
Pan-genomic Analysis of Bradyrhizobium japonicum
Ruihan Ma
a
Northeast Agriculture University, Heilongjiang, 15000, China
Keywords: Bradyrhizobium, Biosphere, Genus.
Abstract: The rhizobium-legume symbiosis is a major source of fixed nitrogen (ammonia) in the biosphere—the
potential for this process to increase agricultural yield while reducing the reliance on nitrogen-based
fertilizers. Bradyrhizobium is an ancient type of soybean nitrogen-fixing symbiotic bacteria. The
Bradyrhizobium japonicum under the bacterial genus classification is widely used as a promotional species
in actual agricultural production. However, the use of rhizobium also faces some practical problems, like Its
nitrogen fixation capacity is unstable. Despite much current research on Bradyrhizobium japonicum, it often
focuses on the molecular mechanism of a certain gene or protein, failing to study the environmental
adaptability of such bacteria, and failing to study the function and characteristics of bacteria from a genomic
perspective. The paper focuses on Pan-genome to study Bradyrhizobium japonicum. Through the obtained
genome information tested for line integrity using CheckM and BUSCO, analysis of the Meverage nucleotide
consistency (similarity) ANI, and analysis of the secondary metabolite, the genomic dynamics of soybean
bradyrhizobium is initially revealed and provides research clues for the analysis of its bacterial functional
evolution mechanism and environmental adaptability.
1 INTRODUCTION
The mutually beneficial symbiosis of rhizobium and
legumes provides plants with rich nitrogen while
actively affecting the soil nitrogen circulation. As a
classic model of bionitrogen fixation, with rhizobium
jointly regulated by plant roots and rhizobium, its
nitrogen fixation occupies more than 60% of the total
bionitrogen fixation (Herridge, 2008) and greatly
alleviates the nitrogen demand in agriculture. It is
worth mentioning that rhizobium
Compound agents (Bradyrhizobium japonicum
and Bacillus subtilis) developed by Indigo (Indigo
Ag, Inc., Charlestown, USA) can increase crop yield
(> 3%) and water absorption efficiency under drought
stress (> 75%) with less nitrogen fertilizer
application. The success of this model has also made
the research and promotion of new bacterial agents or
fertilizers the future development direction of
agriculture. Rhizobium is a special plant tissue
formed by rhizobium and legumes, a process that
involves many chemicals, such as plant-secreted
flavonoids, isoflavones, and terpenoids (Stoksta,
2016). Some certain root secretions (flavonoids-like,
a
https://orcid.org/0000-0001-5028-5620
isoflavones) can bind to the NodD protein secretion
of rhizobium, causing nod gene expression to produce
tuberoma factor, which then acts with the plant root
cells to activate tuberoma-related gene expression to
form rhizobium (BROGHAMMER, 2012). The
genus rhizoma was established in 1889 by B. Frank,
containing three species: pea rhizobium, alfalfa, and
passion. Bradyrhizobium D.C. Jordan differentiated
from rhizobium in 1982. Bradyrhizobium is an
ancient soybean nitrogen-fixing symbiotic bacteria
widely distributed in different habitats and symbiotic
with different legume-specific hosts. Therefore it is
highly cosmopolitan (SPRENT, 2017).
Bradyrhizobium diazoefficiens USDA110 can form
rhizoma with soybean and has excellent symbiotic
nitrogen fixation properties. The Bradyrhizobium
japonicum under the bacterial genus classification is
widely used as a promotional species in agricultural
production. Interestingly, as taxonomy developed, the
well-known Bradyrhizobium japonicum USDA 110
was eventually divided into Bradyrhizobium
diazoefficiens and named Bradyrhizobium
diazoefficiens USDA 110. However, the use of
rhizobium also faces some practical problems. The
Ma, R.
Pan-genomic Analysis of Bradyrhizobium japonicum.
DOI: 10.5220/0011376000003443
In Proceedings of the 4th International Conference on Biomedical Engineering and Bioinformatics (ICBEB 2022), pages 1031-1040
ISBN: 978-989-758-595-1
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
1031

specific identification of host-rhizomomyces causes
rhizobium to be unable to colonize all legume crops.
Their nitrogen fixation capacity is good or bad, not as
predictable as chemical fertilizers. On the one hand,
this is related to rhizobium's genomes and involves
the interaction between rhizobium and plants and
indigenous microorganisms. Traditionally, most
researchers focus on the individual gene or protein to
enhance the nitrogen fixation capacity of the
rhizobium and rare to focus on genomes, but the
effect was not ideal. This paper adopts a new research
method, which is to use Pan-genome to study
Bradyrhizobium. The Pan-genome is defined as the
entire non-redundant gene bank that constitutes the
genome, including the core genome, a set of genes
(almost) present in all genomes; accessory genome,
present in more than two genomes; unique genome,
found only in a bacterial genome. In this paper, Pan-
genome analysis focuses on Bradyrhizobium, first
with identifying core genes, accessory genes, unique
genes, and this-based gene function analysis;
secondly, the openness and closure of the genome are
also important concepts. This paper analyzes the
genome sequence of all 21 Bradyrhizobium
japonicum and one Bradyrhizobium diazoefficiens
USDA 110(from the NCBI due to historical naming).
According to the Heap rule, the closed Pan
genome contains all possible genes, and even
increasing the scale of genome sequencing, only a
small amount of genes are added to the Pan
genome.For an open Pan genome, the sequencing of
the new genome will increase a lot of undiscovered
genes, where its Pan genome is open (L R, V M, P-E
F, 2015).Usually, bacterial biogenomes with multiple
hosts or frequent habitat changes are more open
because their gene islands are more varied; Once
specialized intracellular, pathogens are not in
constant contact with other bacteria and lose large
amounts of genes in evolution to fully adapt to the
host. Thus, their genomes are very compact and more
closed (BARSY, 2016). The differentiation of the
genus rhizobium dates back 200 million years ago (L
M, A M, B D, 2001). However, beumes occurred 60
million years ago (MATT, 2005). This and its
mismatch suggest that rhizobium's symbiotic
nitrogen fixation capacity occurs in modern times,
possibly caused by the horizontal transfer of genes.
However, whether current genomes are open and how
to open rhizobium is still worth studying. The study
of the general genome of the rhizome helps to deepen
the understanding of the soybean-rhizome symbiosis,
provides a theoretical basis for the agricultural
production and application of rhizome, and is of great
significance to the study of the global nitrogen
ecological cycle.
2 METHODS
2.1 Data Source
The genomic data for 21 Bradyrhizobium japonicum
and 1 Bradyrhizobium diazoefficiens analynome
database and the zed in this paper are from NCBI
Gedata format of fasta, is shown in Table 1.In genome
assembly, it is assembled from contig into
scaffold,contig represents the consensus sequences
found from short reads obtained from large-scale
sequencing.The first step in assembly is the assembly
of contig. from a pair-end library.Further based on
mate-pair libraries of different lengths, the originally
isolated contig are connected in order, this step
yielding scaffolds. Finally, scaffold merged adjusted
based on genetic or optical maps to form a
chromosome.
Table 1: The strain information used in the study analysis.
species Bacterial
strain
BioSample
numbe
r
Assembly
level
Genome Size
(Mb)
GC% Scaffolds
numbe
r
Host
information
B. japonicum USDA 6 SAMD00060992 Complete 9.20738 63.7 1 absent
J5 SAMN05890661 Complete 10.1387 63.3 1 soybean
nodulation
5038 SAMN15394813 Complete 9.22625 63.7 1 Soybean
nodulation
E109 SAMN03262953 Complete 9.22421 63.7 1 Farmland
soybean
rhizoma
SEMIA
5079
SAMN02726028 Chromosome 9.58303 63.5 1 Duress soybean
plants
NBRC
14783
SAMD00097546 Contig 9.09656 63.7 177 soybean
5873 SAMN13738681 Scaffold 9.16023 63.7 141 soybean
Is-34 SAMN03083461 Scaffold 10.3266 63 248 soybean
nodulation
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
1032

22 SAMN02441445 Scaffold 7.50456 64.5 4 absent
in8p8 SAMN02440647 Scaffold 7.58992 63.8 52 absent
is5 SAMN02440582 Contig 7.58879 63.8 60 absent
USDA 38 SAMN02440784 Scaffold 9.60897 63.5 107 soybean
FN1 SAMN02666820 Scaffold 9.1385 63.7 87 soil
UBMA197 SAMN06077198 Contig 10.4422 63.3 287 Panleaf pinoma
rhizoma
USDA 123 SAMN02441447 Scaffold 10.4577 63.3 517 soybean
CCBAU
25435
SAMN02469483 Contig 9.46079 63.5 520 absent
CCBAU
15618
SAMN02469476 Contig 9.82401 63.4 691 absent
USDA 135 SAMN02441452 Scaffold 7.70332 64 547 soybean
CCBAU
15354
SAMN02469475 Contig 10.1266 63.3 951 absent
CCBAU
15517
SAMN02469482 Contig 9.91703 63.4 1129 absent
CCBAU
83623
SAMN02469465 Contig 10.0743 63.3 1212 absent
B. diazoefficiens USDA 110 SAMN03573437 Complete 9.10606 64.1 1 absent
2.2 Genomic Integrity
The genomic information obtained is tested with
CheckM (PARKS, 2015) and BUSCO (SIMAO,
2015), and the genome with insufficient
completeness or high contamination is not conducive
to the Pan-genomic subsequent analysis. CheckM
evaluates genome integrity and contamination by
specific to a species lineage and unique genes in the
database. Cds of genome are predicted through
Prodigal (HYATT, 2010) software that Prodigal
shows excellent robustness to gene structure
prediction, translation starting site recognition, and
false positives. BUSCO constructed a single-copy
conserved gene set of genomome Rhizobiales
through the OrthoDB database, compared the
transcript results by Augustus software, and then the
proportion and integrity to evaluate genome integrity.
2.3 Pan-genome Analysis
Genomes were first annotated through Prokka
software, analyzed with gbk files of generated
GeneBank through BPGA (CHAUDHARI, 2016)
(V1.3), selected default parameters. BPGA adopted
Neighbour Joining Tree achievements for core genes
and used USEARCH Clustering Algorithm genome
annotation is mainly based on COG and KEGG
databases. The Pipeline of, panX (DING, 2018),
PGCGAP (https://github.com/yikedou/pgcgap).
2.4 Analysis of Secondary Metabolites
The secondary metabolites of microorganisms are a
class of complex functional compounds synthesized
by primary metabolites through complex synthetic
paths and processes, such as antibiotics, pheromones,
toxins, etc., which is very important for the growth
and competition of microorganisms. The more
complex the life history, the wider the host, the
greater the living environment changes. The more
frequent the communication with the host, the more
metabolites of the secondary level. Secondary
metabolites can be predicted through published
microbial genomic data. Secondary metabolites
analysis can be analyzed based on antiSMASH. Still,
the online version is more abundant, comprehensive
and accurate than the local version, so the online
version of antiSMASH (https: / / antismash.
secondary etabolites.org), the parameters are default.
The strictness of the test is relaxed.
3 RESULTS AND DISCUSSION
3.1 Genomic Integrity
After the genome integrity analysis of 22 soybean
slow biological rhizomas, we found that the selected
genome integrity was higher, all above 97%, but only
a few genomes could reach 100%, as shown in Figure
1. The CheckM results showed that the remaining
genome was relatively complete except for a weak B.
japonicum CCBAU series sequence deletion (Figure
1), consistent with the BUSCO results (Figure 2).In
addition, most of the genome has a small number of
Pan-genomic Analysis of Bradyrhizobium japonicum
1033
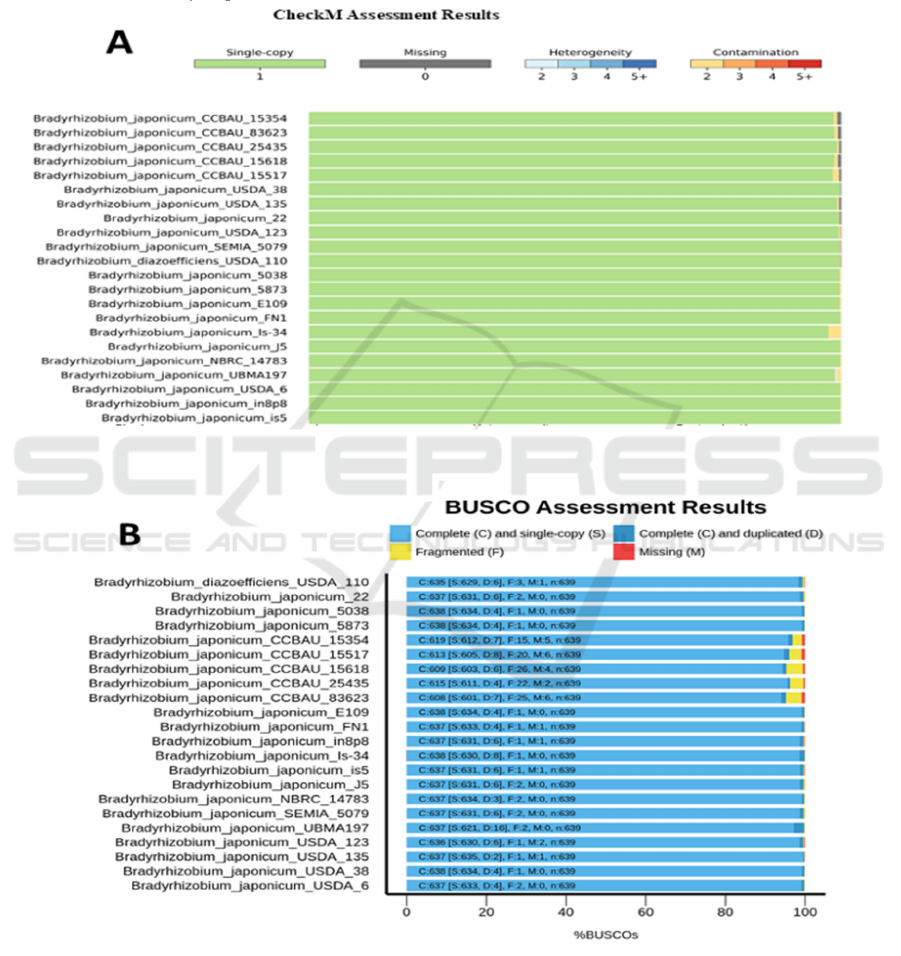
gene pollution, the main reason for this phenomenon
may be wrong sequencing, foreign genes (human,
bacteria in the air, etc.), pollution or sequence splicing
in the sequencing process, but this part is relatively
low and does not affect the subsequent
analysis.BUSCO analysis showed a small number of
genes (Figure 1B yellow section), generally due to
insufficient sequencing depth or incomplete genome
splicing. In addition, the BUSCO results show
multiple copies of parts of the Marker gene in the
rhizobium genome. Overall, the NCBI uploads high
genome integrity and allows downstream generic
genomic and other analysis processes.
Figure 1: 22 Genome integrity assessment of Bradyrhizobium(checkM Assessment Result).
Figure 2: 22 Genome integrity assessment of Bradyrhizobium(BUSCO Assessment result).
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
1034

3.2 Pan-genomic Statistics
This paper counted the number of core, accessory,
and unique genome (specific) and unique deletion
genome of 22 Rhizobium strains, as shown in Table
2. Bradyrhizobium diazoefficiens USDA 110 is
found more common genomics (794) specific
deletion genes (63), so although its genome is
complete, it still varies from Bradyrhizobium
japonicum, so it is understandable to divide it into
other categories. The Pan-genomic statistics of 22
rhizobium counted 3,807 core genes, which is very
small. The core genome is the genes shared by all
strains involved in basic biological processes such as
gene expression, energy production, amino acid
metabolism, etc. Some strains have even more
accessory genomes than the number of core genomes.
Accessory genomes represent some specific
functions and have a relatively strong metabolic
ability. Unique genomes represent that some of their
achievements are more competitive. The more unique
genomes, the stronger the resistance to environmental
adaptability. Thus it can be seen B.japonicum
UBMA197, B. japonicum USDA_135, B. japonicum
22 metabolic ability, environmental adaptability
ability are very strong; B.japonicum 5038, B.
japonicum 5873, B. japonicum E109, B. japonicum
FN1 is highly metabolizing, but it is less
heterophenetic and less adaptable. Many genomes
have more unique genomes like B. japonicum
USDA_135, B. japonicum 22, B. diazoefficiens
USDA_110, B. japonicum UBMA197, which make
each biometabolic process rich and will be beneficial
to the expansion of habitat (Konstantinidis, 2004),
which may be the reason why the wide range of
Bradyrhizobium adapted to (Tian, 2012).
Table 2: 22 Pangenic statistics of rhizoma strains.
Species Core genes accessory
gene
unique gene(specific) Specific
deletion gene
B. diazoefficiens USDA_110 3807 3213 794 63
B. japonicum USDA_123 3807 4745 527 12
B. japonicum USDA_135 3807 2242 717 114
B. japonicum 22 3807 2019 758 114
B. japonicum 5038 3807 4155 1 0
B. japonicum 5873 3807 4148 0 1
B. japonicum CCBAU_15354 3807 5010 132 24
B. japonicum CCBAU_15517 3807 4985 170 22
B. japonicum CCBAU_15618 3807 4422 433 22
B. japonicum CCBAU_25435 3807 4122 347 22
B. japonicum CCBAU_83623 3807 5032 266 21
B. japonicum E109 3807 4152 1 0
B. japonicum FN1 3807 4152 6 1
B. japonicum in8p8 3807 2919 4 1
B. japonicum is5 3807 2918 12 1
B. japonicum Is-34 3807 4409 645 0
B. japonicum J5 3807 4359 400 1
B. japonicum NBRC_14783 3807 4135 10 3
B. japonicum SEMIA_5079 3807 4143 246 1
B. japonicum UBMA197 3807 4176 1048 8
B. japonicum USDA_6 3807 4141 17 5
B. japonicum USDA_38 3807 4276 314 1
3.3 COG Analysis
COG analysis found that,as shown in figure 3, the
genome was mainly focused on related functions such
as [R]General function prediction only, [E]Amino
acid transport and metabolism,[K]Transcription. The
proportion of the core genome, accessory genome,
and unique genome is almost the same in these
functions, directly related to biological trait
expression and basic functions, so the genome plays
a major role in biological trait expression.
Pan-genomic Analysis of Bradyrhizobium japonicum
1035
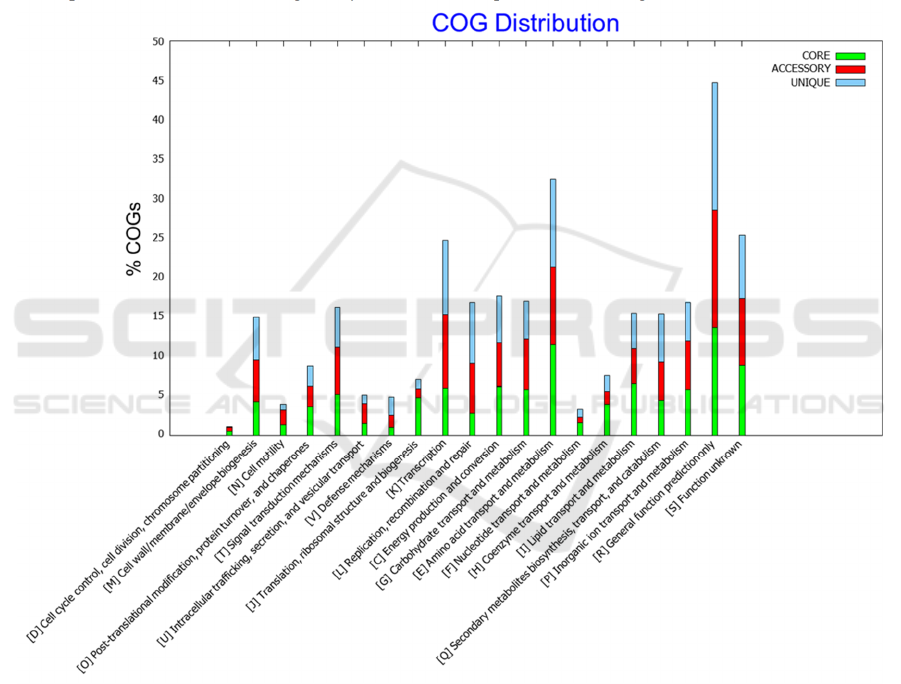
The numbers of genomes related with [M] cell
wall\ membrane\ evelope biogenesis, [T] singal
transduction mechanism, [L] replication,
recombination and repair, [C] energy production and
conversation, [G] Carbohydrate transport and
metabolism, [I] lipid transport and metabolism, [Q]
secondary metabolites biogenesis, transport, and
catabolism, [P]inorganic ion transport and
metabolism are similar. It shows that the strain gene
is mostly concentrated in the metabolic process and
cell differentiation process. It is preliminarily
speculated that this is suitable for the nitrogen
fixation process of rhizobium and legume symbiosis.
The nitrogen fixation process is very complex. It
includes many symbiotic processes, such as rhizoma
infection, bacteria-like differentiation, and tuberous
nitrogen fixation. Their differentiation and
rhizobiums require the expression of related genes in
a large number of cell components. The invasion and
symbiotic nitrogen fixation process with rhizomes
involves biological processes, as well as signal
exchange, interaction, material transportation, and
metabolism, so it requires the participation of many
genes related to molecular function and biological
processes (Wang, 2014). The above inferences now
require further testing.
Figure 3: COG analyse.
3.4 KEGG Analysis
As shown in figure 4, KEGG analysis found a high
proportion of genome distribution in amino acid
metabolism, carbohydrate metabolism, membrane
transport, overview, etc. In these fundamental
functions, like amino acid metabolism, core genomes
play a major role. Amino acid transport and
metabolism are relatively many genes, and it is
preliminarily speculated that this may be related to its
high activity efficiency. Because complex amino acid
circulation is essential for symbiotic nitrogen
fixation, both sides will control the substance
exchange by controlling amino acid metabolism
(Lodwig, 2003); (Prell, 2006). At the same time, the
genes are distributed in cardiovascular diseases,
cellular community, development, digestive system,
excretory. The number of specific functions such as
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
1036
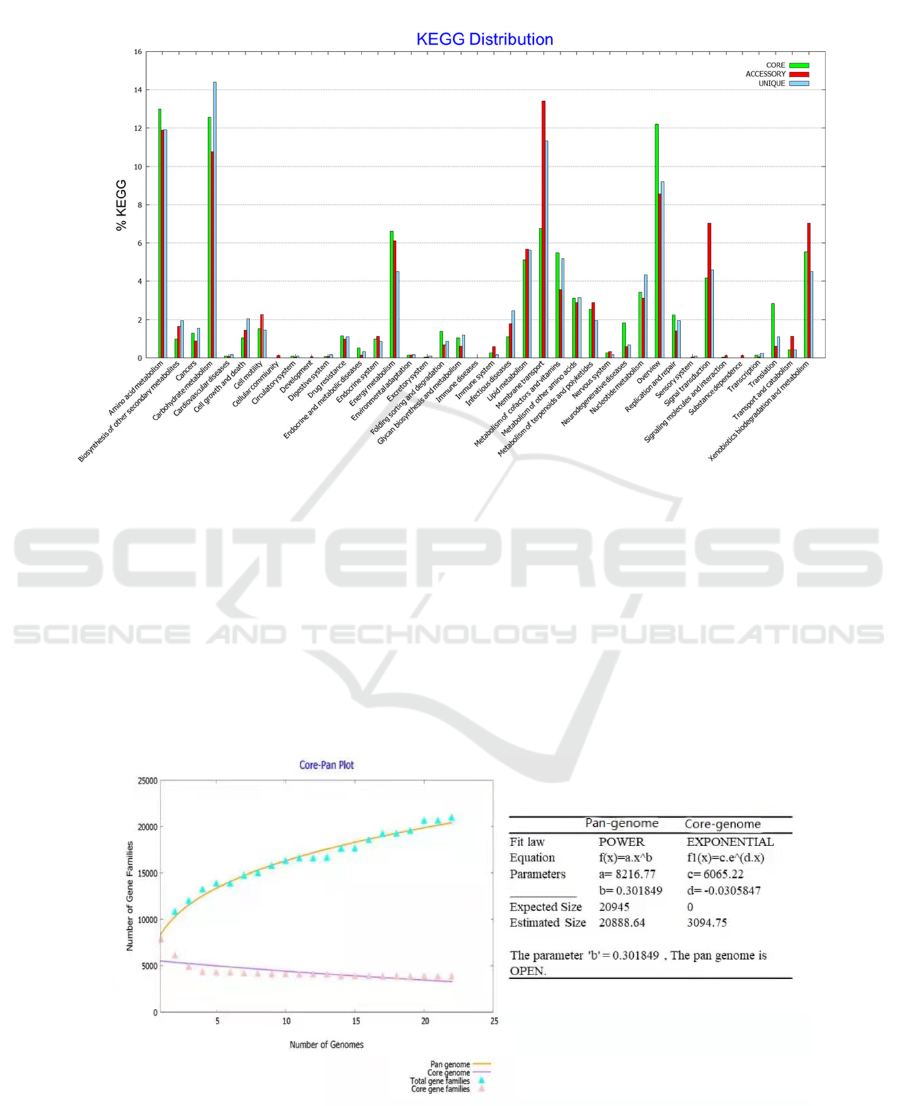
system, sensory system, signaling molecules, and
interaction\ substance dependence\ transcription is
small. Therefore, it can be seen that these bacteria all
have a strong basic function, but the specific
environment is poorly adaptable.
Figure 4. KEGG analyse
3.5 Panogenomic Fitting Equations
The correlation parameters of the fitting equation, as
shown in figure 5, of the generic genome size (T)
relation to genome number (X) are shown in the
figure, showing that the number of genes in the
generic genome of Bradyrhizobium increases as the
number of the genome increases. Different genomes
have the same gene family, and when the number of
genomes in each family increases, the genes in this
family are relatively open (Sun, 2013). The change is
unique number, and the curve shows the core gene
number with an increasing genome. Open here means
that the species is able to exchange genetic material
with other species in many different ways to acquire
new genes. The Bradyrhizobium genome has
extremely high plasticity, suggesting that it may more
readily acquire new genes to accommodate complex
changes in the environment.
Figure 5: Panogenomic fitting equations.
Pan-genomic Analysis of Bradyrhizobium japonicum
1037

3.6 Analysis of Secondary Metabolites
Secondary metabolites are predicted through
published microbial genomic data and are analyzed
using an online version of antiSMASH (https: / /
antismash. secondary etabolites.org). Similar
similarities were found between strains, as shown in
table 3.
lanthipeptide-class-v,
cyanobactin(cyanobacteria), beta lactone, T3PKS,
T1PKS, phosphonate, is compounds do not occur in
other species. Hserlactone and RiPP-like, terpene,
LAP, proteusin all exist to maintain the basic
metabolic process. Lipoids such as beta lactone,
Hserlactone can serve as stored energy in extreme
environments or hungry situations for microbial
growth to provide carbon sources and energy
(Kadouri, 2005). Compounds such as terpene are
present in all types of strains, are associated with the
growth and development of plants, and participate in
the plant defensive response to (LYU, 2017).
Therefore, the enrichment of lipid metabolism and
transport pathway and the participation of compounds
such as terpene may be mechanisms for the effective
environmental adaptation of Bradyrhizobium.
Lanthipeptides and RiPP-like is a large class of
natural peptide products synthesized by ribosomal
and translates modified. Such compounds are widely
produced in different bacteria, with rich structural and
biological activity diversity; T3PKS and T1PKS all
belong to the antimicrobial proteins generated by the
ribosomal pathway. RRE-containing rarely occurs,
only where Bradyrhizobium japonicum CCBAU
15517, Bradyrhizobium japonicum E109, is endemic
to secondary metabolites. RRE-containing is related
to RNA transcription and gene expression, so
Bradyrhizobium japonicum CCBAU 15517,
Bradyrhizobium japonicum E109 will have some
specific functions, representing some specific
functions its strong environmental adaptability.
Table 3: Analysis of the secondary metabolites of 22 rhizoma strains.
Baterial name Number of
secondary
metabolites
g
ene clusters
secondary metabolite
Bradyrhizobium japonicum 22 11 hserlactone, RiPP-like, T1PKS, terpene, lanthipeptide-
class-v, cyanobactin, redox-cofacto
r
, beta lactone
Bradyrhizobium japonicum 5038 10 RiPP-like, proteusin, NRPS, ectoine, NRPS, terpene, LAP,
redox-cofacto
r
, hserlactone
Bradyrhizobium japonicum 5873 10 hserlactone, RiPP-like, NRPS, redox-cofactor, LAP,
p
roteusin, terpene
Bradyrhizobium japonicum CCBAU 15354 15 T3PKS, hserlactone, RiPP-like, terpene, NRPS, redox-
cofacto
r
, LAP, lanthipeptide-class-v, RiPP-like
Bradyrhizobium japonicum CCBAU 15517 13 RRE-containing, hserlactone, NRPS, RiPP-like, LAP,
ter
p
ene, T3PKS,
p
roteusin
Bradyrhizobium japonicum CCBAU 15618 14 hserlactone, terpene, LAP, redox-cofactor, proteusin,
NRPS, NRPS-like
Bradyrhizobium japonicum CCBAU 25435 13 terpene, LAP, hserlactone, redox-cofactor, RiPP-like
NRPS,
p
roteusin
Bradyrhizobium japonicum CCBAU 83623 14 redox-cofactor, hserlactone, NRPS, terpene, RiPP-like,
LAP, T3PKS, ectoine,
p
roteusin
Bradyrhizobium japonicum E109 10 hserlactone, redox-cofactor, terpene, LAP, RiPP-like,
NRPS, ectoine,
p
roteusin, RRE-containin
g
,
Bradyrhizobium japonicum FN1 10 hserlactone, redox-cofactor, LAP, terpene, NRPS, RiPP-
like,
p
roteusin
Bradyrhizobium japonicum in8p8 9 hserlactone, redox-cofactor, terpene, T1PKS, RiPP-like,
NRPS,
b
eta lactone
Bradyrhizobium japonicum is5 9 hserlactone, redox-cofactor, terpene, T1PKS, RiPP-like,
b
eta lactone
Bradyrhizobium japonicum Is-34 12 redox-cofactor, NRPS, LAP, phosphonate, hserlactone,
NRPS, ter
p
ene,
p
roteusin, hserlactone, RiPP-like
Bradyrhizobium japonicum J5 15 hserlactone, redox-cofactor, RiPP-like, terpene, LAP,
NRPS, ectoine,
p
roteusin,
p
hos
p
honate, T3PKS
Bradyrhizobium japonicum NBRC 14783 10 LAP, terpene, hserlactone, RiPP-like, NRPS, redox-
cofacto
r
,
p
roteusin
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
1038

Bradyrhizobium japonicum SEMIA 5079 11 RiPP-like, proteusin, ectoine, NRPS, terpene, LAP,
T1PKS, redox-cofacto
r
, hserlactone
Bradyrhizobium japonicum UBMA197 17 hserlactone, phosphonate, proteusin, LAP, RiPP-like,
NRPS, T3PKS, ter
p
ene, redox-cofacto
r
Bradyrhizobium japonicum USDA 6 10 hserlactone, redox-cofactor, terpene, LAP, RiPP-like,
NRPS, ectoine,
p
roteusin
Bradyrhizobium japonicum USDA 38 12 LAP, RiPP-like, NRPS, hserlactone, proteusin, terpene,
redox-cofacto
r
Bradyrhizobium japonicum USDA 123 20 terpene, redox-cofactor, LAP, hserlactone, T3PKS, NRPS,
RiPP-like, T1PKS,
p
roteusin, ectoine
Brad
y
rhizobium
j
a
p
onicum USDA 135
10 hserlactone, RiPP-like, redox-cofactor, NRPS, ectoine,
ter
p
ene, NRPS
Bradyrhizobium diazoefficiens USDA 110 9 hserlactone, proteusi, RiPP-like, NRPS, ectoine, terpene,
NRPS-like, LAP, redox-cofacto
r
4 CONCLUSION
This paper systematically studied the genome
sequence of 22 Bradyrhizobium strains, which finds
that the genome size is within the range of
7.50456Mb-10.4577Mb, and the selected genome
integrity is high, all above 97%. The genomes all have
3807 core genes, with an open genome.COG analysis
found that the Pseudomonas genome had a higher
proportion of genes related to the underlying
metabolic functions such as General function
prediction only, Amino acid transport and
metabolism, Transcription. Analysis of the secondary
metabolites found that most of the secondary
metabolites of the strain were T3PKS, peptides,
terpene, and esters. However, due to the length and
the small number of reference whole genomes, the
separation environment, and evolutionary
relationship still need to be strengthened. In later
work, broader Bradyrhizobium strains can be
collected, with more systematic and in-depth research
on the relationship between evolutionary history and
environmental adaptation, evolutionary environment,
and genomic characteristics.
REFERENCES
BROGHAMMER A, KRUSELL L, BLAISE M, et al.
Legume receptors perceive the rhizobial lipochitin
oligosaccharide signal molecules by direct binding[J].
PNAS, 109(34): 13859-13864 (2012).
BARSY M D, FRANDI A, PANIS G L, et al. Regulatory
(pan-)genome of an obligate intracellular pathogen in
the PVC superphylum[J]. The ISME Journal:
Multidisciplinary Journal of Microbial Ecology, 10(Pt
2)2016.
CHAUDHARI N M, GUPTA V K, DUTTA C. BPGA- an
ultra-fast pan-genome analysis pipeline [J]. Scientific
Reports, 6 (2016).
DING W, BAUMDICKER F, NEHER R A. panX: pan-
genome analysis and exploration [J]. Nucleic Acids
Research, 46(1) (2018).
Herridge, D.F., Peoples, M.B. & Boddey, R.M. Plant Soil
311, 1–18 (2008).
HYATT D, CHEN G-L, LOCASCIO P F, et al. Prodigal:
prokaryotic gene recognition and translation initiation
site identification [J]. Bmc Bioinformatics, 11 (2010).
Konstantinidis KT, Tiedje JM. Trends between gene
content and genome size in prokaryotic species with
larger genomes [J]. PNAS, 101 (9): 3160-3165 (2004).
Kadouri D, Jurkevitch E, Okon Y, Castro-Sowinski S.
Ecological and agricultural significance of bacterial
polyhydroxyalkanoates[J]. Crit Rev Microbiol, 31 (2):
55-67 (2005)
L R, V M, P-E F, et al. The bacterial pangenome as a new
tool for analysing pathogenic bacteria[J]. New
microbes and new infections, 7 (2015).
L M, A M, B D, et al. Nodulation of legumes by members
of the beta-subclass of Proteobacteria[J]. Nature,
411(6840) (2001),.
Lodwig EM, Hosie AH, Bourdes A, Findlay K, Allaway D,
Karunakaran R, Downie JA, Poole PS. Amino-acid
cycling drives nitrogen fixation in the legume-
Rhizobium symbiosis [J]. Nature, 422 (6933): 722-726
(2003).
LYU HZ, LIU WJ, HEL, etal. Advances on the study of
gene clusters involved in plant secondary metabolism
[J]. Plant science journal, (in Chinese with English
abstract) ,35 (4):609G610,612G621(2017)
MATT L, S H P, F W M. Evolutionary Rates Analysis of
Leguminosae Implicates a Rapid Diversification of
Lineages during the Tertiary [J]. Narnia, 54(4) (2005).
PARKS D H, IMELFORT M, SKENNERTON C T, et al.
CheckM: assessing the quality of microbial genomes
recovered from isolates, single cells, and
metagenomes[J]. Genome Research, 25(7): 1043-1055
(2015).
Prell J, Poole P. Metabolic changes of rhizobia in legume
nodules [J]. Trends Microbiol, 14 (4): 161-168 (2006)
Stoksta E. Science, 353(6305): 1225-1227 (2016).
SPRENT J I, ARDLEY J, JAMES E K. Biogeography of
nodulated legumes and their nitrogen-fixing
symbionts[J]. New Phytologist, 215(1): 40-56 (2017) .
Pan-genomic Analysis of Bradyrhizobium japonicum
1039

SIMAO F A, WATERHOUSE R M, IOANNIDIS P, et al.
BUSCO: assessing genome assembly and annotation
completeness with single-copy orthologs [J].
Bioinformatics, 31(19): 3210-3212 (2015).
Sun C. Research and development of comparative
genomics visualization system (CGVS) [D]. Beijing:
The University of Chinese Academy of Sciences,
(2013).
Tian CF, Zhou YJ, Zhang YM, Li QQ, Zhang YZ, Li DF,
Wang S, Wang J, Gilbert LB, Li YR, Chen WX.
Comparative genomics of rhizobianodulating soybean
suggests extensive recruitment of lineage-specific
genes in adaptations [J]. PNAS,109(22):8629-8634
(2012).
Wang SM. Whole-genome sequencing and comparative
genomic analysis of Mesorhizobium huakuii strain
7653R [D]. Wuhan: Huazhong Agriculture University
(2014).
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
1040
