair,
Elkord, 2018). What’s worse, some of the patients
who are treated with immune checkpoints inhibitors
risk serious immune-related adverse events (irAEs),
and hyper-progression (Feng, Roy, Masson, Chen,
Humphrey, Weber, 2013). Therefore, more effective
and safe molecular targets and drugs are essential for
improvement of ICI discovery and treatments.
NKG2A, an inhibitory receptor that regulates both
innate and adaptive immunity through subsets of T
cells and NK cells, that injects new blood into cancer
immunotherapy. NKG2A often expresses on the
membranes of NK cells and T cell subsets as a
heterodimeric proteins by linking with invariant CD94
polypeptide disulfide (Boyington, Riaz, Patamawenu,
Coligan, Brooks, Sun, 1999). NK cells and T cells
have significant effect on limiting both tumor
progression and metastasis (Zaghi, Calvi, Marcenaro,
Mavilio, Di Vito, 2019). The recognition of human
histocompatibility leukocyte Ag-E (HLA-E) on tumor
cells by CD94/NKG2A complex inhibits certain
functions of NK cells and particular types of T cells,
thus speeding up tumor escape from the immune
system5.In this case, tumor immunity can be greatly
enhanced by the inhibition of CD94/NKG2A or the
ligands, although there are limited approved
medicines, established researches and trials about this
1076
Huang, Q.
NKG2A: A Novel Immune Checkpoint Protein for Cancer Treatment.
DOI: 10.5220/0011377500003443
In Proceedings of the 4th International Conference on Biomedical Engineering and Bioinformatics (ICBEB 2022), pages 1076-1083
ISBN: 978-989-758-595-1
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
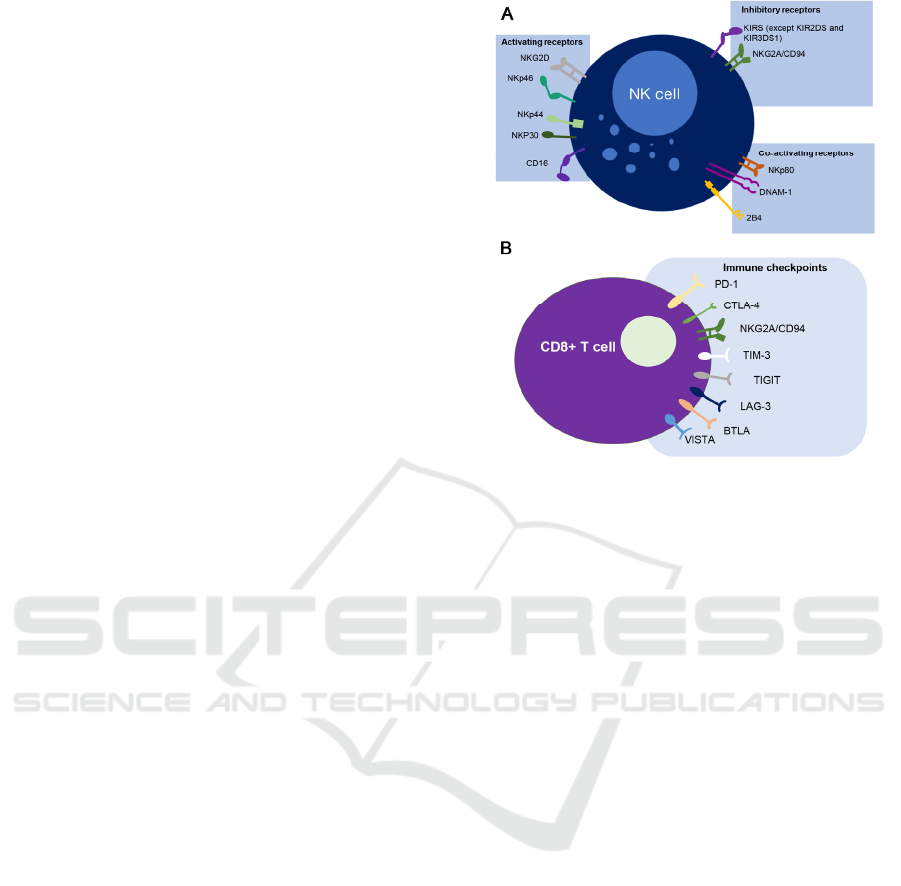
receptor. Here, we review the foundational structure
of NKG2A, involving it genetic information, protein
domain and regulatory mechanisms, as well as its
advances and updates in pan-cancers.
2 NKG2A/CD94
2.1 The Structure of NKG2A
NKG2A is an inhibitory member of the NKG2 family
of receptors6, consisting of 233 amino acid. NKG2 is
a C-type lectin-like receptor family including seven
subunit proteins, NKG2A, B, C, D, E, F, and H
(Sullivan, Clements, Beddoe, et al, 2007). Previous
results have demonstrated that NKG2A shows great
similarity in genomic organization and sequence with
NKG2-C, -E and -F, having the same transcriptional
orientation as theirs (Borrego, Kabat, Kim, et al.
2002). However, NKG2A transcript involves an
unique extra 5 untranslated exon (Plougastel,
Trowsdale, 1998). Its C-type lectin domain is
possessed by a type II integral membrane protein
(Glienke, Sobanov, Brostjan, et al, 1998). The
NKG2A molecule carries two immunoreceptor
tyrosine-based inhibitory motifs (ITIMS) in the
intracytoplasmic tail nearly identical to those of the
inhibitory KIRs (Borrego, Kabat, Kim, et al. 2002)
and forms lengthened disulfide-linked heterodimers
with the invariant CD94 protein (Walter, Petersen,
2017). Recent researches have also shown that
NKG2A is expressed in combination with other
proteins in some NK cells (Figure 1A) and T cell
subsets (Figure 1B). The NKG2A receptor has been
found on nearly half of the peripheral NK cells which
are predominantly presented in the CD56high fraction
that contains the more immature cells (Borst L, Burg
SH van der, Hall T van. 2020). Moreover, intra-
tumoral NK cells express rather high frequencies of
NKG2A (van Montfoort, Borst, Korrer, et al, 2018).
The expression of NKG2A In CD8 T cells has been
strictly regulated since peripheral cells hardly display
this receptor, while the majority of intra-tumoral T
cells, particularly those in the tumor
microenvironment, exhibit NKG2A (van Montfoort,
Borst, Korrer, et al, 2018, Hamid, Wang, Yao, et al,
2019).
Figure 1. The expression and interaction of NKG2A in NK
cell (A) and Tcell (B).
The NKG2A ectodomain, with the overall
dimensions 54*33*29 A, is consist of two
perpendicular beta sheets formed by 8 Beta strands,
plus 2 alpha helices (residues 140–149 and 160–169)
on the opposite face of the promotor (Sullivan,
Clements, Beddoe, et al, 2007). The three bonds
stabilizing the NKG2A fold are Cys119-Cys130 for
the connection between the beta2 strand and the N-
terminal loop; Cys147-Cys229 for the connection
between beta7 strand and alpha1 helix; and Cys208-
Cys221 for the connection between beta5 strand to
loop 6 (Sullivan, Clements, Beddoe, et al, 2007).
Therefore, NKG2A is in the same structural
homologous series with CD94 (Sullivan, Clements,
Beddoe, et al, 2007).
The CD94 molecule, a subunit of CD94/NKG2A
receptor with general dimension of 42*37*33 A in
approximation, is made up of an antiparallel beta sheet
with strands 1, 2, 7, an antiparallel beta sheet with
strands 4, 3, 5, and 6, and an alpha helix after strand 2
(Boyington, Riaz, Patamawenu, Coligan, Brooks,
Sun, 1999). It has four intrachain disulfide linkages,
including three invariant disulfides (Cys61–Cys72,
Cys89–Cys174, and Cys152–Cys166) which
presented in long-form C-type lectin (Day, 1994).
Cys59-Cys70 which is the fourth disulfide, links with
N-terminal beta strands and is found uniquely in the
molecular structure of CD94 (Boyington, Riaz,
Patamawenu, Coligan, Brooks, Sun, 1999). Cys58,
the lone interstitial cysteine not found in interchain
disulfide pairing, is supposed to combine with the
NKG2A: A Novel Immune Checkpoint Protein for Cancer Treatment
1077

Cys116 in NKG2A, resulting in the interchain
disulfide in the CD94/NKG2A heterodimer
(Boyington, Riaz, Patamawenu, Coligan, Brooks,
Sun, 1999).
The CD94/NKG2A dimer has the dimension
75*42*38 A with a large dimer interface containing
roughly 1500 A2 of buried surface area (BSA)
(Sullivan, Clements, Beddoe, et al, 2007), at which
CD94 and NKG2A contribute 69% and 31%
respectively (Petrie, Clements, Lin, et al, 2008). The
interaction between the alpha2 helix of NKG2A and
the complementary prolonged loop region of CD94
greatly leads to the asymmetry at the CD94/NKG2A
interface (Sullivan, Clements, Beddoe, et al, 2007).
Additionally, there are two of the amino acids in
CD94/NKG2A locating on the presumed HLA-E
binding site, position 197 in loop 5 (Glu) and position
225 within loop 7 (Ile) (Sullivan, Clements, Beddoe,
et al, 2007).
According to recent researches, tumor infiltration
NK cells and CD8+ T cells express an abnormal
amount of NKG2A which contributes to the poor
cancer prognosis (Zaghi, Calvi, Marcenaro, Mavilio,
Di Vito, 2019). By analyzing the tissue-infiltrating
leukocyte (ITL) from normal livers, intratumor tissues
(IT), peritumor tissues (PT) and intratumor tissues
(IT), Cheng Sun et al had found that the expression of
NKG2A in NK cells from IT was dramatically
increased in comparison with those cells in healthy
livers and PT which also relates to NK cell exhaustion
and to great extent, results in a shorter overall survival
(OS) of patients with hepatocellular carcinoma (Sun,
Xu, Huang, et al, 2017). Furthermore, the
upregulation on NK cells by NKG2A in patients with
lung cancers can act as a biomarker of tumor
metastatization (NK Cell Phenotypic Modulation in
Lung Cancer Environment, 2021). Besides, the direct
interaction between NK cells and intratumor stromal
cells gives rise to the pathogenic and phenotypic
mutation of NK cell in lung cancer as well as invasive
breast cancer where an increment in the expression of
NKG2A and a lessened expression of the NKRs
NKp30, NKG2D, DNAM-1, and CD16 have been
observed (Galland, Vuille, Martin, et al, 2017,
Mamessier, Sylvain, Thibult, et al, 2011).
2.2 The Ligand of NKG2A in Both NK
and T Cells
The primary ligand for CD94/NKG2A inhibitory
receptor is the human major histocompatibility
complex class Ib (MHC-Ib) molecule, HLA-E (Braud,
Allan, O’Callaghan, et al, Borrego, Ulbrecht, Weiss,
Coligan, Brooks, 1998,)
and its mouse ortholog Qa-1b
(Borst L, Burg SH van der, Hall T van. 2020). This
class Ib protein specifically binds and presents an
immensely associated set of nonameric peptides
generated from the signal sequences of class I
molecules (Braud, Allan, O’Callaghan, et al, 1998,
Braud, Yvonne Jones, McMichael. 1997, Lee, Llano,
Carretero, et al, 1998), which is different from class Ia
molecules that exhibit a broad range of peptide
ligands. In addition, it contains only two functional
alleles present in humans (the HLA-E*01:01 and the
HLA-E*01:03 variants
)
(Borst L, Burg SH van der,
Hall T van. 2020). These 2 alleles can be distinguished
from each other only by an individual amino acid at
position 107 which is arginine (01:01) or glycine
(01:03) (Borst L, Burg SH van der, Hall T van. 2020).
The expression of HLA-E is overall common whereas
relatively low in normal tissues with exceptions of
high level of expression in trophoblast cells in the
placenta and ductal epithelial cells in the testis and
epididymis due to the effect of HLA-E in immune
tolerance (Wei, Orr, 1990, van Hall, André,
Horowitz, et al, 2019). In contrast, the amount of
HLA-E exert on tumor cells are abnormally increased
in lung, kidney, pancreas, stomach, colon, head and
neck, liver, melanoma, prostate, and rectal tumor
tissues (van Montfoort, Borst, Korrer, et al, 2018,
Gooden, Lampen, Jordanova, et al, 2011). It has been
reported that high HLA-E expression can be
associated with a poor prognosis in colorectal
carcinoma, breast and ovarian carcinoma (Gooden,
Lampen, Jordanova, et al, 2011, Levy, Bianchini,
Von Euw, et al, 2008). Nevertheless, a favorable
connection between expression of HLA-E and
survival time has been recognized in patients with
glioblastoma (Kren, Slaby, Muckova, et al, 2011).
Joseph D.Miller et al have indicated that the
position 5 Arg side chain in HLA-E performs as a
dominant contact for interaction with CD94/NKG2A
receptor, which acts as one of the main contact
residues together with P8 amino acids for this
interaction (Miller, Weber, Ibegbu, Pohl, Altman,
Jensen, 2003). For mechanism, the tyrosine
phosphorylation of ITIMs and following recruitment
and activation of phosphatases (SHP-1 and SHP-2)
then characterizes the ligation of inhibitory receptors,
thus causing the inhibition of various NK cell-
mediated effector functions (Burshtyn, Scharenberg,
Wagtmann, et al, 1996). The length of amino acid
between the two ITIMs in NKG2A is about 25
peptides, which is regarded to be appropriate for the
occupation of tandem SH2 with phosphatases6. Also,
for the maximum phosphatase catalytic activity, it is
necessary to activate SH2 domains of SHP-1/-2
simultaneously (Pluskey, Wandless, Walsh,
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
1078
Shoelson, 1995). Previous discovery had shown that
HLA-E complexed to the peptide correlating to the
leader sequence peptide derived from HLA-G-bound
CD94-NKG2A has the affinity of 0.94 mM (Kaiser,
Barahmand-pour, Paulsene, Medley, Geraghty,
Strong, 2005) and the peptide structure was found to
affect binding affinity (Miller, Weber, Ibegbu, Pohl,
Altman, Jensen, 2003).
2.3 Current Statue of NKG2A in Basic
Research
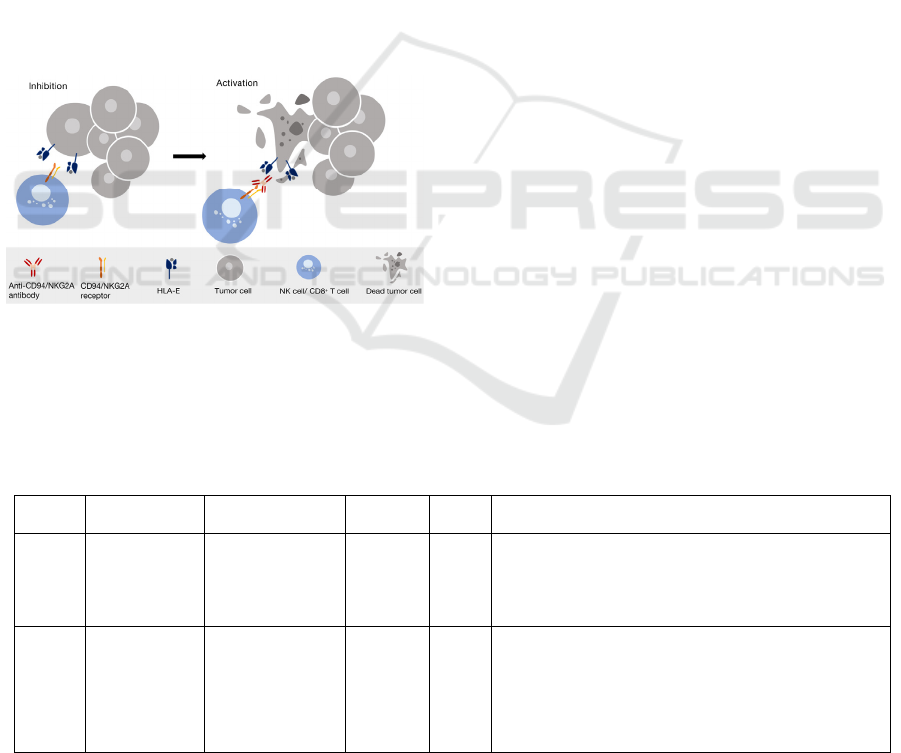
Since CD94/NKG2A receptor is expressed by not
only NK cells but also a subsets of T cells such as
activated αβ CD8pos T-cells, 𝛾𝛿 T cells, and NK-
T cells34, the blockade of CD94/NKG2A can
effectively unleash the reactivity of immune cells
involving several types of cytotoxic lymphocytes
(Figure2)., resulting in triggering their antitumor
potentials and strengthening tumor control (Zaghi,
Calvi, Marcenaro, Mavilio, Di Vito, 2019).
Figure 2. The mechanism of NKG2A inhibitor for cancer
treatment
Monalizumab/IPH2201 is a humanized and
clinical stage anti-NKG2A monoclonal
antibody(mAb) which was initially developed in mice
(Zaghi, Calvi, Marcenaro, Mavilio, Di Vito, 2019). It
have revealed therapeutic effect on immunodeficient
mice with human leukemia (Effects of anti-NKG2A
antibody administration on leukemia and normal
hematopoietic cells, 2021), leading to its possibility of
its development in phase I-III clinical trials targeting
solid tumors and hematologic. According to the Tg32
mouse PK assay, the monalizumab has a binding
affinity of 48.1 ± 3.1nM, NK cell killing efficacy of
1.5 ± 0.78 μg/ml and an approximately 17-day plasma
PK half‐life in Tg32 mouse. It is recently investigated
for its efficacy and toxicity in the treatment of
different forms of cancers, such as gynecological and
squamous cell carcinoma of the head and neck
(SCCHN) (Spinosa, Musial‐Siwek, Presler, et al,
2021). Since CD94/NKG2A receptors usually co-
express with PD-1 (André, Denis, Soulas, et al, 2018),
monalizumab are recently examined with anti-PD(L)1
antibodies (durvalumab and nivolumab) (Spinosa,
Musial‐Siwek, Presler, et al, 2021), which have also
been registered in NIH gov.clinical trial
(https://clinicaltrials. gov/ct2/home)
3 CLINICAL APPLICATION OF
NKG2A INHIBITOR,
MONALIZUMAB
Nowadays, the safety and efficacy of monalizumab is
investigated and tested. A number of clinical trials for
the treatment of different types of cancer have already
completed and shown effective results, including
Gynecologic cancer (NCT02459301), colorectal
cancer (NCT02671435), recurrent or metastatic head
and neck cancer (NCT02643550) and chronic
lymphocytic leukemia (NCT02643550), also listed in
table1 (Table 1).
Table 1: Completed clinical trials related to monalizumab (IPH2201).
Target Drug
Pivotal
Indication
Trials
No.
Phase Most recent result
NKG2
A
Monalizumab
(IPH2201)
Gynecologic
cancer
NCT024
59301
2
Monolizumab (10 mg/kg i.v. every two weeks) is well
tolerated in individuals who have already been treated for
with gynecologic malignancies. There are mild related
adverse events and no dose-limiting cytotoxicity. Short-
term disease stabilization is observed.
PD-L1
NKG2
A
VEGF
EGFR
Durvalumab
Monalizumab
mFOLFOX6
Bevacizumab
Cetuximab
metastatic
microsatellite-
stable colorectal
cancer (MSS-
CRC)
NCT026
71435
1 and
2
In advanced/metastatic MSS-CRC, the combined therapy
had a manageable safety profile, with no dose-limiting
toxicity and DMCB showed promising preliminary
activity. Most of the patients treated give partial response
or have a chronic disease.
NKG2A: A Novel Immune Checkpoint Protein for Cancer Treatment
1079
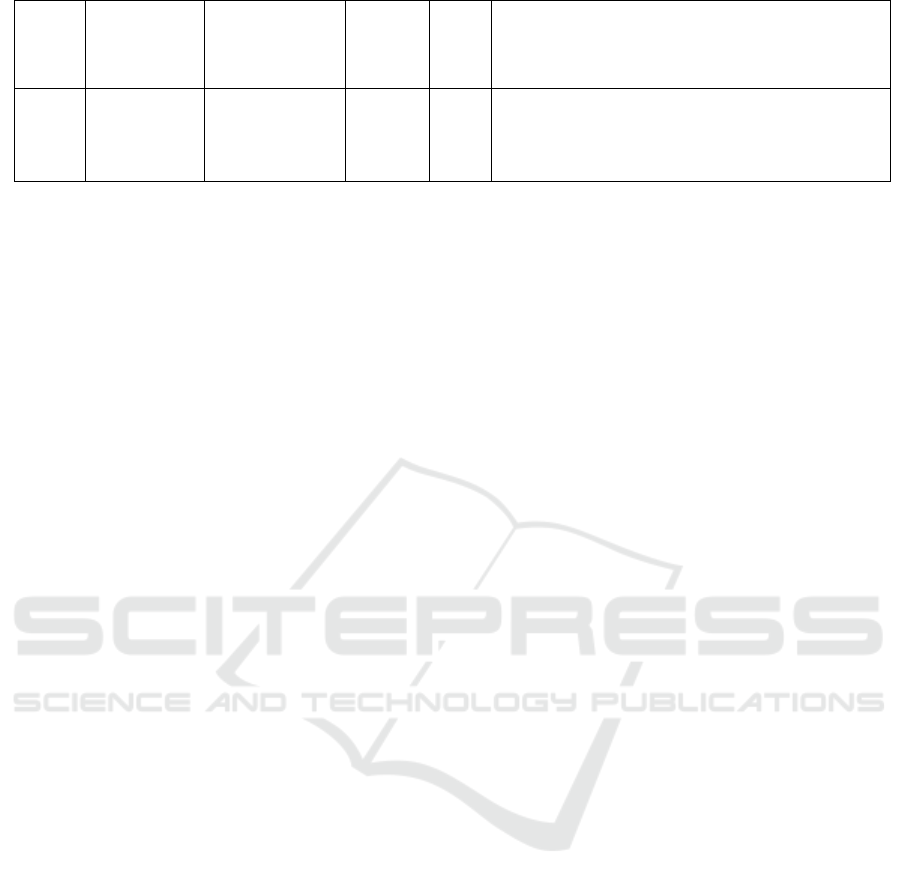
NKG2
A
EGFR
Monalizumab
Cetuximab
recurrent or
metastatic head
and neck cancer
NCT026
43550
1 and
2
the combination of monalizumab and cetuximab is safe
for the patients with recurrent or metastatic head and neck
cancer. Most of the patients respond partially or have a
stable disease.
NKG2
A
monalizumab
Ibrutinib
Chronic
Lymphocytic
Leukemia
NCT025
57516
1/2
The combination of monalizumab and ibrutinib shows
dose-limiting toxicity. Most patients show partial response
or have a stable disease. The most common adverse events
is Diarrhea. Due to its termination, the results have not
b
een completed.
The purpose of NCT02459301 is to explore and
determine the recommended phase II dose (RP2D) of
monalizumab which is analyzed as a separate agent for
treatment for patients with advanced, recurrent, or
metastatic gynecologic malignancies and its clinical
activity, pharmacokinetics, pharmacodynamics,
safety, and immunogenicity (Tinker, Hirte,
Provencher, et al, 2019). The data from this trial
indicates the recommended dose to be 10 mg/kg every
2 weeks (Tinker, Hirte, Provencher, et al, 2019).
Moreover, although monalizumab solely did not
induce treatment responses, it shows promising
activities: short-term stabilization, minimal drug
toxicities and high treatment efficacy (Tinker, Hirte,
Provencher, et al, 2019). The clinical trials of
NCT02671435 combining monalizumab with
durvalumab (MEDI4736, anti-PD-L-1 mAb) which
also investigates solid tumors demonstrates favorable
results again. The toxicity and tolerability of
monalizumab as a single agent and in combination
with durvalumab had been tested in a non-responder
cancer to single-agent anti-PD-1/PD-L-1 therapy
called metastatic microsatellite-stable colorectal
cancer (MSS-CRC), (Wainberg, Diamond,
Curigliano, et al, 2020). The updated data confirms
the excellent tolerance of this therapy without dose-
limiting toxicity and the majority of participants
display partial responses or have stable diseases
(Wainberg, Diamond, Curigliano, et al, 2020).
Another clinical trial related to solid tumor is the trial
NCT02643550, a multicenter single arm study to
evaluate the combination of monalizumab and
cetuximab(anti-EGFR) in patients with recurrent
and/or metastatic head and neck cancer (R/M
SCCHN) (Cohen, Bauman, Salas, et al, 2020). The
preliminary data revealed the acceptable safety of this
therapy and early, deep and durable responses in
participants (Cohen, Bauman, Salas, et al, 2020). The
therapy showed encouragement of progress free
survival (PFS) and overall survival (OS) in both 10
naïve and 10 pretreated patients, as well as higher
activity in platinum-resistant, HPV positive and
negative patients than cetuximab alone based on
historical data (Cohen, Bauman, Salas, et al, 2020).
Importantly, these information warrant the increased
attempt of combination of monalizumab and
cetuximab in the treatment for SCCHN.
Different from these clinical trials studying solid
tumors, phase I/II clinical trial (NCT02557516) which
analyses the combination of monalizumab with
irutinib, a Bruton’s tyrosine kinase inhibitor already
used in the treatment of the Chronic Lymphocytic
Leukemia (CLL) which is a hematologic malignancy,
aims at seeking for a long-run therapeutic benefit for
patients suffering from CLL (Innate Pharma, 2021).
The trial was designed to justify the assumption that
the coeffect of ibrutinib and monalizumab will give
rise to complete response (CR) rate, particularly CR
without minimal residual disease (MRD), since this
has been proved to be correlated with permanent
clinical benefit (Innate Pharma, 2021). Unfortunately,
it had been terminated due to different factors
including adverse events, disease progression,
physician decision and sponsor decision (Innate
Pharma, 2021). But it still left valuable data: most of
the treated patients have a reverse event of diarrhea
and show partial responses or have a stable disease.
Furthermore, the result revealed dose-limiting toxicity
(Innate Pharma, 2021).
Apart from the clinical trials above, there are
several ongoing trials for the investigation of
monalizumab which are still enrolling patients (Table
2). Different combinations with anti-EGFR, anti-PD-
L1, morpholino-pyrimidine-based inhibitor and
chemotherapy are tested in different cancer
indications, including patients with unresectable stage
III NSCLC (NCT03822351) and patients with PD-1
therapy-resistant NSCLC (NCT03833440), as well as
resectable early-stage (II-IIIA) NSCLC, or patients
with advanced squamous cell carcinoma of the head
and neck (NCT04590963, NCT03088059), and also a
trial tested monalizumab alone in patients with
Advanced or Metastatic Hematological or Solid
Malignancies (NCT04333914).
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
1080
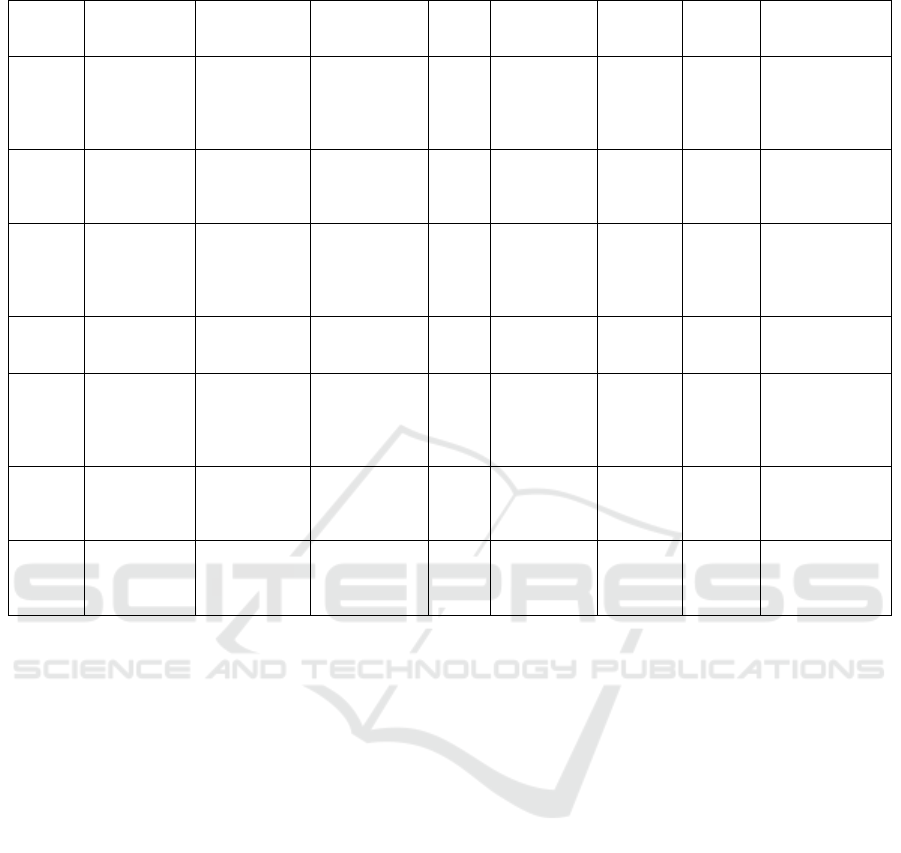
Table 2: The ongoing clinical trials related to monalizumab (IPH2201).
Target Drug Indication
Clinical trial
no.
Phase
Recruitment
status
First
posted
date
Last
update
posted
Estimated
Study
completion date
NKG2A
EGFR
Monalizumab
Cetuximab
Squamous
Cell
Carcinoma of
the Head and
Nec
k
NCT04590963 3 Recruiting
Oct.19
th
2020
Oct.29
th
2021
Mar.28
th
2024
PD-L1
CD73
NKG2A
Durvalumab
Oleclumab
Monalizumab
Stage III Non-
Small Cell
Lung Cancer
Unresectable
NCT03822351 2
Active, not
recruiting
Jan.30
th
2019
Aug.4
th
2021
Jul.11
th
2023
NKG2A Monalizumab
Advanced or
Metastatic
Hematological
or Solid
Tumo
r
NCT04333914 2
Active, not
recruiting
Apr.3
rd
2020
Aug.5
th
2021
Dec.2021
PD-L1
CD73
NKG2A
Durvalumab
Oleclumab
Monalizumab
Non-small
Cell Lung
Cance
r
NCT05061550 2
Not yet
recruiting
Sept.29
th
2021
Sept.29
th
2021
Feb.19
th
2025
PD-L1
NKG2A
CD73
Durvalumab
Monalizumab
Oleclumab
Ceralasertib
Docetaxel
Non-small
Cell Lung
Cancer
NCT03833440 2 Recruiting
Feb.7
th
2019
Apr.1
st
2021
Feb.2024
NKG2A
EGFR
PD-L1
PD-1
Monalizumab
Cetuximab
Anti-PD(L)1
Head and
Neck
Neoplasms
NCT02643550 1,2
Active, not
recruiting
Dec.31
st
2015
Feb.9
th
2021
Sept,2022
NKG2A
PD-L1
Monalizumab
Duvalumab
Carcinoma,
Squamous
Cell of Head
and Nec
k
NCT03088059 2 Recruiting
Mar.23
rd
2017
Nov.6
th
2019
Dec.2021
4 CONCLUSIONS
The main challenges of immunotherapy are to raise
the population of responding patients, as well as
overcoming tumor resistance (Borst L, Burg SH van
der, Hall T van. 2020). The NK cell and T cell
immunotherapy are fast-growing field with
remarkable contribution to cancer treatment. Here,
CD94/NKG2A, as a novel immune checkpoint protein
expressed on subsets of NK and T cells, has its
upregulation related to upregulation of its ligand,
HLA-E, and immune cell exhaustion. The abnormal
expression of both CD94/NKG2A and its ligand in
intratumor region is negatively correlated to cancer
prognosis, OS and DFS of patients, providing an idea
that blockade of the inhibitory receptor or its ligand
has the potential to unleash immunity against tumor
(Sun, Xu, Huang, et al, 2017, NK Cell Phenotypic
Modulation in Lung Cancer Environment, 2021,
Gooden, Lampen, Jordanova, et al, 2011, Levy,
Bianchini, Von Euw, et al, 2008, (Kren, Slaby,
Muckova, et al, 2011) Monalizumab is the humanized
NKG2A-targeting monoclonal antibody that is now
under investigation for its safety and efficacy. Data
from the results of clinical trials about various cancer
indication reveals its possibility and reliability as the
treatment for solid and hematologic malignancies,
especially in combination with other monoclonal
antibodies or cancer therapy.
ACKNOWLEDGMENTS
I thank my biological teacher, Xuan Zhang, for
provided me with some basic knowledge of cancer
treatment, and revised the manuscript.
REFERENCES
André P, Denis C, Soulas C, et al. Anti-NKG2A mAb Is a
Checkpoint Inhibitor that Promotes Anti-tumor
Immunity by Unleashing Both T and NK Cells. Cell.
2018;175(7):1731-1743.e13.
doi:10.1016/j.cell.2018.10.014.
Arlettaz L, Villard J, de Rham C, et al. Activating
CD94:NKG2C and inhibitory CD94:NKG2A receptors
NKG2A: A Novel Immune Checkpoint Protein for Cancer Treatment
1081

are expressed by distinct subsets of committed CD8+
TCR alphabeta lymphocytes. Eur J Immunol.
2004;34(12):3456-3464. doi:10.1002/eji.200425210.
Borrego F, Kabat J, Kim DK, et al. Structure and function
of major histocompatibility complex (MHC) class I
specific receptors expressed on human natural killer
(NK) cells. Molecular Immunology. 2002;38(9):637-
660. doi:10.1016/S0161-5890(01)00107-9.
Borrego F, Ulbrecht M, Weiss EH, Coligan JE, Brooks AG.
Recognition of human histocompatibility leukocyte
antigen (HLA)-E complexed with HLA class I signal
sequence-derived peptides by CD94/NKG2 confers
protection from natural killer cell-mediated lysis. J Exp
Med. 1998;187(5):813-818.
doi:10.1084/jem.187.5.813.
Borst L, Burg SH van der, Hall T van. The NKG2A–HLA-
E Axis as a Novel Checkpoint in the Tumor
Microenvironment. Clin Cancer Res.
2020;26(21):5549-5556.doi:10.1158/1078-0432.CCR-
19-2095.
Boyington JC, Riaz AN, Patamawenu A, Coligan JE,
Brooks AG, Sun PD. Structure of CD94 Reveals a
Novel C-Type Lectin Fold: Implications for the NK
Cell–Associated CD94/NKG2 Receptors. Immunity.
1999;10(1):75-82. doi:10.1016/S1074-7613(00)80008-
4.
Boyington JC, Riaz AN, Patamawenu A, Coligan JE,
Brooks AG, Sun PD. Structure of CD94 reveals a novel
C-type lectin fold: implications for the NK cell-
associated CD94/NKG2 receptors. Immunity.
1999;10(1):75-82. doi:10.1016/s1074-7613(00)80008-
4.
Braud V, Yvonne Jones E, McMichael A. The human major
histocompatibility complex class Ib molecule HLA-E
binds signal sequence-derived peptides with primary
anchor residues at positions 2 and 9. European Journal
of Immunology. 1997;27(5):1164-1169.
doi:10.1002/eji.1830270517.
Braud VM, Allan DS, O’Callaghan CA, et al. HLA-E binds
to natural killer cell receptors CD94/NKG2A, B and C.
Nature. 1998;391(6669):795-799. doi:10.1038/35869.
Burshtyn DN, Scharenberg AM, Wagtmann N, et al.
Recruitment of Tyrosine Phosphatase HCP by the
Killer Cell Inhibitory Receptor. Immunity.
1996;4(1):77-85. doi:10.1016/S1074-7613(00)80300-
3.
Cohen RB, Bauman JR, Salas S, et al. Combination of
monalizumab and cetuximab in recurrent or metastatic
head and neck cancer patients previously treated with
platinum-based chemotherapy and PD-(L)1 inhibitors.
JCO. 2020;38(15_suppl):6516-6516.
doi:10.1200/JCO.2020.38.15_suppl.6516.
Darvin P, Toor SM, Sasidharan Nair V, Elkord E. Immune
checkpoint inhibitors: recent progress and potential
biomarkers. Exp Mol Med. 2018;50(12):1-11.
doi:10.1038/s12276-018-0191-1.
Day AJ. The C-type carbohydrate recognition domain
(CRD) superfamily. Biochem Soc Trans.
1994;22(1):83-88. doi:10.1042/bst0220083.
Effects of anti-NKG2A antibody administration on
leukemia and normal hematopoietic cells. Accessed
November5, 2021.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5004
363/.
Feng Y, Roy A, Masson E, Chen TT, Humphrey R, Weber
JS. Exposure-response relationships of the efficacy and
safety of ipilimumab in patients with advanced
melanoma. Clin Cancer Res. 2013;19(14):3977-3986.
doi:10.1158/1078-0432.CCR-12-3243.
Galland S, Vuille J, Martin P, et al. Tumor-Derived
Mesenchymal Stem Cells Use Distinct Mechanisms to
Block the Activity of Natural Killer Cell Subsets. Cell
Rep. 2017;20(12):2891-2905.
doi:10.1016/j.celrep.2017.08.089.
Glienke J, Sobanov Y, Brostjan C, et al. The genomic
organization of NKG2C , E , F , and D receptor genes
in the human natural killer gene complex.
Immunogenetics. 1998;48(3):163-173.
doi:10.1007/s002510050420.
Gooden M, Lampen M, Jordanova ES, et al. HLA-E
expression by gynecological cancers restrains tumor-
infiltrating CD8+ T lymphocytes. Proc Natl Acad Sci U
S A. 2011;108(26):10656-10661.
doi:10.1073/pnas.1100354108.
Hamid MA, Wang RZ, Yao X, et al. Enriched HLA-E and
CD94/NKG2A Interaction Limits Antitumor CD8+
Tumor-Infiltrating T Lymphocyte Responses. Cancer
Immunol Res. 2019;7(8):1293-1306.
doi:10.1158/2326-6066.CIR-18-0885.
Innate Pharma. Open Label 1b/2a Trial of a Combination of
IPH2201 and Ibrutinib in Patients With Relapsed,
Refractory or Previously Untreated Chronic
Lymphocytic Leukemia. clinicaltrials.gov; 2019.
Accessed November 4,
2021.https://clinicaltrials.gov/ct2/show/NCT02557516
.
Kaiser BK, Barahmand-pour F, Paulsene W, Medley S,
Geraghty DE, Strong RK. Interactions between NKG2x
Immunoreceptors and HLA-E Ligands Display
Overlapping Affinities and Thermodynamics. J
Immunol. 2005;174(5):2878-2884.
doi:10.4049/jimmunol.174.5.2878.
Kren L, Slaby O, Muckova K, et al. Expression of immune-
modulatory molecules HLA-G and HLA-E by tumor
cells in glioblastomas: an unexpected prognostic
significance? Neuropathology. 2011;31(2):129-134.
doi:10.1111/j.1440-1789.2010.01149.x.
Lee N, Llano M, Carretero M, et al. HLA-E is a major
ligand for the natural killer inhibitory receptor
CD94/NKG2A. PNAS. 1998;95(9):5199-5204.
doi:10.1073/pnas.95.9.5199.
Levy EM, Bianchini M, Von Euw EM, et al. Human
leukocyte antigen-E protein is overexpressed in
primary human colorectal cancer. Int J Oncol.
2008;32(3):633-641.
Mamessier E, Sylvain A, Thibult ML, et al. Human breast
cancer cells enhance self tolerance by promoting
evasion from NK cell antitumor immunity. J Clin
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
1082

Invest. 2011;121(9):3609-3622.
doi:10.1172/JCI45816.
Miller JD, Weber DA, Ibegbu C, Pohl J, Altman JD, Jensen
PE. Analysis of HLA-E Peptide-Binding Specificity
and Contact Residues in Bound Peptide Required for
Recognition by CD94/NKG2. The Journal of
Immunology. 2003;171(3):1369-1375.
doi:10.4049/jimmunol.171.3.1369.
NK Cell Phenotypic Modulation in Lung Cancer
Environment. Accessed November 4, 2021.
https://journals.plos.org/plosone/article?id=10.1371/jo
urnal.pone.0109976.
Petrie EJ, Clements CS, Lin J, et al. CD94-NKG2A
recognition of human leukocyte antigen (HLA)-E
bound to an HLA class I leader sequence. Journal of
Experimental Medicine. 2008;205(3):725-735.
doi:10.1084/jem.20072525.
Plougastel B, Trowsdale J. Sequence Analysis of a 62-kb
Region Overlapping the HumanKLRCCluster of
Genes. Genomics. 1998;49(2):193-199.
doi:10.1006/geno.1997.5197.
Pluskey S, Wandless TJ, Walsh CT, Shoelson SE. Potent
Stimulation of SH-PTP2 Phosphatase Activity by
Simultaneous Occupancy of Both SH2 Domains.
Journal of Biological Chemistry. 1995;270(7):2897-
2900. doi:10.1074/jbc.270.7.2897.
Spinosa P, Musial‐Siwek M, Presler M, et al. Quantitative
modeling predicts competitive advantages of a next
generation anti‐NKG2A monoclonal antibody over
monalizumab for the treatment of cancer. CPT
Pharmacometrics Syst Pharmacol. 2021;10(3):220-
229. doi:10.1002/psp4.12592.
Sullivan LC, Clements CS, Beddoe T, et al. The
heterodimeric assembly of the CD94-NKG2 receptor
family and implications for human leukocyte antigen-E
recognition. Immunity. 2007;27(6):900-911.
doi:10.1016/j.immuni.2007.10.013.
Sun C, Xu J, Huang Q, et al. High NKG2A expression
contributes to NK cell exhaustion and predicts a poor
prognosis of patients with liver cancer.
Oncoimmunology. 2017;6(1):e1264562.
doi:10.1080/2162402X.2016.1264562.
Tinker AV, Hirte HW, Provencher D, et al. Dose-Ranging
and Cohort-Expansion Study of Monalizumab
(IPH2201) in Patients with Advanced Gynecologic
Malignancies: A Trial of the Canadian Cancer Trials
Group (CCTG): IND221. Clin Cancer Res.
2019;25(20):6052-6060. doi:10.1158/1078-0432.CCR-
19-0298.
van Hall T, André P, Horowitz A, et al. Monalizumab:
inhibiting the novel immune checkpoint NKG2A. j
immunotherapy cancer. 2019;7(1):263.
doi:10.1186/s40425-019-0761-3.
van Montfoort N, Borst L, Korrer MJ, et al. NKG2A
Blockade Potentiates CD8 T Cell Immunity Induced by
Cancer Vaccines. Cell. 2018;175(7):1744-1755.e15.
doi:10.1016/j.cell.2018.10.028.
Verma V, Sprave T, Haque W, et al. A systematic review
of the cost and cost-effectiveness studies of immune
checkpoint inhibitors. j immunotherapy cancer.
2018;6(1):128. doi:10.1186/s40425-018-0442-7.
Wainberg ZA, Diamond JR, Curigliano G, et al. First-line
durvalumab + monalizumab, mFOLFOX6, and
bevacizumab or cetuximab for metastatic
microsatellite-stable colorectal cancer (MSS-CRC).
JCO. 2020;38(4_suppl):128-128.
doi:10.1200/JCO.2020.38.4_suppl.128.
Walter L, Petersen B. Diversification of both KIR and
NKG2 natural killer cell receptor genes in macaques –
implications for highly complex MHC‐dependent
regulation of natural killer cells. Immunology.
2017;150(2):139-145. doi:10.1111/imm.12666.
Wei XH, Orr HT. Differential expression of HLA-E, HLA-
F, and HLA-G transcripts in human tissue. Hum
Immunol. 1990;29(2):131-142. doi:10.1016/0198-
8859(90)90076-2.
Zaghi E, Calvi M, Marcenaro E, Mavilio D, Di Vito C.
Targeting NKG2A to elucidate natural killer cell
ontogenesis and to develop novel immune-therapeutic
strategies in cancer therapy. Journal of Leukocyte
Biology. 2019;105(6):1243-1251.
doi:10.1002/JLB.MR0718-300R.
NKG2A: A Novel Immune Checkpoint Protein for Cancer Treatment
1083
