
Preparation of Chemically Modified Residue-based Bio-adsorbents in
Astragalus and Study on Its Adsorption Performance
Xiaochun Yin
a
, Hongtao Li, Nadi Zhang, Hai Zhu, Ting Ke, Yi Zhao, Xingmin Wei,
Jianjun Wu
*
and Yongfeng Wang
*
School of Public Health, Gansu University of Traditional Chinese Medicine, Lanzhou, Gansu, China
119261751@qq.com, 11704014@qq.com,
*
591806561@qq.com,
*
331220684@qq.com
Keywords: Astragalus Residues, Chemical Modification, Adsorbent, Heavy Metal Removal.
Abstract: [Objective] Astragalus residues were prepared with NaOH, Na
2
CO
3
and citric acid modification, and their
adsorption capacity for Cu
2+
was studied for the removal of metal ions from simulated wastewater. [Method]
Fourier transform infrared spectroscopy (FTIR), scanning electron microscope (SEM), thermogravimetry
(TG) and X-ray diffraction (XRD) were adopted to characterize the morphology and surface structure of
bio-adsorbents; through static adsorption experiments, the effects of adsorbent dosage, pH, adsorption time
and initial concentration of Cu
2+
solution on the adsorption performance of the adsorbent were investigated;
the desorption performance of the adsorbent was studied by dynamic experiments, the adsorption principle
of the adsorbent was clarified, and a certain theoretical basis was provided for the reuse of Astragalus
residues. [Result] The modification of NaOH, Na
2
CO
3
and citric acid can improve the adsorption capacity
of Astragalus residues to Cu
2+
in water body, thereby reducing its pollution to the environment and realizing
the resource utilization of Astragalus residues. [Conclusion] Astragalus can be used as a raw material for the
preparation of adsorbents for removing heavy metal pollutants in water, and the modified adsorbent has
excellent performance.
1 INTRODUCTION
a
With the rapid economic development and the
continuous progress of society, the living standards
of people have gradually improved. At the same
time, the amount of wastewater discharged has
increased year by year, causing serious pollution to
the environment. Cu
2+
is one of the most common
heavy metal ions in wastewater. As one of the
essential trace elements of human body and animals
and plants, a minute amount of Cu
2+
can promote the
growth of animals and plants and normal life
activities of human body. However, long-term
accumulation of Cu
2+
will cause physiological
obstruction of animals and plants, development
stagnation, and even will result in a large number of
deaths, so that the whole aquatic ecosystem will
disorder or even will collapse. Through the
enrichment of the food chain, the human body
ingests a large number of contaminated animals and
a
https://orcid.org/0000-0002-9749-2608
plants, causing toxicity accumulates and damage to
the human body, such as Wilson’s disease (WD),
which is a chromosomal recessive disease,
dominated by adolescents and is a congenital copper
metabolic disorder. CuEXC is considered to be a
specific marker of copper overload in WD (Estela
2021). Excessive copper content in adults can lead to
many undesirable consequences such as high blood
pressure, coronary heart disease, arteriosclerosis,
and even endanger human health (Wang 2015).
In recent years, China and most other countries
have realized the hazards of wastewater pollution
and the urgency of water shortages, and have begun
to take various measures to re-treat wastewater for
cyclic utilization and reducing environmental
hazards, such as electric flocculation method (Bian
2021), microbiological method (Zhang 2021),
electrodeposition method (Chen 2021), biochar
adsorption method (Das 2021), etc. Compared with
other methods, the adsorption method has the
advantages of a wide application range, no
secondary pollution, high treatment efficiency, low
operating cost, and relatively simple operation (Shao
1022
Yin, X., Li, H., Zhang, N., Zhu, H., Ke, T., Zhao, Y., Wei, X., Wu, J. and Wang, Y.
Preparation of Chemically Modified Residue-based Bio-adsorbents in Astragalus and Study on Its Adsorption Performance.
DOI: 10.5220/0011375700003443
In Proceedings of the 4th International Conference on Biomedical Engineering and Bioinformatics (ICBEB 2022), pages 1022-1030
ISBN: 978-989-758-595-1
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

2019, Lan 2020). Therefore, the adsorption method
is currently the most promising method. In recent
years, researches on adsorbents have emerged one
after another. Among them, traditional Chinese
medicine residue contains a large amount of lignin
and cellulose, which can effectively adsorb heavy
metals and is one of the emerging mainstreams in
the preparation of adsorbents.
Astragalus is a legume plant often used as a
traditional medicine in China, Japan, Korea and
Southeast Asia (Wang 2018, Zhu 2020). Astragalus
has anti-tumor effect and can enhance resistance
(Wu 2001, Meng 2016, Deng 2018, Yu 2019, Huang
2019, Ma 2020, Zhu 2020). A variety of active
substances can be extracted by processing
Astragalus, such as flavonoids, saponins,
polysaccharides and amino acids (Zheng 2019, Li
2019, Zheng 2019, Chu 2019). In recent years, with
the gradual improvement of Astragalus processing
industry technology, the output of Astragalus
residues has also increased. If these drug residues
are not treated in time, they will cause serious
pollution to the environment.
Studies have shown that Astragalus residues are
rich in lignocellulose and other substances (Wang
2019). Lignocellulose is combined by cellulose,
hemicellulose and lignin through non-covalent force
and covalent cross-linking (Yang 2018). Because the
surface of lignin, cellulose and hemicellulose
contains many active functional groups such as
carbonyl groups and hydroxyl groups, they can be
combined with heavy metal ions in various ways
such as surface precipitation (Wang 2020, Qiu 2020,
Tan 2020) to remove heavy metals in the water
environment. Feng (Feng 2017) used the waste
residue of Astragalus to prepare a bio-adsorbent,
which can adsorb Pb
2+
well. However, the cellulose
surface functional groups in Astragalus residues are
often encapsulated in it, which affects the adsorption
performance of Astragalus residues on heavy metal
ions. Therefore, chemical modification is necessary
to increase the adsorption performance of Astragalus
residues.
In this study, NaOH, Na
2
CO
3
and citric acid
were used to modify the Astragalus residues, and the
Astragalus residue-based bio-adsorbent was
prepared for the adsorption of heavy metal Cu
2+
in
the aqueous solution. The samples were
characterized by Fourier transform infrared
spectroscopy (FTIR), scanning electron microscope
(SEM), thermogravimetry (TG) and X-ray
diffraction (XRD). Through static adsorption
experiments, the effects of adsorbent dosage,
solution pH, adsorption time and initial Cu
2+
concentration on the adsorption performance of the
adsorbent were investigated; the desorption
performance of the adsorbent was studied through
dynamic experiments, and the adsorption
mechanism of the adsorbent was clarified, which can
provide a theoretical basis for the reuse of
Astragalus residues.
2 MATERIALS AND
EXPERIMENTS
2.1 Materials
The Astragalus residues used in the experiment
came from Huirentang Pharmacy (Lanzhou, China),
and were repeatedly washed to remove residual
organic components. Water used in the experiment
was deionized water.
2.2 Experiments
2.2.1 Adsorbent Preparation
(1) Decolorization treatment: Astragalus was bought
from a pharmacy, and washed with deionized water
for 1-2 times, changing the water and boiling for 3
times (30 min/time), and drying at 65°C, crush, then
passing through a 40-mesh sieve. The prepared drug
residue and methanol were mixed and stirred at a
solid-liquid ratio of 1:5, and stirred until the pigment
and biologically active ingredients in the drug
residue were completely removed. The drug residue
was washed with distilled water and dried at 65°C.
The prepared pre-treatment drug residue is referred
to as Astragalus residues for short, denoted as AR.
(2) Modification:
①NaOH modification: Weigh a certain amount
of AR, add 1 mol/L NaOH, mix in a solid-to-liquid
ratio of 10:1, stir magnetically for 4 h (120 r/min),
shake the residue with deionized water and wash it
to neutrality, filter the medicine residue with a sand
filter, dry it, and name it as NaOH modified
Astragalus residues, denoted as AR-NaOH.
②Na
2
CO
3
modification: Weigh a certain amount
of AR, add 1 mol/L Na
2
CO
3
, mix at a solid-liquid
ratio of 10:1, and magnetically stir for 4 h (120
r/min). Then it was washed with deionized water to
neutrality, filtered with a sand filter, dried, and
named as Na
2
CO
3
modified Astragalus residues,
denoted as: AR-Na
2
CO
3
.
③ Citric acid modification: AR was firstly
pre-treated with 0.1 mol/L NaOH for 30 min, then
Preparation of Chemically Modified Residue-based Bio-adsorbents in Astragalus and Study on Its Adsorption Performance
1023

mixing 0.6 mol/L citric acid solution with the
pre-treated dregs at a ratio of 10:1 for modification,
and magnetic stirring for 4h (120 r/min). Finally, the
drug residue was washed with deionized water,
washed to neutrality, dried, and named as citric acid
modified Astragalus residues, denoted as AR-CA.
All Astragalus residue-based bio-adsorbents were
collectively named AR-Ts.
2.2.2 AR-Ts Structure Characterization
(1) FTIR: Fourier infrared spectrometer (FTIR,
Nicolet Nexus, USA) was used to study the structure
and chemical bonds of its molecules. The samples
for analysis were dried before use, ground in a
mortar, mixed with potassium bromide powder and
then pressed into a transparent sheet. The
experiment was carried out in the spectral range
(4000-400cm
-1
).
(2) SEM: Scanning electron microscopy (SEM;
JSM-6701F, JEOL, Japan) was used to study the
morphology of lignocellulosic compounds. The
samples for analysis were stored in an oven at a
temperature of 50°C overnight.
(3) TG: Perkin Elmer TGA-7 thermogravimetric
analyzer (Perkin-Elmer Cetus Instruments, Norwalk,
CT) was used to study the thermal stability and
composition of the adsorbents. About 10 mg of the
sample was weighed in a crucible and placed in a
sample holder. The sample was heated from room
temperature to 800°C (heating rate 10°C/min), the
purge gas was nitrogen, and the flow rate was 20
ml/min.
(4) XRD: X-ray diffractometer (JEOL,
JDX-3530, 2 kW, Tokyo, Japan) was used to study
the crystalline properties of the cellulose component.
Before analysis, the sample was ground into a finer
and uniform particle size powder, stored in an oven
at 50°C overnight, using CuK pulsed radiation with
a wavelength of 0.154 nm, and determining the
crystallinity degree of the compound by monitoring
the position, shape and intensity of the reflection
from the distribution structure substrate.
2.2.3 Adsorption Performance
First, a set of Cu(NO
3
)
2
solutions was prepared with
a concentration gradient, and a Cu
2+
standard curve
was established by flame atomic absorption
spectrophotometry. Then, a certain concentration of
Cu(NO
3
)
2
solution was prepared to adjust the pH of
the solution. A certain quality of Astragalus
residue-based adsorbent was placed in a conical
flask with a certain pH and a certain concentration of
Cu(NO
3
)
2
solution, and oscillated on a constant
temperature oscillator for a certain period of time.
The adsorption capacity, adsorption kinetic model
parameters and adsorption isotherm parameters of
the adsorbent can be obtained by measurement and
calculation.
3 RESULTS AND ANALYSIS
3.1 Characterization of Chemically
Modified Astragalus Residue-based
Bio-adsorbent
3.1.1 SEM Analysis
The scanning electron micrographs of AR,
AR-NaOH, AR-Na
2
CO
3
and AR-CA are shown in
Figure 1(a). It can be seen from the figure that the
structure of AR is relatively dense, and the surface
of AR-NaOH, AR-Na
2
CO
3
and AR-CA has more
pores and loose structure, which may be caused by
the erosion and fragmentation of lignocellulose. The
results show that the modifiers have a certain effect
on the AR surface, and the modified Astragalus
residue adsorbent is easier to adsorb Cu
2+
in water.
3.1.2 FTIR Analysis
Figure 1(b) is the infrared spectrum of AR,
AR-NaOH, AR-Na
2
CO
3
and AR-CA. It can be seen
from the figure that the absorption peak of AR: the
peak at 3411 cm
-1
is stronger, indicating that the
surface of residue-based bio-adsorbent in Astragalus
is rich in O-H; the peak at 2927 cm
-1
is the stretching
vibration of the C-H bond in methyl and methylene;
the peak at 1739 cm
-1
is the C=O stretching vibration
in the acid ester; the peak at 1639 cm
-1
is the C=O
stretching vibration in the protein, and the peak at
1373 cm
-1
is the CO stretching vibration of the
phenyl-hydroxyl group in lignin, the peak at
1249cm
-1
is the C-O-C stretching vibration of lignin,
the peak at 1157 cm
-1
is the C-O-C stretching
vibration of the cellulose ester group. The peak at
1030cm
-1
is the bending vibration of –OH (Li 2019).
In AR-NaOH, the peak at 1739 cm
-1
disappeared,
indicating that NaOH can cleave the ether ester bond,
and the ether ester bond in lignocellulose is the main
chemical bond connecting lignin and hemicellulose.
Comparing the peaks before and after the
modification, the peak intensity of some groups of
the Astragalus residue adsorbent after the
modification decreased, and the amplitude decreased,
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
1024
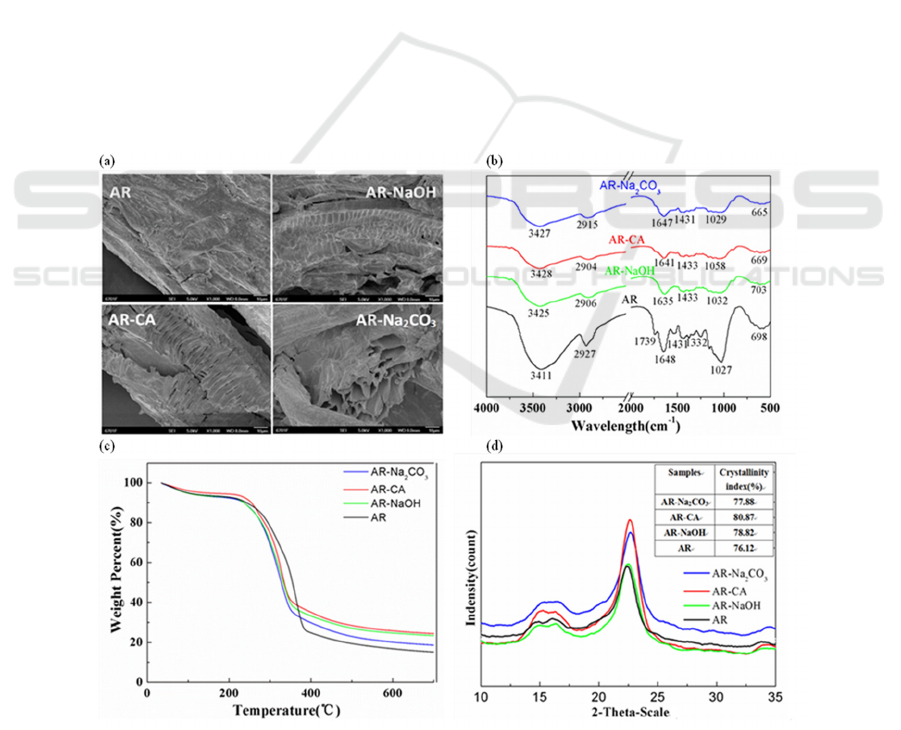
indicating that the content of chemical groups in the
Astragalus residue adsorbent decreased after the
modification.
3.1.3 TG Analysis
Figure 1(c) is the thermogravimetric diagram of AR,
AR-NaOH, AR-Na
2
CO
3
and AR-CA. The thermal
degradation of cellulose can be divided into three
stages: (1) AR, AR-NaOH, AR-Na
2
CO
3
and AR-
CA began to decrease slowly at 40-240℃, mainly
due to the evaporation of water in the sample and the
loss of hemicellulose; (2) The rapid weight loss of
AR-NaOH, AR-Na
2
CO
3
and AR-CA at 240-330℃,
and AR at 240-380℃ may be due to the
decomposition of the cellulose molecular skeleton
and the degradation of hemicellulose. The weight
loss rate of AR-NaOH, AR-Na
2
CO
3
and AR-CA is
smaller than AR, indicating that the cellulose and
hemicellulose content in AR-NaOH, AR-Na
2
CO
3
and AR-CA is lower than AR; (3) In the final stage,
the solid residue continues to decompose at a very
slow rate. In addition, it can also be found that the
thermal stability of the adsorbent after modification
is lower than that before modification.
3.1.4 XRD Analysis
Figures 1(d) are XRD patterns of AR, AR-NaOH,
AR-Na
2
CO
3
and AR-CA. Studies have shown that
different treatment methods break the hydrogen
bonds between and within the fiber chains of
lignocellulose, resulting in different changes in the
cellulose crystal structure. In the adsorbent,
lignocellulose is mainly used for adsorption, and
lignocellulose usually exists in the form of
amorphous and crystalline states. Among them,
cellulose is mainly present in a crystalline state.
Therefore, the crystallinity increases when lignin
and hemicellulose are destroyed to a certain extent.
The results showed that compared with the
unmodified sample (crystallinity index 76.12%), the
crystallinity index after citric acid treatment,
Na
2
CO
3
treatment and NaOH treatment increased to
80.87%, 77.88% and 78.82%, respectively, which
means that the adsorption capacity of the three
Astragalus residue-based adsorbents after
modification is essentially the same.
Figure 1: Characterization of AR, AR-NaOH, AR-Na2CO3 and AR-CA. (a) Electron micrographs of AR, AR-NaOH,
AR-Na2CO3 and AR-CA. (b) Infrared spectra of AR, AR-NaOH, AR-Na2CO3 and AR-CA. (c) TG results of AR,
AR-NaOH, AR-Na2CO3 and AR-CA. (d) XRD results of AR, AR-NaOH, AR-Na2CO3 and AR-CA.
Preparation of Chemically Modified Residue-based Bio-adsorbents in Astragalus and Study on Its Adsorption Performance
1025
3.2 Study on the Adsorption Behavior
of Astragalus Residue-based
Bio-adsorbent on Cu
2+
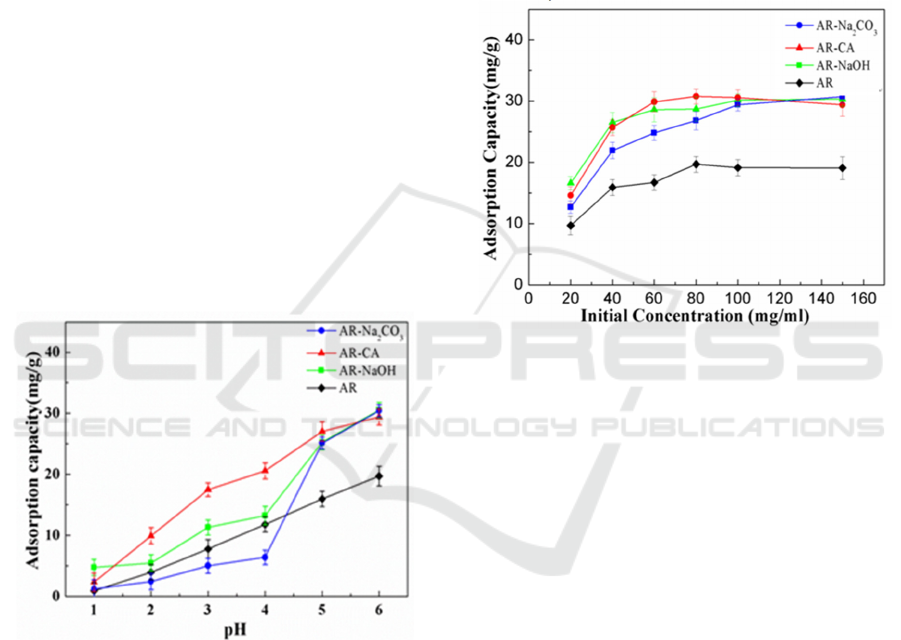
3.2.1 Effect of Solution pH on Adsorption
Figure 2 shows the effect of solution pH on Cu
2+
adsorption capacity. It can be seen from the figure
that, firstly, as the pH of the solution increases, the
adsorption capacity of the four adsorbents for Cu
2+
tends to increase, which can be related to the high
concentration of H
+
inhibiting the reaction and the
low concentration of H
+
promoting the reaction.
When the solution pH=6, the adsorption capacity of
the four adsorbents is the largest. The maximum
adsorption capacities of AR, AR-NaOH,
AR-Na
2
CO
3
and AR-CA are 19.7 mg/g, 31.05 mg/g,
30.53 mg/g and 30.55 mg/g, respectively. In addition,
in the whole adsorption process, the adsorption
capacity of AR-NaOH and AR-CA are both higher
than AR, and when the solution pH=1 and pH=6, the
maximum adsorption capacity of AR-NaOH is
slightly higher than AR-CA. When the pH=2~5, the
maximum adsorption capacity of AR-CA is higher
than that of AR-NaOH.
Figure 2: The effect of solution pH on the adsorption
capacity of residue-based bio-adsorbent in Astragalus.
3.2.2 Effect of Initial Concentration of the
Solution on Adsorption
Figure 3 shows the results of the adsorption capacity
of AR, AR-NaOH, AR-Na
2
CO
3
and AR-CA on Cu
2+
with the different initial Cu
2+
concentrations. It can
be seen from the figure that, first of all, with the
increase of the initial concentration of the Cu
2+
, the
adsorption capacity of the four adsorbents all shows
a trend of increasing first and then unchanged or
slightly decreasing, which may be mainly related to
the change of the Cu
2+
concentration in the solution
during the adsorption process and the fixed number
of adsorption sites, and the optimal initial Cu
2+
concentrations of the solution that can be adsorbed
by AR, AR-NaOH, AR-Na
2
CO
3
and AR-CA are 80
mg/L, 100 mg/L, 100 mg/L and 80 mg/L,
respectively. Secondly, as the initial Cu
2+
concentration increases, the adsorption capacities of
AR-NaOH, AR-Na
2
CO
3
and AR-CA are all higher
than AR, and the adsorption capacities of AR-NaOH,
AR-Na
2
CO
3
and AR-CA at equilibrium are
essentially the same.
Figure 3: The effect of initial solution concentration on the
adsorption capacity of residue-based bio-adsorbent in
Astragalus.
3.2.3 Effect of Adsorption Time on
Adsorption
Figure 4 shows the results of the adsorption capacity
of AR, AR-NaOH, AR-Na
2
CO
3
and AR-CA for
Cu
2+
with the change of adsorption time. It can be
seen from the figure that, first of all, as the
adsorption process progresses, the adsorption
capacity of the four adsorbents for Cu
2+
all presents
a trend of rapid increase and then equilibrium, which
maybe related to the emptier adsorption sites on the
surface of the adsorbent at the beginning of
adsorption. In the reaction progresses, the adsorption
capacity increases rapidly. When the adsorption sites
reach saturation, the adsorption capacity no longer
changes. In the whole adsorption process, the
adsorption capacities of AR-NaOH, AR-Na
2
CO
3
and
AR-CA are all higher than AR, and the adsorption
capacities of AR-NaOH, AR-Na
2
CO
3
and AR-CA
are basically the same when the adsorption
equilibrium is reached. The effects of these
modifiers on the modification of Astragalus are
basically the same. Secondly, the time for AR,
AR-NaOH, AR-Na
2
CO
3
and AR-CA to reach
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
1026
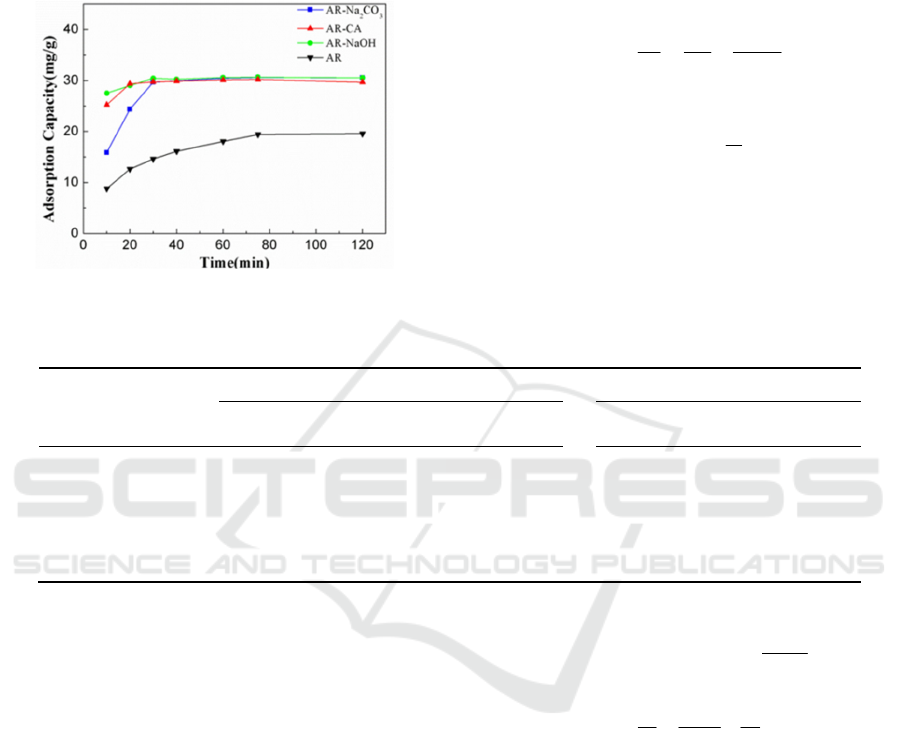
adsorption equilibrium is 80min, 30min, 30min and
20min, respectively, that is, the two modification
methods shorten the adsorption time when the
adsorption equilibrium is reached to varying
degrees.
Figure 4: The effect of adsorption time on the adsorption
capacity of residue-based bio-adsorbent in Astragalus.
3.2.4 Adsorption Isotherm
The Langmuir model (1) and Freundlich model (2)
were used to fit the adsorption data of the
residue-based adsorbent for Astragalus, and the
formulae are as follows:
Langmuir model:
(1)
Freundlich model:
(2)
Table 1: Astragalus drug residue-based bio-adsorbent adsorption isotherm models and parameters.
Adsorbent
Langmuir model
Freundlich model
q
max
(mg/g) K(L/mg) R
L
2
K
F
n R
F
2
AR 22.15 5.42×10
-2
0.980
2.18 2.96 0.768
AR-NaOH 33.91 6.85×10
-2
0.990
1.93 3.50 0.731
AR-Na
2
CO
3
38.65 2.87×10
-2
0.990 1.07 2.30 0.883
AR-CA
33.85 6.78×10
-2
0.965
2.22 2.89 0.654
It can be seen from Table 1 that, compared with
the Freundlich model, the Langmuir model has a
larger correlation coefficient, which shows that the
quasi-Langmuir model can better fit the adsorption
process of Cu
2+
by the residue-based adsorbent for
Astragalus, that is, the adsorption of the four
residue-based adsorbents to heavy metal ions Cu
2+
is
dominated by single-layer adsorption. According to
Langmuir adsorption isotherm, the saturated
adsorption capacities of AR, AR-NaOH,
AR-Na
2
CO
3
and AR-CA for Cu
2+
are 22.15 mg/g,
33.91 mg/g, 38.65 mg/g and 33.85 mg/g,
respectively. It can be seen that the three modified
methods of adsorbents have comparable adsorption
effects on Cu
2+
in water.
3.2.5 Adsorption Kinetics
The first-order adsorption kinetics (3) and
second-order adsorption kinetics (4) equations were
used to fit the adsorption data of the adsorbent, and
the formulae are as follows:
First-order adsorption kinetics:
t
k
qqq ete )
303.2
()log()log(
1
−=−
(3)
Second-order adsorption kinetics:
e
e
t
q
t
qkq
t
+=
2
2
1
(4)
It can be seen from Table 2 that, compared with
the quasi-first-order kinetic model, the correlation
coefficient of the quasi-second-order kinetic model
is larger, above 0.990. It shows that the
quasi-second-order kinetic model can better fit the
adsorption process of Cu
2+
by the adsorbent, that is,
the adsorptions of Cu
2+
by the three residue-based
adsorbents for Astragalus are mainly chemical
adsorption.
3.2.6 Reusability of Adsorbent
AR-Na
2
CO
3
is the best bio-adsorbent for studying
adsorption and desorption conditions in this
experiment. Through four consecutive cycles of
Lmm
e
e
e
KQQ
C
q
C 1
+=
eFe C
n
Kq log
1
loglog ×+=
Preparation of Chemically Modified Residue-based Bio-adsorbents in Astragalus and Study on Its Adsorption Performance
1027
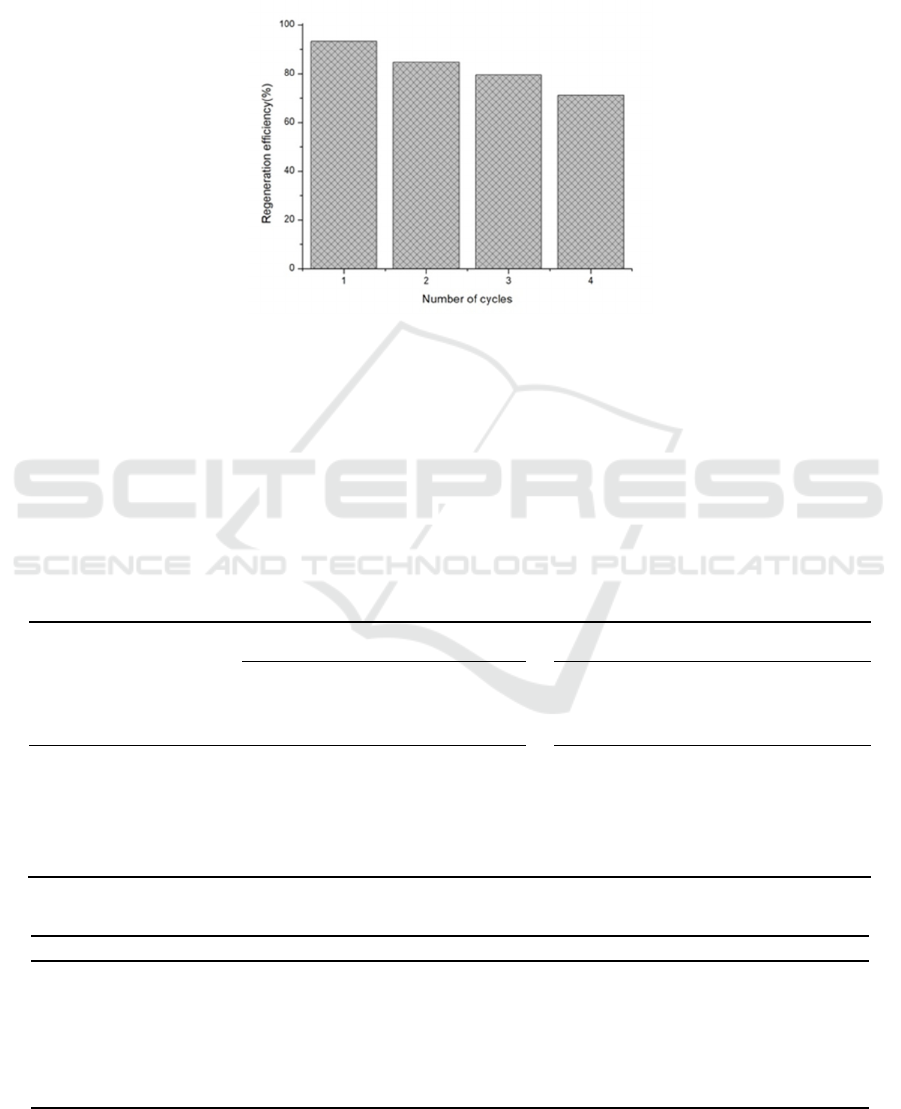
adsorption and desorption, the regeneration and
repeatability of AR-NaOH on heavy metal Cu
2+
were evaluated. As shown in Figure 3, the results
show that AR-Na
2
CO
3
has good reusability. After
four consecutive adsorption-desorption cycles, the
adsorption rate is still higher than 70%, which is
consistent with other reports (Chct 2020, Shi 2020,
Mok 2020, Maaloul 2021, Pavithra 2021). The
report shows that AR-Na
2
CO
3
is a suitable potential
adsorbent for removing heavy metal ions Cu
2+
in
water.
Figure 5: Reusability of AR-Na
2
CO
3
.
3.3 Comparison with Other
Adsorbents
The q
max
value obtained in this study was compared
with various celluloses reported in the literature for
removing Cu
2+
as bio-adsorbents, as shown in Table
3. The results showed that, except that the
adsorption capacity of saponified polygonum
cuspidatum fiber is slightly higher than AR-NaOH,
the adsorption capacity of AR-NaOH and
AR-Na
2
CO
3
are higher than other bio-adsorbents
(such as activated carbon fiber and coffee
grounds).
4 CONCLUSIONS
Table 2: Adsorption kinetic model and parameters of residue-based bio-adsorbent for Astragalus.
Adsorbent q
e, exp
(mg/g)
Quasi-first-order kinetic model
Quasi-second-order kinetic model
q
e, cal
(mg/g) k
1
(min
−1
) R
1
2
q
e, cal
(mg/g) k
2
(g/mg·min
-1
) R
2
2
AR 19.70 20.23 4.16×10
-2
0.931 22.31 3.01×10
-3
0.997
AR-NaOH 31.05 1.94 1.59×10
-2
0.443
30.86 3.69×10
-3
0.999
AR-Na
2
CO
3
30.72 6.88 3.94×10
-2
0.657
32.65 5.13×10
-3
0.994
AR-CA 30.55 1.67 1.21×10
-2
0.127
30.06 5.69×10
-2
0.997
Table 3: Comparison of Cu
2+
adsorption capacity of various bio-adsorbents.
Bio-adsorbent Metal ion
q
max
(mg/g)
References
AR-NaOH
Cu
2+
33.91 This research
AR-Na
2
CO
3
38.65 This research
Activated carbon fibe
r
25.51 Yu 2019
Waste coffee
g
rounds 13.33 Sadok 2019
Papermaking sludge 28.788 Dai 2019
Modified sawdust cellulose 4.33 Ulfa 2019
Saponified polygonum cuspidatum residue 34.482 Liu 2017
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
1028

Using Astragalus as raw material, three kinds of
residue-based bio-adsorbents for Astragalus
modified by NaOH, Na
2
CO
3
and citric acid were
successfully prepared. By using the prepared
adsorbent to adsorb Cu
2+
in water, the adsorption
performance of Cu
2+
in water was studied. Their
structures were characterized by FTIR, SEM, TG
and XRD, and it was found that the structures of the
modified bio-adsorbents are looser than before. The
crystallinities of the three adsorbents after
modification are basically the same, indicating that
the contents of crystalline cellulose of the three
adsorbents after modification are basically the same,
that is, the adsorption performances are also
basically the same. The results show that the
adsorption performances of AR-NaOH, AR-Na
2
CO
3
and AR-CA are better than AR, the adsorption
capacities of AR-NaOH, AR-Na
2
CO
3
and AR-CA to
Cu
2+
are equivalent, and the maximum adsorption
capacities are about 30 mg/g; The adsorption
equilibrium time of AR-NaOH (30 min),
AR-Na
2
CO
3
(30 min) and AR-CA (20 min) is
shorter than AR (80 min). The adsorption processes
of these adsorbents to Cu
2+
accord with the
quasi-first-order kinetic model and Langmuir model.
In summary, the modification of NaOH, Na
2
CO
3
and
citric acid can not only improve the adsorption
capacity of Astragalus residues on Cu
2+
in water, but
also reduce the environmental pollution caused by
Astragalus residues and realize the resource
utilization of it.
ACKNOWLEDGEMENT
This work was supported by NSFC (82160900),
Innovation Fund Project of Higher Education in
Gansu Province (2021B-159), Open Foundation of
Collaborative Innovation Center for Prevention and
Control by Chinese Medicine on Disease Related
Northwestern Environment and Nutrition
(998/99860202), Open Foundation of Traditional
Chinese Medicine Research Center of Gansu
Province (ZYZX-2020-ZX16) and Research on
prevention and control of COVID-19 by integrated
Traditional Chinese and Western Medicine
(2020C-36).
REFERENCES
Bian C Y, Ling X, Wu K L, Lu C H. (2021). Advances in
the Treatment of Radioactive Wastewater Containing
Heavy Metals by Electroflocculation. J. International
Core Journal of Engineering. 9, 2414-1895.
Chu C J, Jin Y. (2019). Analysis of flavonoids in
Astragalus in different growth years based on
one-test-multiple-evaluation method. J. Chinese
Medicinal Materials. 8,74-77.
Chct, A., Ss, B., Mkmh, C., Zng, D., and Mhh, A.. (2020).
The improved adsorbent properties of microcrystalline
cellulose from oil palm fronds through immobilization
technique. J. Surfaces and Interfaces. 20,2468-0230.
Chen H B, Lin Y H, Meng L Y, Lin M Y. (2021).
Research on the treatment of heavy metal wastewater
by Fe/C electrodeposition method. J. China Resources
Comprehensive Utilization. 05,18-20.
Deng C, Zhao H Y, Gao M L. (2018). Research progress
in anti-tumor activity of Astragalus active components.
J. Foreign Medicine (Medical Geography). 3,276-280.
Dai C S, Zhang Y D. (2019). Study on the preparation of
functional adsorption materials from papermaking
sludge and its adsorption performance for Cu2+. J.
Environmental Pollution & Control.006,621-625, 630.
Das S K, Ghosh G K, Avasthe R. (2021). Conversion of
crop, weed and tree biomass into biochar for heavy
metal removal and wastewater treatment. J. Biomass
Conversion and Biorefinery. 3, 2190-6823.
Estela, D., Cuello-Nunez, S., Ward-Deitrich, C., Morley,
T., and Goenaga-Infante, H. (2021). A fit-for-purpose
copper speciation method for the determination of
exchangeable copper relevant to Wilson’s disease. J.
Analytical and Bioanalytical Chemistry. 1-13.
Feng N C, Fan W, Zhu M L, Shi Y. (2017). Preparation of
Astragalus waste residue bio-adsorbent and its
adsorption to Pb2+. J. Journal of Lanzhou University
of Technology.4, 71-76.
Huang Y, Du Z C, Hou X T, Hao E W, Qin H Z, Deng J G.
(2019). Research progress in chemical constituents,
pharmacology and application of Astragalus residues.
J. Chinese Journal of Information on Traditional
Chinese Medicine. 6,140-144.
Liu Z Y, Mao B Y, Pan M, Dong W, Ma L A, Yu Z H,
and Cheng X H. (2017). Adsorption performance of
saponified Polygonum cuspidatum residue on Cu2+. J.
Jiangsu Agricultural Sciences.023,299-302.
Li X, He F, Hou Y. (2019). Study on the main components
and pharmacological effects of astragalus. J. Diet
Health Care. 4, 85.
Li Q, Sha S L, An Y S, Meng W, Bu J J, Zhou G L. (2019).
Study on infrared spectroscopy of Astragalus at
variable temperature. J. Coal and Chemical Industry.
12, 136-140.
Lan X, Guo S T, Li B. (2020). Research progress on the
desorption of heavy metals in water treatment by
adsorption method. J. Shandong Chemical Industry. 10,
81-82.
Meng C, Wu J S, Ma Y M. (2016). Research progress on
liver-protecting effects of Astragalus components and
main components. J. Chinese Medicine. 2, 63-70.
Ma Y F, Li M J. (2020). The efficacy of Astragalus
polysaccharide combined with cisplatin in the
treatment of ovarian cancer-related malignant ascites
Preparation of Chemically Modified Residue-based Bio-adsorbents in Astragalus and Study on Its Adsorption Performance
1029

and its influence on drug resistance genes. J. Chinese
Journal of Postgraduates of Medicine. 8, 685-690.
Mok, C. F., Chee, Y., Azuan, N., Osman, N., and C
Rosmani Hassan. (2020). Adsorbents for removal of
cationic dye: nanocellulose reinforced biopolymer
composites. J. Journal of Polymer
Research.12,1022-9760.
Maaloul, N., Oulego, P., Rendueles, M., Ghorbal, A., and
M Díaz. (2021). Biopolymer composite from cellulose
nanocrystals of almond (Prunus dulcis) shell as
effective adsorbents for Cu2+ ions from aqueous
solutions. J. Journal of Environmental Chemical
Engineering. 2, 2213-3437.
Pavithra S; Thandapani Gomathi; S Sugashini; P N Sudha;
Alkhamis Hussein H; Alrefaei Abdulwahed F;
Almutairi Mikhlid H. (2021). Batch adsorption studies
on surface tailored chitosan/orange peel hydrogel
composite for the removal of Cr(VI) and Cu(II) ions
from synthetic wastewater. J. Chemosphere. 271.
doi:10.1016/J.CHEMOSPHERE.2020.129415.
Qiu H Y, Wang J W, Xue S S, Lu Y C, Jin L, Xu B.
(2020). Study on the adsorption properties of modified
cellulose superabsorbent resin for Cu2+, Pb2+. J.
Modern Chemical Industry. 3, 182-186+191.
Shao D Y. (2019). Research progress in the treatment of
heavy metal wastewater pollution by adsorption
method. J. Shandong Chemical Industry. 7, 57-59.
Sadok, H., Wali, A., Mseddi, S., and Zouari, N.. (2019).
Adsorption of Cu(II) ions from aqueous solutions on
waste coffee residues: sorption kinetics, equilibrium
isotherms, and thermodynamic parameters. J. Arabian
Journal of Geosciences. 24, 1866-7511.
Shi, Y Z, Yin, X C, Si, G H, Zhang, N D, and Wang, X H.
(2020). Bio-adsorbent preparation based on Chinese
Radix isatidis residue for Pb(II) removal. J. Water
Practice & Technology. 4.
Tan S L, Bai X Y, Wang X, An Y H, Shen Y L, Zhang X
T. (2020). Study on the adsorption performance of
lignocellulose-based composites for Mn2+. J. Journal
of Inner Mongolia Agricultural University (Natural
Science Edition). 3, 74-80.
Ulfa S M, Chamidah N, Kurniawan A. (2019). Adsorption
of Cu(II) in Aqueous Solution by Modified Sawdust
Cellulose. J. IOP Conference Series Earth and
Environmental Science.1, 1755-1315.
Wu Y P, Cao Y, Cao Z Z. (2001). Discussion on the best
extraction process of the active ingredients of
Astragalus membranaceus. J. Li Shizhen Traditional
Chinese Medicine and Materia Medica. 10, 876-877.
Wang X F. (2015). Study on the Current Situation and
Countermeasures of Cu2+ Harm to the Environment. J.
Land and Natural Resources Research. 01, 55-57.
Wang J. (2018). Research progress on the
pharmacological effects of astragalus. J. Medical
Equipment. 14, 203-203.
Wang C M, Wang M C, Liang H W, Zhao C, Li B, Wu S J.
(2019). Determination of main feed nutrients of seven
kinds of traditional Chinese medicine residues. J.
Heilongjiang Animal Science and Veterinary
Medicine. 2, 111-113.
Wang L X, Zhou X T, Luo Z Q, Mu W H, Ma Y, Shao Z J.
(2020). Research progress on the performance and
mechanism of the adsorption of heavy metals Pb2+ in
wastewater by agricultural and forestry wastes. J.
Materials Review. 17, 119-127.
Yang J, Duan J J. (2018). Resource utilization of
lignocellulosic waste in the environment. J. Modern
Engineering. 1, 58- 61.
Yu G H, Shi X H. (2019). Research progress on the
anti-tumor mechanism of active components of
Astragalus membranaceus. J. Frontiers of Medicine.
12, 25-26.
Yu J, Chi C. Zhu B, Qiao K, Yan S. (2019). High
adsorptivity and recycling performance activated
carbon fibers for Cu (II) adsorption. J. Science of The
Total Environment. 700, 134412-134435.
Zheng D. (2019). The clinical study of Xiaoban granule
combined with facial peripheral acupuncture and
Astragalus injection in the treatment of
spleen-deficiency chloasma. J. Journal of Liaoning
University of Traditional Chinese Medicine. 2,
200-203.
Zheng W C. (2019). Research and analysis of chemical
constituents and pharmacological activities of
astragalus, a traditional Chinese medicine. J. Diet
Health Care. 22, 82-83.
Zhu L J, Huang Q Y, Wu J M. (2020). Research progress
on the role of Astragalus and its components in viral
diseases. J. Journal of Southwest Medical University.
5, 100-105.
Zhang L, Xu Q Q, Su C L. (2021). Research progress on
the mechanism and influencing factors of microbial
treatment of heavy metal water pollution. J. Modern
Preventive Medicine. 14, 2631-2634+2663.
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
1030
