
The Roles of Cytoskeleton in Huntington’s Disease
Jiayin Wu
School of Laboratory Medicine and Biotechnology, Southern Medicine University, Guangzhou, China
Keywords: Huntington’s Disease, Kinesin, Dynein, Actinin, Tau.
Abstract: This article indicates partly roles of cytoskeleton in Huntington’s Disease. First, mutant huntingtin may
change the vesicle transport due to the fact that phosphorylation of huntingtin as a switch to regulate the
anterograde/retrograde transport in neurons. Secondly, dysfunction of cellular morphology regulated by
huntingtin, actinin and growth factor may be related in the occurrence of the Huntington’s Disease. Finally,
the alterations of Tau in total level, imbalance of isoforms produced by alternative splicing or by post-
translational modifications imply that Huntington’s Disease and Alzheimer’s Disease may have similar
occurrence mechanism. According to specific description of these, this article hopes to provide new treatments
for Huntington’s Disease or new research orientations for diseases with similar characteristic of Huntington’s
Disease.
1 INTRODUCTION
With the continuous improvement of medical level
and health environment, the average life expectancy
of the population is gradually increasing and some
diseases associated with the aging of the population
are also gradually highlighted, especially in
neurodegenerative diseases such as Alzheimer's
disease and Huntington's disease, which cause huge
economic burden to the society and family.
Huntington’s Disease is a rare genetic autosomal-
dominant neurodegenerative disease that results from
expansion of a CAG trinucleotide repeat (>35) in the
HTT(Huntingtin) gene on the short (p) arm of
chromosome 4 at position 16.3 and first involves
basal ganglia (caudate nucleus and putamen) (Taran
et al. 2020). In neuropathology, Huntington’s Disease
is characterized by neuron death, primarily a
progressive atrophy of the basal ganglia produced by
medium-sized spiny neurons of the striatum, and the
presence of spherical inclusions due to aggregation of
mutant Huntingtin (Htt) in the neuronal nucleus and
cytoplasm (Marta et al. 2020). The clinical
manifestations of this disease are movement
disorders, cognitive decline and a range of somatic
symptoms. Progressive worsening comes with a
bedridden state and patients finally die in 20 years
after the onset of symptoms (Anne-Catherine et al.
2019). Huntington's disease affects approximately 1
in 10,000 people worldwide, and the average age of
onset is between 40 and 50 years (An et al. 2018) .
Although etiology of Huntington’s Disease is
clear, there is no radical treatment for it currently and
the mechanism of its occurrence and development is
unequivocal. In recent years, through the further
understanding of the cytoskeleton, more and more
studies have proposed that the cytoskeleton plays an
important role in the pathogenesis of Huntington's
disease.
This review is designed to integrate the
remarkable recent advances that have led to new
insights into the possible pathogenesis of
Huntington’s disease from the perspective of
cytoskeleton.
2 THE CYTOSKELETON IN
HUNTINGTON’S DISEASE
2.1 Huntingtin, Kinesins and Dyneins
Dynein is a minus end-directed microtubule motor
protein, while kinesin is a plus end-directed
microtubule motor protein. The property of motor
protein that carry vesicles along MTs determines the
correct intracellular transport of membranous
organelles and cargoes. In neurons, kinesins are
responsible for anterograde transprot of vesicles from
572
Wu, J.
The Roles of Cytoskeleton in Huntington’s Disease.
DOI: 10.5220/0011374400003438
In Proceedings of the 1st International Conference on Health Big Data and Intelligent Healthcare (ICHIH 2022), pages 572-576
ISBN: 978-989-758-596-8
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
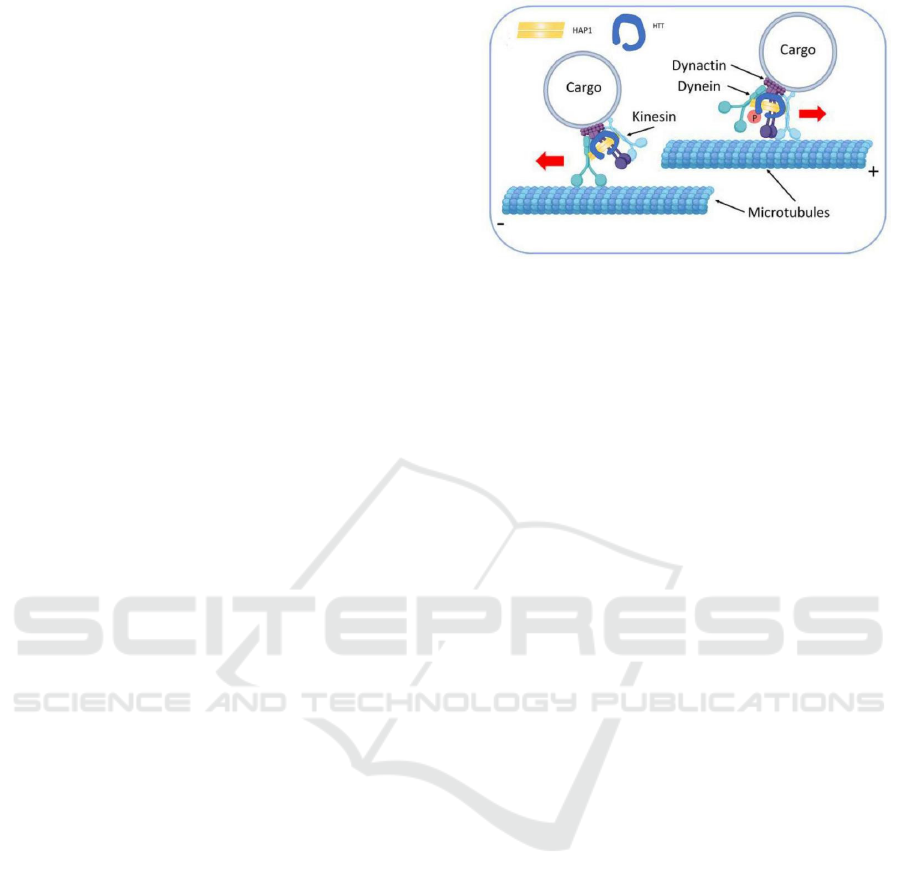
cell centre to the end of neurites. On the contrary,
dyneins are responsible for the retrograde transport of
vesicles and organelles back towards the cell centre.
Dynactin is a dynein activator that binds to both
dynein and MT to form the complex which plays a
critical role in intracellular transport, such as
vesicular transport from the endoplasmic reticulum to
the Golgi and lysosomal motility. Besides, Juliane et
al found that proper localization of huntingtin in the
cell depends on functional dynein/dynactin complex
(Caviston et al. 2007).
Huntingtin, a protein with 3,144 amino acids, is
present in all cells especially neurons and has a large
number of huntingtin partner proteins (Taran et al.
2020) involved in vesicales in neurons and endocytic
vesicles through directly binding to intermediate
chain of dyneins and to huntingtin-associated protein-
1(HAP1). HAP1 interacts with the subunit
p150Glued of dynactin and the heavy chain of
kinesin-1 by its coiled-coil domain. What is more, the
subunit p150Glued of dynactin can also interact with
kinesin-1. The fact that mutant huntingtin can cause
disturbance in axonal transport also indirectly
demonstrates the important role of Huntington
proteins in vesicle transport along microtubules. The
function of huntingtin to facilitate anterograde and
retrograde transport along MT in neurons is regulated
by phosphorylation of huntingtin at serine 421 by Akt
upon IGF-1 stimulation. When huntingtin S421 is
phosphorylated, it recruits kinesin-1 to vesicles and
MTs to facilitate anterograde transport. In contrast,
when huntingtin S421 is not phosphorylated,
huntingtin directly combine with intermediate chain
of dynein to facilitate dynein/dynactin-mediated
retrograde transport of vesicle along the axon like
Figure 1 shows. Furthermore, phosphorylation of
huntingtin does not affect basic characters of MT
such as nucleation, dynamics or stability (Colin et al.
2008).
Although phosphorylation of huntingtin S421
indicates no differences in binding between
huntingtin and HAP1 or between HAP1 and
p150Glued of dynactin or between HAP1 and
kinesin-1, the signal marked the interaction of
p150glued of dynactin and kinesin-1 increase. It
shows that the phosphorylation of huntingtin S421
leads to the stable interaction between dynactin and
kinesin-1 (Colin et al. 2008).
Figure 1. Participation of Huntingtin in vesicle transport
along microtubule (Taran et al. 2020).
Huntingtin as a scaffold for the dynein-dynactin
complex and its phosphorylation determines the
direction of vesicle transport along the microtubules.
2.2 Huntingtin and Actin
Huntingtin is essential for cellular adhesion and for
actin cytoskeleton in response to stimulation of
growth factor, like platelet derived growth factor
(PDGF), to change the normal morphology. The
deletion of huntingtin results in reduced adhesion and
altered morphology. Moreover, mutant huntingtin in
Huntington’s Disease inhibits the regulation of
growth factor stimulation in morphology changes and
increases numbers of vinculin-positive focal
adhesion. Immunoreactivity indicates huntingtin is
localized to actin stress fibers, vinculin-positive
adhesion contacts and membrane ruffles in
fibroblasts. Other studies further to show that first 14
amino acids of a purified fragment of huntingtin have
a direct interaction with actin in vitro. Besides,
huntingtin co-localizes with α-actinin and regulates
its localization at membranes to impact on
maintenance of adhesion and cellular morphologic
changes (Tousley et al. 2019). The Figure 2 shows the
co-localizations between huntingtin with α-actinin-1
in primary human fibroblasts (a and b).
The Roles of Cytoskeleton in Huntington’s Disease
573
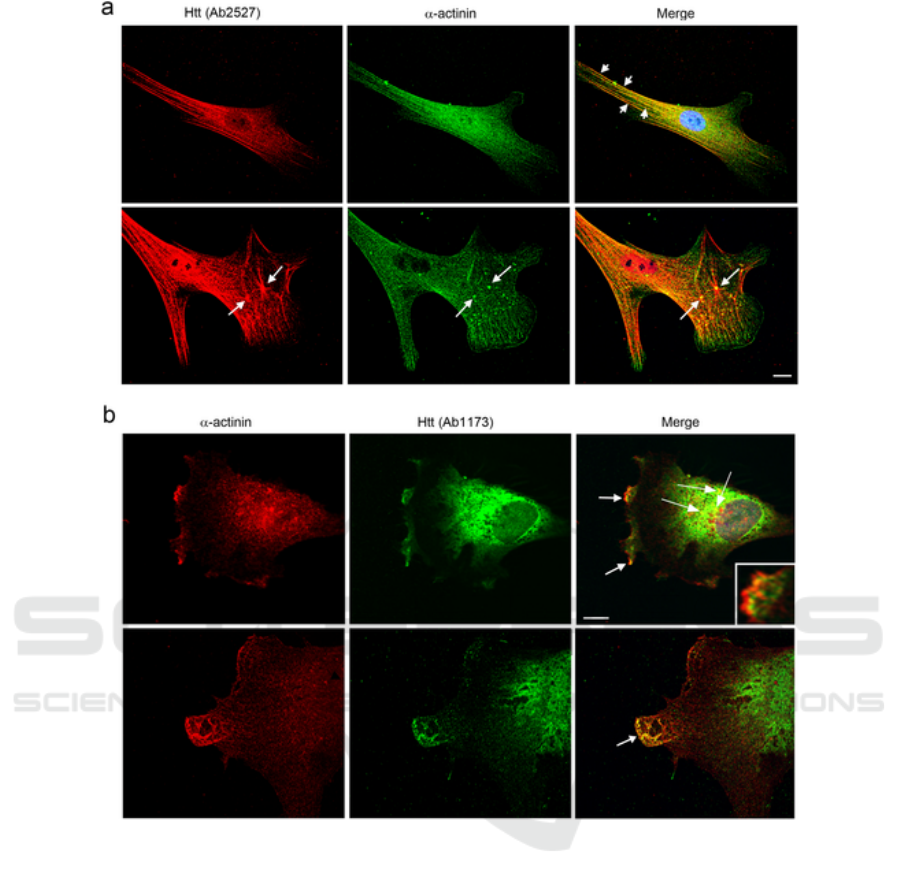
Figure 2: Co-localizations between huntingtin with α-actinin-1 in primary human fibroblasts (a and b) by double-label
immunofluorescence (Tousley et al. 2019).
Red shows huntingtin detected with Ab2527,
green shows α-actinin detected by a monoclonal
antibody and yellow show them co-localize at stress
fibers in serum-starved cells(top, short arrows). (b)
Green shows huntingtin detected with Ab1173, red
shows α-actinin detected by a monoclonal antibody
and yellow shows them co-localize in lamellipodia at
ruffled membranes(top panel, arrows and inset) and
in protrusions at the leading edge of
lamellipodia(bottom panel, arrow). 60x oil objective.
Scale bars = 10µm.
α-actinins are actin binding proteins including
different isoforms, like α-actinin-1 and α-actinin-2
(Tousley et al. 2019). Huntingtin can bind to α-
actinin-1, 2 and 4. The function of α-actinin is to
bundle and crosslink actin filaments in both
contractile and non-contractile cells and link actin
filaments to integrins in focal complexes and focal
adhesions that persists during hierarchical assembly.
Moreover, Actin and α-actinin-2 are concentrated in
the dendritic spines of neurons and play a role in
regulating the morphology of the spine and
stabilizing postsynaptic membrane proteins (Tousley
et al. 2019).
α-actinin-1 and huntingtin co-localized to stress
fibers, membrane and ruffles and lamellar protrusions
in fibroblasts through double-label
immunofluorescence. Proximity ligation assays
indicate that α-actinin-1 have a close interaction with
huntingtin in human fibroblasts and neurons (Tousley
ICHIH 2022 - International Conference on Health Big Data and Intelligent Healthcare
574

et al. 2019). Adelaide et al. found that huntingtin is
responsible for regulation of α-actinin-1 proper
localization on the membrane and combination of
growth factor with actin polymerization at new sites
of adhesion (Taran et al. 2020).
α-actinin-2 interacts with 399-969 amino acids
region of huntingtin. However, full interaction
between α-actinin-2 and huntingtin demands
additional amino acids N-terminal to huntingtin
residue 399. Highly dynamic α-actinin-2 is
concentrated in dendritic spines of neurons in brain,
where it regulates morphology and maturation of
dendritic spines and the transport of the AMPA
subtype of glutamate receptors to post-synaptic.
Huntingtin is also essential for development of
excitatory synapses in the cortical-striatal pathways
in brain. Hence, the interaction between huntingtin
and α-actinin-2 may induce maturation and function
of excitatory synapses on neurons (Taran et al. 2020).
On one hand, IP 3-kinase in respond to growth
factor stimulation activates Akt and produces PI
(3,4,5) P3 and PI (3,4) P2. Activated Akt can
phosphorylate huntingtin at serines 419 and 421 to
interfere the combination between huntingtin and α-
actinin-2 or facilitate their dissociation. On the other
hand, α-actinins can bind to both PI (4,5) P2 and PI
(3,4,5) P3. Huntingtin binds to PI (4,5) P2 with a low
affinity while binds to PI (3,4) P2 and PI (3,4,5) P3
with a high affinity. As the result of the fact that PI
(4,5) P2 is more abundant in the membrane than PI
(3,4,5) P3, α-actinins bind to PI (4,5) P2 with lack of
huntingtin. With the interaction of huntingtin, α-
actinins bind to PI (3,4,5) P3 with a high affinity at
highly specialized regions. These may be pathways
for huntingtin interacting with actin and actinin to
impact the cellular morphology, induced adhesion
and neuronal maturation and they may alter in
Huntington’s Disease. Nevertheless, it still requires
more relevant experiments and studies to prove
(Taran et al. 2020).
It is worth noting that α-actinin-2 and dynein have
the same region S421 of interaction on huntingtin and
their competitive binding to huntingtin perhaps play
a critical role in regulating the transport of vesicle
from MTs to actin filaments (Tousley et al. 2019).
2.3 Alternation of Tau in Huntington's
Disease
Recently, with further studies of Huntington's
Disease, increasing evidences of multiple alterations
of Tau have been found in brains of Huntington's
Disease patients, which implies that abnormal
alterations of Tau is likely to pathogenic for
contributing to the process of Huntington's Disease.
Tau, a microtubule-associated protein, is encode
by the MAPT gene that is located in the long(q) arm
of chromosome 17 at position 21.31 and contains 16
exons. Multiple Tau isoforms is generated by
alternative splicing. For instance, the exclusion of
exon 10 results in 3R isoform of Tau while inclusion
of exon 10 results in 4R isoform. The difference
between 3R and 4R is in the C-terminal region of Tau,
where exon 10 encodes a 31 amino acid sequence and
provides one of the four probable tubulin-binding
repeats. The proportion of Tau isoforms as well as
post-translational modifications such as
phosphorylation and acetylation influence the affinity
of Tau for microtubules (Marta et al. 2020).
The MAPT gene is mainly expressed in neurons
of the central nervous system, which is related to its
function of maintaining neuronal polarity by
regulating microtubule assembly and stability. In
general, Tau is almost exclusively located in the axon
of healthy neurons. The N-terminal region of Tau
binds to plasma membrane components and
participates in the formation of microtubule bundles
as a spacer between microtubule bundles while the C-
terminal region binds to microtubules to regulate their
dynamic assembly. Besides, Tau is involved in the
transport of mRNA and proteins along axons in
intracellular, neurite extension and synaptic plasticity
(Marta et al. 2020).
The alterations of Tau are mainly reflected in
increased total levels, imbalance of isoforms
produced by alternative splicing or by post-
translational modifications and the presence of Tau
nuclear rods (TNRs) or Tau-positive nuclear
indentations (TNIs) (Marta et al. 2020).
Marked by Tau-5 antibody, Tau showed a high
increase in the cortex of Huntington’s Disease
patients while no changes were found in the striatum.
Moreover, elevated Tau total mRNA levels in the
putamen of Huntington’s Disease patients and
attenuate motor abnormalities by Tau knock-down in
an HD mouse model also demonstrate that excess of
Tau contributes to the process of Huntington’s
Disease (Marta et al. 2020).
In Huntington’s Disease patients, another
prominent manifestation of alteration of Tau is an
increase in the ratio 4R-Tau/3R-Tau isoforms, which
is regulated by alternative splicing of exon 10. It
shows an increase of the level of 4R-Tau protein in
the cortex while in the striatum, an increase of the
level of 4R-Tau is accompanied by a decrease of the
level of 3R-Tau. Alternative splicing of exon 10 is
mainly regulated by the family of the serine- and
The Roles of Cytoskeleton in Huntington’s Disease
575

arginine-rich (SR) proteins, especially SRSF6. Post-
translational modifications of SR proteins, like
phosphorylation of serine and threonine residues,
regulate their activity and localization. In general,
phosphorylation of SR proteins is benefit to its
translocation from the cytoplasm to the nucleus. In
Huntington’s Disease, increased levels of
phosphorylation of SRSF6 in the striatum and cortex,
as well as sequestration by mutant Huntingtin
inclusions result in a decrease of SRSF6 activity. In
addition, SRSF6 regulates alternative splicing of
MAP-2, which also alters in Huntington’s Disease
(Marta et al. 2020).
Marking by AT-8 antibody, it found that an
increase of phosphorylation of Tau at Ser396, 404,
199 and Thr205 epitopes in the putamen of
Huntington’s Disease patients. GSK-3 is one of the
main kinases to phosphorylate Tau. Further studies
found that the level and activity of GSK-3 decreased
and the phosphorylation of GSK-3β Ser9, inactive
form of the kinase, increased in Huntington’s
Disease. What’s more, a decrease of phosphatases
(PP1, PP2A and PP2B) implicated in
dephosphorylation of Tau is detected in the R6/2
mouse model. All of these implicate that the reason
of Tau hyperphosphorylation in Huntington’s Disease
is the deficiency of dephosphorylation of Tau (Marta
et al. 2020).
Finally, some research has found the presence of
TNIs, known as TNRs, in the striatum and cortex of
Huntington’s Disease patients by antibodies that
recognize 4R-Tau isoforms, 3R-Tau isoforms, total
Tau or Tau oligomers. The ordered filamentous
ultrastructure of TNIs/TNRs fills neuronal
invaginations of nuclear envelope and partially or
totally span the neuronal nuclear space (Marta et al.
2020).
3 CONCLUSIONS
All in all, this review describes the roles of
cytoskeleton in Huntington's Disease from three
aspects. When huntingtin S421 is phosphorylated, it
recruits kinesin-1 to vesicles and MTs to facilitate
anterograde transport. In contrast, when huntingtin
S421 is not phosphorylated, huntingtin directly
combine with intermediate chain of dynein to
facilitate dynein/dynactin-mediated retrograde
transport of vesicle along the axon. Huntingtin
interacts with actin and actinin to impact the cellular
morphology, induced adhesion and neuronal
maturation. Moreover, the competitive binding of α-
actinin-2 and dynein to huntingtin S421 may be the
switch on vesicle transport from MT to actin. As a
result, mutant huntingtin in Huntington's Disease
may changes its function above. In addition, the
alterations of Tau like total level, alternative splicing
and post-translational modification suggest that Tau
may be an independent factor that results in
Huntington's Disease, or together with Huntingtin
leads to the occurrence and development of the
disease.
This article through to partly roles of cytoskeleton
in Huntington’s Disease, on the one hand, hopes to
provide new targets for clinical treatment and
method. On the other hand, such as Alzheimer's and
Huntington's part neurodegenerative diseases are
characterized by accumulation of protein misfolding,
which may have a certain similarity in pathogenesis,
this article may provide help for researches on these
diseases with similar characteristics.
REFERENCES
An, P. Lu, B. (2018). The research status of Huntington's
disease. Chinese Journal of Cell Biology, 40(10): 1621-
1632.
Anne-Catherine, B. L. et al. (2019). International
Guidelines for the Treatment of Huntington's Disease.
Frontiers in neurology. (10): 710.
Doi:10.3389/fneur.2019.00710
Caviston, J. P. et al. (2007). Huntingtin facilitates
dynein/dynactin-mediated vesicle transport.
Proceedings of the National Academy of Sciences of
the United States of America, 104(24): 10045-10050.
Doi:10.1073/pnas.0610628104
Colin, E. et al. (2008). Huntingtin phosphorylation acts as
a molecular switch for anterograde/retrograde transport
in neurons. The EMBO journal. 27(15): 2124-2134.
Doi:10.1038/emboj.2008.133
Marta, F. N. and Lucas, J. J. (2020). Altered Levels and
Isoforms of Tau and Nuclear Membrane Invaginations
in Huntington's Disease. Frontiers in cellular
neuroscience. 13. Doi:10.3389/fncel.2019.00574
Taran, A. S. et al. (2020). Huntington's Disease-An Outlook
on the Interplay of the HTT Protein, Microtubules and
Actin Cytoskeletal Components. Cells vol. 9(6): 1514.
Doi:10.3390/cells9061514
Tousley, A. et al. (2019). Huntingtin associates with the
actin cytoskeleton and α-actinin isoforms to influence
stimulus dependent morphology changes. PloS one.
14(2): e0212337. Doi:10.1371/journal.pone.0212337
ICHIH 2022 - International Conference on Health Big Data and Intelligent Healthcare
576
