
Anticancer Effects and Mechanisms of Artemisinin and Its
Derivatives on Hematological Malignancies
Xinlei An
1,
†
a
, Qiao Huang
2,
†
b
and Yuheng Wu
3,
†
c1
1
College of Grassland Science, Xinjiang Agricultural University, Xinjiang, 830052, China
2
Department of Applied Biology and Chemical Technology, Hong Kong Polytechnic University, Hong Kong, 999077, China
3
College of Chemistry and Chemical Engineering, Huazhong University of Science and Technology, Wuhan, 430074, China
†
These authors contributted equally
Keywords:
Artemisinin, Dihydroartemisinin, Hematological Maglignancies.
Abstract:
Traditional Chinese medicine believes that artemisinin (ART) could treat malaria which are extracted from
artemisia. Modern medicine found that except the use of curing malaria, artemisinin and its derivatives also
show anticancer activities in vitro and in vivo by reducing the proliferation, migration, invasion,
tumorigenesis and metastasis of cancer cells. As the components of natural plants, artemisinin and its
derivatives demonstrated multi-specific manner in the treatment of hematological malignancies. The major
mechanisms of effects of artemisinin and its derivatives on anticancer activities include induction of apoptosis,
inhibition of angiogenesis, inhibition of proliferation, etc., through regulating multiple pathways, such as JNK,
KDR / Flk-1, MAPK, STAT3 and Wnt/β-catenin signalling pathways. This review discusses the anticancer
activity of artemisinin, artesunate and dihydroartemisinin (DHA) in the treatment of hematological
malignancies, and from which it is demonstrated that ART and its derivatives are effective in vitro and in vivo.
Future research is required in this promising field of cancer drug discovery.
1 INTRODUCTION
Hematological malignancies is the general name of a
large class of malignant tumors originated from
hematopoietic system, mainly including leukemia,
lymphoma and myeloma. It has become a severe
challenge to public health and public hygiene.
According to the statistics of common malignant
tumors in China, the acute leukemia and lymphoma
in hematological malignancies rank the top ten in the
"ten common malignant tumors", and the incidence
rate is increasing year by year. Multiple myeloma is
the second most common malignancy in the blood
system. The incidence rate has also increased in
recent years. Except the old way of treating cancer
such as chemotherapy, World Health Organization
publicated that artemisinin is one of the most efficient
drugs for the treatment of resistant malaria (World
Health Organization 1998). In recent years,
increasing amount of artemisinin's other functions
a
https://orcid.org/0000-0002-3172-9537
b
https://orcid.org/0000-0002-5043-8372
c
https://orcid.org/0000-0001-7415-043X
have been discovered and applied, such as treatment
potential on pulmonary hypertension, anti-diabetes
effects, anti-fungal, immune regulation, antiviral
effects (Kapepula 2020). Importantly, anti-cancer
effects of artemisinin within the scientific and
medical community are evidenced by the fact that the
Nobel Prize in Medicine and Physiology was
awarded in 2015 for the discovery of artemisinin in
China by the pharmaceutical chemist Tu Youyou (Su
2015). Hence, among these numerous effects,
anticancer effect of artemisinin and its derivatives
attracted attention of researchers with its properties of
well-tolerance by human body and no significant
side-effect.
As a derivant of artemisinin, artsunate also
presents potential anticancer activity. Several studies
showed varying degrees of inhibitory effect on liver
cancer cells, breast cancer cells as well as lung cancer
cells in vitro (Sun 2015, Dong 2014). This review
summarizes its potential anticancer mechanisms on
An, X., Huang, Q. and Wu, Y.
Anticancer Effects and Mechanisms of Artemisinin and Its Derivatives on Hematological Malignancies.
DOI: 10.5220/0011377300003443
In Proceedings of the 4th International Conference on Biomedical Engineering and Bioinformatics (ICBEB 2022), pages 1057-1064
ISBN: 978-989-758-595-1
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
1057
inducing tumor apoptosis and inhibiting
angiogenesis.
Dihydroartemisinin (DHA), which is another
derivative of artemisinin with better water solubility,
could be more easily absorbed by the human body
(Adam 2018). This review discusses the treatment
with hematological malignancies with DHA and the
potential mechanisms including its role of inhibiting
cancer cell proliferation (Zhang 2019), as well as
inducing cancer apoptosis (Yan 2018, Hu 2018).
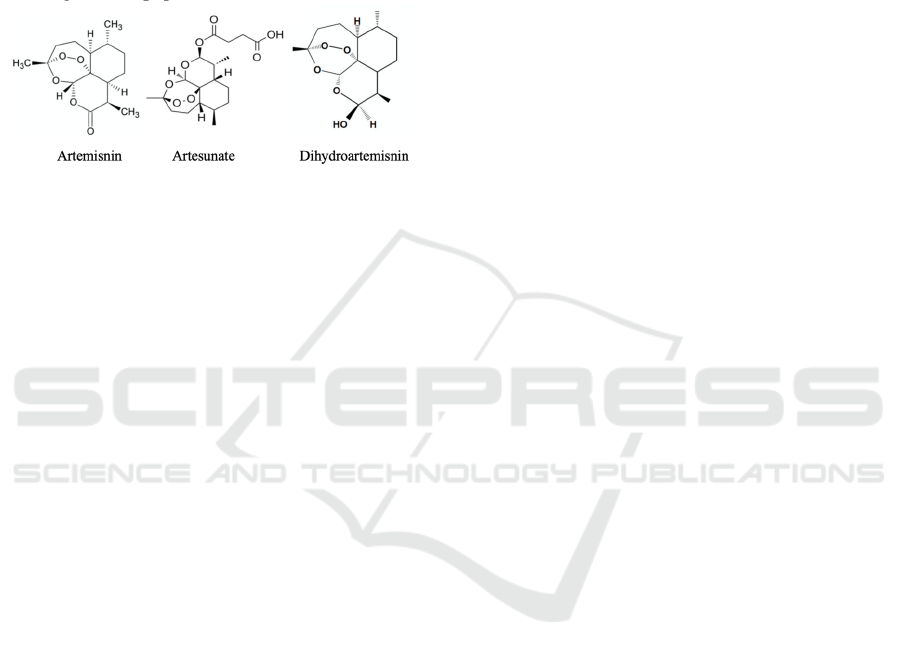
Figure 1: The chemical structure of artemisinin, artesunate
and dihydroartemisinin.
2 CONSTRUCTION AND
ANTICANCER EFFECT OF
ARTEMISININ
2.1
Description of Specimens
Artemisinin, with the formula of C
15
H
22
O
5
, is a
sesquiterpene (Fig1) (Kumar 2017). High-resolution
mass spectrometry (HRMS) showed a molecular ion
at m/z 282.1470 which is corresponding to the
formula C15H20 (Zheng 1994). The reaction with
triphenylphosphine to give the phosphine oxide
proved that there's a peroxide group in artemisinin
(Kumar 2017). However, research showed that there
existed some shortcomings such as short half-life and
poor solubility when artemisinin was applied to
cancer cells (Zhang 2020). Hence, efforts have been
made to synthesis hybridization of artemisinin as well
as other anticancer physicochemical for triggering a
solution toward these problems (Meunier 2008).
2.1.1 The Cytotoxicity Activity of
Artemisinin
Nine sesquiterpene compounds were tested for their
cytotoxicity toward several cancer cell lines, though
only artemisinin exhibited potent cytotoxicity toward
A-549 (human lung carcinoma), P-388 (murine
lymphocytic leukemia) and HT-29 (human colon
adenocarcinoma) cells with ED
50
values of 0.0962,
4.16, and 4.41μg/ml, respectively (Zheng 1994).
Apart from these cell lines, another group tested the
cytotoxicity of artemisinin to Ehrlich ascites cancer
cells by microculture tetrazolium (MTT) assay and
found a dramatic inhibition of cell proliferation with
IC
50
of 29.8μM (Woerdenbag 1993).
Hence, Artemisinin showed higher cytotoxicity
toward most of cancer cells, implying a potential
therapeutic way to treat cancers in clinical medicine.
However, importantly, artemisinin has stronger
cytotoxicity effects on normal cells than its
derivatives which may lead to the death of normal
tissues and consequently potential side effect, which
makes it urgent to find more derivatives with efficient
anticancer effects while less cytotoxicity on normal
cells.
2.1.2 Artemisinin Induces Apoptosis
It was found that B cell-specific Moloney murine
leukemia virus integration site 1 (BMI1) inhibitors
induced apoptosis with a fluorescence-activated cell
sorting (FACS) assay (Ohtaka 2017). Artemisinin, as
one of the BMI1 inhibitors, was found potential
therapeutic effects on six acute myeloid leukaemia
and two normal lymphoblastic cell lines (Hu 2018).
It’s showed in the Dose-response curves that ART
suppressed multiplication of Jurkat cells, which
provided another evidence that artemisinin inhibits
some certain cancer cells proliferation (Ohtaka
2017).
Besides the inhibition function, ART also didn’t
weaken the multiplication of normal cells within the
concentration range used (Ohtaka 2017). In this case,
a potential advantage of using artemisinin as a
treatment toward leukemia is that it may minimize
leukemia cell proliferation while seldom infect the
normal lymphocyte. Studies on patients with
artemisinin also showed well-tolerance and did not
show severe side effects based on a large number of
clinical trials of artemisinin (Efferth 2010).
Artemisinin decreased the expression of BMI1,
NOTCH1, and in Jurkat cells it nullified NOTCH1
and the downstream targets of NOTCH, indicating
that a potential mechanism of artemisinin inducing
apoptosis is to down-regulate the expression of BMI1
and NOTCH1 (Ohtaka 2017). BMI inhibitors might
be used as drugs targeting leukemia stem cells owing
to its ability to regulate cell stemness (Ohtaka 2017).
However, there should be more experiments to clarify
molecular pathways targeted by BMI inhibitors, as
well as test their effects for normal hematopoietic
stem cells.
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
1058

2.2 Artesunate
Artesunate is a water-soluble esterification derivative
of artemisinin. The anticancer mechanisms of
artesunate involve induction of reactive oxygen
species (ROS) production and ROS dependent DNA
damage, inhibition of angiogenesis, induction of
apoptosis and cell cycle stagnation (Zhang 2015).
2.2.1 Pro-apoptosis Effect
It was found that ART may induce cell proliferation
and programmed cell death in human leukemia cells,
which have been proven in in vitro tests on cell line
K562 (Sun 2015). The effects of different
concentrations of Artesunate on the viability of K562
cells decreased (98.9 ± 2.3 % to 37.9 ± 6.2 %) with
the increase of drug concentration (0 to 400 μmol/L)
as a dose-dependent manner (Sun 2015). Specifically,
Artesunate played an pro-apoptosis effect of K562
cells, as the study showed that after 24 hours of
treatment with 100μmol/L Artesunate, there were
increasing number of cells in early apoptosis (2.48 ±
0.9%), late apoptosis (9.96 ± 1.5)% and necrotic cells
(3.25 ± 0.5)% compared with the control group (1.46
± 0.7)%, (2.79 ± 0.6)%, and (1.68 ± 0.4)%,
respectively (Sun 2015). Hence, the Bcl-2 family
members play an important role in regulating
apoptosis in intrinsic pathway, which can be divided
into anti-apoptosis including Bcl-2 and Bcl XL, as
well as pro-apoptosis including Bid, Bax, Bad, and
Bim. Bcl-2 may suppress the apoptosis-inducing
signals while Bax may antagonize Bcl-2 by
promoting apoptosis. Furthermore, the ratio of these
two molecules determines the survival or apoptosis of
cells. Artesunate was displayed the dual role for the
anti-apoptotic Bcl-2 protein in mitochondria and
endoplasmic reticulum of cancer cells (Alk 2014).
Studies showed that after 24 hours with 100μmol/L
of Artesunate treatment, it exerted a positive effect on
the expression of Bcl-2 while did not change the
expression of Bcl-2 significantly (Alk 2014),
indicating that the pro-apoptosis effect of Artesunate
may be related to the regulation of Bcl-2 family and
potentially the intrinsic pathway.
The cell cycle of K562 cell lines was also affected
under Artesunate treatment, with a decreasing cell
population in the S phase while increasing the cell
population in the G2M phase in a dose-dependent
manner. After the treatment with a higher
concentration of Artesunate (200μmol/L), the
percentage of apoptosis was up to 39.65%, and the
cells in the S and G2M phase were reduced (Sun
2015). Moreover, with an increase of drug
concentration, cell proliferation was significantly
inhibited in a dose-dependent manner. Applying
12.5, 25, 50, 100, 200 and 400 μmol/L ART inhibits
rates of cell proliferation by (6.90 ± 3.3)%, (17.8 ±
5.5)%, (38.4 ± 5.8)%, (52.8 ± 6.1)%, (64.0 ± 5.8)%,
(69.9 ± 7.2)%, respectively, and the 50%
proliferation inhibition concentration (IC
50
) was
about 95.0 μmol/L (Sun 2015).
In summary, Artesunate could regulate cell cycle
change, inhibit cell proliferation, and induce
apoptosis through the intrinsic pathway, which may
be related to the down-regulating expression level of
Bcl-2 and the up-regulating expression level of Bax
(Solaini 2011). The in vitro methods that usually used
in related studies include cell culture, cell vitality
detection, apoptosis detection, cell proliferation
detection, cell cycle analysis, etc. (Sun 2015), while
further in vivo studies as well as potential clinical
research are required to further understanding the
influence of Artesunate on cell proliferation process.
2.2.2 Anti-angiogenesis Effects
Angiogenesis is one of the critical process to
migration, division, and differentiation of vascular
endothelial cells or stromal stem cells, followed by
the formation of lumen structure, and consequently
malignant growth and metastasis of tumors (Zhang
2007). Various research showed an inhibition effect
of Artesunate on angiogenesis. For instance, at the
concentration range of 0.5-50 μmol/L, Artesunate
significantly supressed angiogenesis in a
concentration-dependent manner (Li 2013).
However, the mechanisms of Artesunate induced
anti-angiogenesis effects have not been fully
recognized.
Tumor cells and endothelial cells may interact
with each other to regulate tumor angiogenesis.
During this process, the vascular endothelial growth
factor (VEGF) is one of the most important major
ligand for angiogenesis leading to malformation and
dysfunctional vascular system, activates two tyrosine
kinase receptors, VEGFR-1 (Flt-1) and VEGFR-2
(KDR / Flk-1). After the activation of these two
receptors, the sign for angiogenesis will be enabled
(Shibuya 2011). Studies have found that Artesunate
may play a role in mitigating the activation of KDR /
Flk-1 and consequently affects the process of
angiogenesis with limited production of pro-
angiogenic cytokines from tumor cells (Wei 2017). It
was found that Artesunate significantly inhibited the
proliferation, migration, and subsequent tube
formation of human umbilical vein endothelial cells
(HUVEC), with a significantly decreased expression
Anticancer Effects and Mechanisms of Artemisinin and Its Derivatives on Hematological Malignancies
1059
of Flt-1 and KDR / Flk-1 in endothelial cells.
Moreover, in embryonic samples derived from mouse
embryonic stem cells, Artesunate down-regulated
HUVEC Bcl-2 while up-regulated Bax levels, caused
changes in the proportion of Bcl-2 and Bax, and
significantly induced HUVEC apoptosis (Wu 2004).
Importantly, the mRNA expression of more than 6
angiogenic genes is related to the sensitivity and drug
resistance of tumor cells to eight artemisinin
derivatives (Anfosso 2006), which provided a clue as
to potential clinical precise therapy.
Taken together, the anti-angiogenesis
mechanisms of Artesunate may be related to
disarranging pathways including JNK, p38 MAPK,
KDR / Flk-1 and Akt, etc. (Table 1). Subsequently,
Artesunate could inhibit endothelial cell
proliferation, induce endothelial cell apoptosis, and
play an anti-tumor angiogenesis role by inhibiting
VEGF expression, downregulating Flt-1 and KDR /
Flk-1 expression levels, downregulating Bcl-2, as
well as upregulating Bax levels, and in turn
suppression the vessel formation.
Table 1. Mechanisms underlying the anti-angiogenesis effects of Artesunate (modified from Ref. (Wei 2017)).
Cell types Mechanisms Artesunate effect Ref(s)
HUVECs JNK activation ↓
p38 MAPK activation ↑
KDR / Flk-1 activation ↓
Proliferation ↓
Apoptosis ↑
Angiogenesis ↓
(Cheng 2013)
(Cheng 2013)
(Wu 2004)
RAFLS Akt phosphorylation ↓
Akt phosphorylation ↓
Production of VEGF
and IL-8↓ IL-8
production↓
(Uckun 2021)
(Xu 2007)
2.3
Dihydroartemisinin
Dihydroartemisinin (DHA) is semi-synthesized from
artemisinin which is modified to retain the
antimalarial active group. Thus, DHA contains the
hydroxyl group, which greatly improves its
antimalarial effect. As shown in Fig.1, its anticancer
effect mainly depends on its unique peroxide bridge
structure. The DHA is more water-soluble than
artemisinin and is easier to be absorbed by the human
body. Hence, it has been showing the great
advantages of a faster metabolism rate, more efficient
effect, and lower toxicity (Adam 2018).
The anticancer activity of DHA in the treatment
of hematologic malignancies was discussed from
three aspects: inhibition of cancer cell proliferation
and DHA induction of cancer cell death.
2.3.1 DHA Inhibits Cancer Cell
Proliferation
DHA can inhibit the proliferation of leukemia cell
K562 by inhibiting aerobic glycolysis mediated by
Pyruvate kinase M2 (PKM2) and glucose transporter
1 (GLUT1). Gao et al. illustrated the inhibitory effect
of DHA on the proliferation of human chronic
myeloid leukemia cells by presenting an example of
the inhibitory effect of DHA on human chronic
myeloid leukemia K562 cells (Gao 2020).
The rapid growth of cancer cells requires a
dramatic increase in glucose and glucose metabolites.
Normal cells obtain energy mainly through oxidative
phosphorylation of mitochondria, while most cancer
cells rely on aerobic glycolysis. Glycolysis produces
large amounts of lactic acid and induces metabolic
waste. Lactic acid, in turn, promotes the development
of cancers (Zhang 2019). Thus, the specific
dependence of cancer cells on glycolysis makes them
susceptible to specific glycolysis target inhibitors.
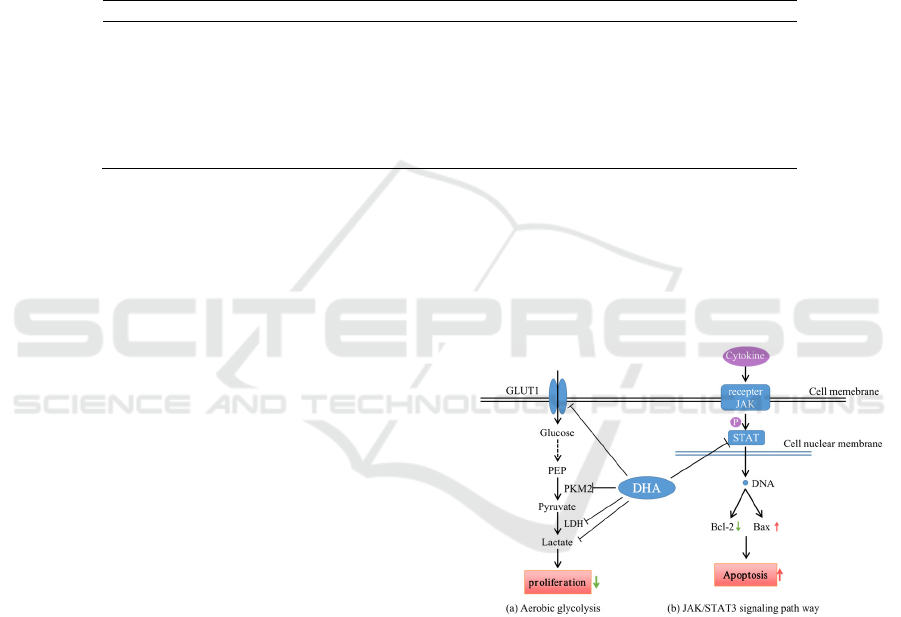
Figure 2: mechanisms of DHA induced proliferation
inhibition and apoptosis.
Overexpression of glucose transporters (GLUTs)
can be usually observed in cancer cells to regulate the
"Warburg effect" in cancer cells. It was found that
DHA can gradually inhibit the expression of GLUT1
at protein levels (Gao 2020). These results indicated
that DHA could inhibit lactate secretion, block
glucose uptake and inhibit GLUT1 expression in
K562 cells through inhibition. At the same time,
DHA may also inhibit PKM2, which is important for
phosphoenolpyruvate (PEP) to generate pyruvate.
Therefore, DHA may regulate the metabolism of
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
1060

cancer cells by inhibiting the expression of GLUT1
and PKM2, thus inhibiting the proliferation of cancer
cells as shown in Fig.2a (modified from Ref. (Gao
2020)).
2.3.2 DHA Induced Cancer Apoptosis
The increase of abnormal cell response is mainly
through increasing the transmembrane response of
GLUT1. Studies have shown that the low expression
level of cells GLUT1 is related to abnormal
conditions. GLUT1 is the most widely distributed
glucose transporter known, and is highly expressed in
brain, blood-brain barrier, myocardium, adipose
tissue and skeletal muscle, which are adapted to the
glucose needs of the body's microenvironment (Cai
2004, Takata 1990). It has been showing that GLUT1
played an important role in cancer progression and
abnormal expression of GLUT1 has been found in
many cancers. Studies have found that GLUT was
overexpressed in juvenile hemangioma, and an
abnormally elevated expression of GLUT1 was found
in multiple cancers such as pancreatic cancer, gastric
cancer, ovarian cancer, cervical cancer, lung cancer,
and nasopharyngeal cancer (Drut 2004). Thus,
GLUT1 could be regarded as a potential biomarker of
cancer early diagnosis, differentiation of benign and
malignant, as well as prognostic evaluation.
To better understand the anti-cancer effects of
DHA, it has been found that its mechanism of
inducing cancer cell apoptosis is related to multiple
signal pathways, such as PI3K/Akt, MAPK, STAT3,
Wnt/β-catenin, NF-κB and other signal pathways
(Table 2).
Table 2: DHA targets in cancer cell signaling.
Reported target Function/pathway of
tar
g
et
DHA
Effect
Ref(s)
PI3K/Akt Activates downstream
target of rapamycin,
mTOR
Inhibits proliferation;
Promotes apoptosis;
Abnormal invasion
(Li 2017)
(Tang 2014)
MAPK Decreases DNA repair
enzyme (PARP)
expression
Down-regulates mRNA
and protein expression
Induces caspase-
dependent apoptosis
Inhibits proliferation
Promotes apoptosis
(Dong 2015)
(Zhang
2017)
STAT3 Regulates of transcription
of target genes
Activates Bax and leads to
programmed cell death
Inhibits proliferation
Promotes apoptosis
(Hu 2018)
Wnt/β-catenin Reduces the adhesion
between cells
Promotes the interstitial
transformation of cells
Promotes apoptosis (Qiao 2016)
Notch Down-regulatesof mRNA
expression in cells
Promotes apoptosis (Liu 2014)
NF-κB Leads to the accumulation
of ROS
Promotes apoptosis (Hu 2014)
Anticancer Effects and Mechanisms of Artemisinin and Its Derivatives on Hematological Malignancies
1061

JAK-STAT signaling pathway is one of the
critical pathways during tumorigenesis by which
DHA induces apoptosis. JAK/STAT signaling
pathway is a widely expressed intracellular signal
transduction pathway stimulated by a variety of
cytokines, which is mainly involved in many
important biological processes such as cell
proliferation, differentiation, and apoptosis.
Lymphocyte adaptor protein (LNK) gene was found
to play an important role in regulating hematopoietic
stem regeneration and proliferation. The protein
encoded by the LNK gene is lymphocyte linker
(SH2B3), which belongs to the SH2B connexin
family and is a key factor in normal hematopoietic.
LNK was highly expressed in hematologic cancer
cells. It was found that LNK mutation could cause
mutations in corresponding domains such as SH2
or/and PH, which may weaken or lose the inhibitory
function of activated JAK receptor and its
downstream genes, and in turn leads to the high
expression of STAT3, as a consequence causing
abnormal proliferation of hematopoietic cells and
accelerating the occurrence and development of
hematologic cancer cells (Vainchenker 2011).
Importantly, studies have showing that expression
level of LNK protein increased after DHA
application, and LNK protein inhibited STAT3
protein expression, so DHA further inhibited STAT3
protein expression. Furthermore, the Bcl-2 protein
level was decreased while Bax protein level was
increased, which promoted the apoptosis of AML
cells (Fig2a) (Yan 2018, Hu 2018).
The effect of DHA on laryngeal cancer was
investigated and it was demonstrated that the
treatment of DHA can prolong the survival time of
mice and inhibit the activation of STAT3 in cancer
cells. These results indicated that DHA inhibits the
invasion and metastasis induced by cancer STEM
cells by inhibiting the activation of STAT3 in
laryngeal cancer (Wang 2020). It was found that
DHA inhibited melanoma proliferation in a time- and
dose-dependent manner by studying the effect of
DHA on melanoma (Yu 2020). Moreover, DHA
significantly promoted mitochondrial apoptosis in
melanoma by regulating the STAT3 pathway.
Researchers studied the antitumor activity of DHA in
head and neck squamous cell carcinoma and found
that DHA showed significant specific inhibitory
effect on STAT3 activation through selective
blocking of Jak2/STAT3 signaling pathway (Jia
2016). In addition, DHA could also inhibit the growth
of squamous cell carcinoma of the head and neck in
vitro and in vivo, possibly by inducing apoptosis and
inhibiting cell migration (Jia 2016).
In summary, DHA may be a STAT3 inhibitor and
may represent a new effective drug for the treatment
of cancer and for the treatment of sensitization in
cancer patients.
3 CONCLUSION
Artemisinin and its derivatives, including artemisinin,
DHA, and artesunate, have been showing remarkable
anticancer effects on hematological malignancies.
Artemisinin and its derivatives may regulate multiple
pathways, such as JNK, KDR / Flk-1, MAPK,
STAT3 and Wnt/β-catenin. A better understanding of
the common mechanisms under similar conditions in
different cell systems would greatly contribute to the
development of targeted artemisinin derivativesas
well as improving the cytotoxicity of artemisinin by
reducing IC
50
, emergence of drug resistance, drug-
related toxicity and enhancing drug interaction. At
present, there have been a large number of studies
applying artemisinin and its derivatives to the
treatment of various types of cancers. This article
reviews the latest advances in the research of
artemisinin and its derivatives in hematological
malignancies. Various studies have shown that it can
play a role through a variety of mechanisms, such as
inducing cell cycle arrest, inducing autophagy and
apoptosis. In addition, artemisinin and its derivatives
also show anti-cancer effects in many drug-resistant
hematological malignancies, and have a synergistic
effect with other drugs. Nevertheless, the potential
drug reaction, drug interaction, drug resistance, as
well as the side effects toward normal cells remains a
concern. An increasing number of studies have been
focusing on determining the biological activation
mechanism and molecular events behind the
artemisinin effect. However, how artemisinin exerts
its antitumor activity after activation remains unclear.
Besides, future investigation may be required to
futher understand the effects of artemisinin to reveal
potential of artemisinin as a clinical drug on not only
malaria, but also hematological malignancies.
REFERENCES
Adam, I., Ibrahim, Y., Gasim, G.I. (2018) Efficacy and
Safety of Artemisinin-based Combination Therapy for
Uncomplicated Plasmodium Falciparum Malaria in
Sudan: a Systematic Review and Meta-Analysis. Malar
J, 17:110.
Akl, H., Vervloessem, T., Kiviluoto, S., Bittremieux, M.,
Parys, J.B., De Smedt, H., Bultynck, G. (2014) a Dual
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
1062

Role for the Anti-Apoptotic Bcl-2 Protein in Cancer:
Mitochondria Versus Endoplasmic Reticulum. Biochim
Biophys Acta, 1843:2240-52.
Anfosso, L., Efferth, T., Albini, a., Pfeffer, U. (2006)
Microarray Expression Profiles of Angiogenesis-
Related Genes Predict Tumor Cell Response To
Artemisinins. Pharmacogenomics J, 6:269-78.
Cai, Y., Shao, S.L. (2004) Research Progress of GLUT1.
Journal of International Oncology, (S1):50-52..
Cheng, R., Li, C., Li, C., Wei, L., Li, L., Zhang, Y., Yao, Y.,
Gu, X., Cai, W., Yang, Z., Ma, J., Et Al. (2013) the
Artemisinin Derivative Artesunate Inhibits Corneal
Neovascularization by Inducing ROS-Dependent
Apoptosis in Vascular Endothelial Cells. Invest
Ophthalmol Vis Sci, 54:3400-9.
Dong, F., Tian, H., Yan, S., Li, L., Dong, X., Wang, F., Li,
J., Li, C., Cao, Z., Liu, X., Liu, J. (2015)
Dihydroartemisinin Inhibits Endothelial Cell
Proliferation through the Suppression of the ERK
Signaling Pathway. Int J Mol Med, 35:1381-7.
Dong, F., Zhou, X., Li, C., Yan, S., Deng, X., Cao, Z., Li,
L., Tang, B., Allen, T.D., Liu, J. (2014)
Dihydroartemisinin Targets VEGFR2 Via the NF-
Kappab Pathway in Endothelial Cells To Inhibit
Angiogenesis. Cancer Biol Ther, 15:1479-88.
Drut, R.M., Drut, R. (2004) Extracutaneous Infantile
Haemangioma Is Also Glut1 Positive. J Clin Pathol,
57:1197-200.
Efferth, T., Kaina, B. (2010) Toxicity of the Antimalarial
Artemisinin and Its Dervatives. Critical Reviews in
Toxicology, 40:405.
Gao, P., Shen, S., Li, X., Liu, D., Meng, Y., Liu, Y., Zhu, Y.,
Zhang, J., Luo, P., Gu, L. (2020) Dihydroartemisinin
Inhibits the Proliferation of Leukemia Cells K562 by
Suppressing PKM2 and GLUT1 Mediated Aerobic
Glycolysis. Drug Des Devel Ther, 14:2091-2100.
Hu, W., Chen, S.S., Zhang, J.L., Lou, X.E., Zhou, H.J.
(2014) Dihydroartemisinin Induces Autophagy by
Suppressing NF-Kappab Activation. Cancer Lett,
343:239-48.
Hu, Y.J., Zhang, J.Y., Luo, Q., Xu, J.R., Yan, Y., Mu, L.M.,
Bai, J., Lu, W.L. (2018) Nanostructured
Dihydroartemisinin plus Epirubicin Liposomes
Enhance Treatment Efficacy of Breast Cancer by
Inducing Autophagy and Apoptosis. Nanomaterials
(Basel), 8.
Jia, L., Song, Q., Zhou, C., Li, X., Pi, L., Ma, X., Li, H., Lu,
X., Shen, Y. (2016) Dihydroartemisinin as a Putative
STAT3 Inhibitor, Suppresses the Growth of Head and
Neck Squamous Cell Carcinoma by Targeting
Jak2/STAT3 Signaling. Plos One, 11:E0147157.
Kapepula, P.M., Kabengele, J.K., Kingombe, M.,
Bambeke, F.V., Nachega, J.B. (2020) Artemisia Spp.
Derivatives for COVID-19 Treatment: Anecdotal Use,
Political Hype, Treatment Potential, Challenges, and
Road Map To Randomized Clinical Trials. the
American Journal of Tropical Medicine and Hygiene,
103.
Kumar, B., Kalvala, a., Chu, S., Rosen, S., Forman, S.J.,
Marcucci, G., Chen, C.C., Pullarkat, V. (2017)
Antileukemic Activity and Cellular Effects of the
Antimalarial Agent Artesunate in Acute Myeloid
Leukemia. Leukemia Research, 59.
Li, Q., Weina, P., Hickma, M. (2013) the Use of Artemisinin
Compounds as Angiogenesis Inhibitors To Treat
Cancer. Research Directions in Tumor
Angiogenesis:175-259.
Li, X., Ba, Q., Liu, Y., Yue, Q., Chen, P., Li, J., Zhang, H.,
Ying, H., Ding, Q., Song, H., Liu, H., Et Al. (2017)
Dihydroartemisinin Selectively Inhibits Pdgfralpha-
Positive Ovarian Cancer Growth and Metastasis
through Inducing Degradation of Pdgfralpha Protein.
Cell Discov, 3:17042.
Liu, Z.X., Wang, D., Ding, J., Tan, B.Z. (2014) Inhibition
of Notch1 Expression by Dihydroartemisinin on
Human Ovarian Cancer Skov3 Cells. Acta Medicinae
Universitatis Scientiae Et Technologiae Huazhong,
43(02):215-218.
Meunier, B. (2008) Hybrid Molecules With a Dual Mode of
Action: Dream or Reality? Accounts of Chemical
Research. 41(1), 69–77.
Ohtaka, M., Mai, I., Toh-Da, S. (2017) BMI1 Inhibitors
down-Regulate NOTCH Signaling and Suppress
Proliferation of Acute Leukemia Cells. Anticancer
Research, 37:6047.
Qiao, D., Song J.H. (2016) Recent Advances in the Study
of Activation of Wnt/Β-Catenin Signaling Pathway and
Cervical Lesions. Journal of International Reproductive
Health/Family Planning, 35(06):510-514.
Shibuya, M. (2011) Involvement of Flt-1 (VEGF Receptor-
1) in Cancer and Preeclampsia. Proc Jpn Acad Ser B
Phys Biol Sci, 87:167-78.
Solaini, G., Sgarbi, G., Baracca, a. (2011) Oxidative
Phosphorylation in Cancer Cells. Biochim Biophys
Acta, 1807:534-42.
Su, X.Z., Miller, L.H., Li, J. (2015) Discovery of
Artemisinin and Nobel Prize in Physiology or Medicine.
Scientia Sinica(Vitae), 45:1148-1152.
Sun Y.M., Zhao, L.Z., Li, Q. (2015) the Effects of Cell
Proliferation and Apoptosis Induced by Artesunate on
Leukemia Cell. Academy of Laboratory, Jilin Medical
University:1005-9202.
Sun, Y.M., Zhao, L.Z., Li, Q., Et Al. (2015) the Effects of
Cell Proliferation and Apoptosis Induced by Artesunate
on Leukemia Cell. Academy of Laboratory, Jilin
Medical University:1005-9202.
Takata, K., Kasahara, T., Kasahara, M., Ezaki, O., Hirano,
H. (1990) Erythrocyte/Hepg2-Type Glucose
Transporter Is Concentrated in Cells of Blood-Tissue
Barriers. Biochemical and Biophysical Research
Communications, 173.
Tang, L., Luo, Z.G., Zhu, Q.M., Zou, W. Q. (2014) Study
on the Regulation Mechanism of Dihydroartemisinin
on UCHL1 Gene Expression in PC-3 Cells of Prostate
Cancer. Tumor, 34(12):1082-1089.
Uckun, F.M., Saund, S., Windlass, H., Trieu, V. (2021)
Repurposing Anti-Malaria Phytomedicine Artemisinin
as a COVID-19 Drug. Front Pharmacol, 12:649532.
Anticancer Effects and Mechanisms of Artemisinin and Its Derivatives on Hematological Malignancies
1063

Vainchenker, W., Delhommeau, F., Constantinescu, S.N.,
Bernard, O.a. (2011) New Mutations and Pathogenesis
of Myeloproliferative Neoplasms. Blood, 118:1723-35.
Wang, W., Sun, Y., Li, X., Shi, X., Li, Z., Lu, X. (2020)
Dihydroartemisinin Prevents Distant Metastasis of
Laryngeal Carcinoma by Inactivating STAT3 in Cancer
Stem Cells. Med Sci Monit, 26:E922348.
Wei, T., Liu, J. (2017) Anti-Angiogenic Properties of
Artemisinin Derivatives (Review). Int J Mol Med,
40:972-978.
Woerdenbag, H.J., Moskal, T.a., Pras, N., Malingré, T., El-
Feraly, F.S., Kampinga, H.H., Konings, a. (1993)
Cytotoxicity of Artemisinin-Related Endoperoxides To
Ehrlich Ascites Tumor Cells. Journal of Natural
Products, 56:849.
World Health Organization. (1998) the Use of Artemisinin
and Its Derivatives as Anti-Malarial Drugs (Archived).
Geneva: World Health Organization.
Wu, G.D., Zhou, H.J., Wu, X.H. (2004) Apoptosis of
Human Umbilical Vein Endothelial Cells Induced by
Artesunate. Vascul Pharmacol, 41:205-12.
Xu, H., He, Y., Yang, X., Liang, L., Zhan, Z., Ye, Y., Yang,
X., Lian, F., Sun, L. (2007) Anti-Malarial Agent
Artesunate Inhibits TNF-Alpha-Induced Production of
Proinflammatory Cytokines Via Inhibition of NF-
Kappab and PI3 Kinase/Akt Signal Pathway in Human
Rheumatoid Arthritis Fibroblast-like Synoviocytes.
Rheumatology (Oxford), 46:920-6.
Yan, X., Li, P., Zhan, Y., Qi, M., Liu, J., an, Z., Yang, W.,
Xiao, H., Wu, H., Qi, Y., Shao, H. (2018)
Dihydroartemisinin Suppresses STAT3 Signaling and
Mcl-1 and Survivin Expression To Potentiate ABT-263-
Induced Apoptosis in Non-Small Cell Lung Cancer
Cells Harboring EGFR or RAS Mutation. Biochem
Pharmacol, 150:72-85.
Yu, R., Jin, L., Li, F., Fujimoto, M., Wei, Q., Lin, Z., Ren,
X., Jin, Q., Li, H., Meng, F., Jin, G. (2020)
Dihydroartemisinin Inhibits Melanoma by Regulating
CTL/Treg Anti-Tumor Immunity and STAT3-Mediated
Apoptosis Via IL-10 Dependent Manner. J Dermatol
Sci, 99:193-202.
Zhang, B. (2020) Artemisinin‐Derived Dimers as Potential
Anticancer Agents: Current Developments, Action
Mechanisms, and Structure–Activity Relationships.
Archiv Der Pharmazie, 353:-.
Zhang, D., Tang, Z., Huang, H., Zhou, G., Cui, C., Weng,
Y., Liu, W., Kim, S., Lee, S., Perez-Neut, M., Ding, J.,
Et Al. (2019) Metabolic Regulation of Gene Expression
by Histone Lactylation. Nature, 574:575-580.
Zhang, D., Wang, M.Y., Yang, L. (2015) Progress in New
Formulation Studies of Artemisinins. Chinese
Pharmaceutical Journal, 50:189-193.
Zhang, S., Shi, L., Ma, H., Li, H., Li, Y., Lu, Y., Wang, Q.,
Li, W. (2017) Dihydroartemisinin Induces Apoptosis in
Human Gastric Cancer Cell Line BGC-823 through
Activation of JNK1/2 and P38 MAPK Signaling
Pathways. J Recept Signal Transduct Res, 37:174-180.
Zhang, X.P., Zhang, C.C. (2007) Research Progress on the
Mechanisms of Artemisinin and Its Derivatives'
Anticancer Activity. Scientific Research Dept. of
College of Medical Science, Three Gorges University,
Yichang 443002, Hubei, China, 9(5).
Zheng, G.Q. (1994) Cytotoxic Terpenoids and Flavonoids
from Artemisia Annua. Planta Med, 60.
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
1064
