
Toxoplasma Gondii Infection and Toxoplasmosis in Different Species:
A Review
Jinyi Li
a
College of Life science, Sichuan Agricultural University, Xikang Street, Ya’an City, China
Keywords: Toxoplasmosis, Prevalence, Severity, Diagnosis.
Abstract: Toxoplasmosis is a zoonotic parasitic disease caused by Toxoplasmosis gondii – a protozoan of important
medical and veterinary significance. Its unique sexual cycle (transmission between intermediate and definitive
hosts) and asexual cycle (transmission between intermediate hosts via carnivorism) make transmission and
infection vary according to complex outer and inner environment. IFA, ELISA and qPCR are the mainstream
detection method. In this article, the strengths and weaknesses of diverse commonly used detection
approaches were compared based on their specificity and sensitivity. Though no licensed vaccines have been
applied to human clinical treatment, a few potential antigens for vaccine development are discussed in this
article. Overall, this article aims to summarize the knowledge on the prevalence and effects of infections with
T. gondii in the most important species, including human being and livestock, and how the Toxoplasmosis is
detected and treated.
1 INTRODUCTION
1
Almost every homoeothermic vertebrates worldwide
share a common parasitic zoonosis which caused by
Toxoplasma gondii - an obligate intracellular
protozoan parasite, and it is assumed that T. gondii
have infected over one-third of human population
(Hosseini et al. 2019). Almost every infection
happens in intermediate hosts of T. gondii will
experience three stages: a rapidly dividing invasive
tachyzoite stage which causes tissue destruction and
pathogen proliferation; a slowly localized dividing
stage in CNS or muscle tissue where tachyzoites
convert to tissue cysts or bradyzoites; and eventually
achieve an environmental stage which characterized
by sporozoites (contained within oocysts) shedding
(Gangneux et al. 2012). And for the only definite host
– felines, since they lack enzyme delta-6-desaturase,
which is required during linoleic acid metabolism in
intestine, this deprivation results in systemic linoleic
acid accumulation which is unique in mammals and
makes T. gondii’s sexual development possible.
Feline’s parasited epithelial cells can remove from
original tissue and release oocysts into feline’s feces,
a
https://orcid.org/0000-0003-3777-968X
from which oocysts can spread to wide range of
environment (Weiss et al. 2004).
Ascribing to high vitality of feline’s oocysts,
which can survive and remain parasitic in extreme
condition for several months, we need to pay attention
on the foodborne transmission routes. The
contaminated food includes meat (especially chicken,
lamb, and pork) or shellfish (such as clams, mussels,
and oysters) or unwashed vegetable are highly likely
to be pathogenic when expose to this parasite (Dubey
wt al. 2011). What’s more, for people keeping felines
as family pet, accidentally ingesting oocysts after
cleaning a feline’s litter box is regarded as a highly
possible approach to this parasite. For other people
who do not contact feline directly, ingesting oocyst
via contaminated soil or water can also lead to the
infection of T. gondii. In addition to food
contamination and contact with oocysts via infected
feline, congenital transmission is also regarded as a
fatal route. That is, because women during or just
before pregnancy may not show any symptoms,
without effective pregnancy check-up T. gondii can
remain undetected and possibly result in miscarriage,
stillborn and child disability including mental
retardation, liver damage etc. (Dubey et al. 2004).
524
Li, J.
Toxoplasma Gondii Infection and Toxoplasmosis in Different Species: a Review.
DOI: 10.5220/0011373300003438
In Proceedings of the 1st International Conference on Health Big Data and Intelligent Healthcare (ICHIH 2022) , pages 524-532
ISBN: 978-989-758-596-8
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

2 SEVERITY AND SYMPTOM
It is estimated that the global prevalence of
toxoplasmosis is around 30%, affecting more than 1
million people annually and is ranked as the third
highest burden of foodborne disease, about 17% of
the total foodborne disease burden in the European
Region (WHO 2017). The main symptoms and
severity will be illustrated by diverse species,
including family pets, human beings and farm
animals.
2.1 Toxoplasmosis in Family Pet
Felines are the most commonly known carrier of
T. gondii, and they are the only natural species that
excrete environmentally resistant oocysts. The global
pool of T. gondii seroprevalence suggests positive
samples take up 35% (95% Cl: 32-38%) (Montazeri
et al. 2020). Apart from felines, dogs are also
susceptible to this ubiquitous parasite, even though
the toxoplasmosis is more common in felines than in
dogs which promote the potential cross infection to
the families that keep felines and dogs at the same
time. The high infection rate of T. gondii ascribes to
low morbidity and mortality in these two species.
Meanwhile, the high-efficiency of direct fecal-oral
cycle also contribute to ascending seropositivity
(Lindsay 2009). Furthermore, once infected with T.
gondii, animals will carry toxoplasmic cysts lifetime
long, and worse, their oocyst-shedding periods are
highly unpredictable (Lindsay et al. 2009), which
makes the infected domestic felines of greater
potential danger. Other kind of pets that occupy
smaller niche such as rabbits and birds are also
susceptible to T. gondii and its effect to human being
are often being underestimated. For instance, a cross-
sectional study in Egypt - ELISA was used to analyze
a total number of 150 rabbits for T. gondii IgM and
IgG antibodies, the seropositivity is 40 (26.7%) of
150 rabbits raised in Cairo, Qalyubia, and Sharkia
Governorates (Hassanen et al. 2017). This index
suggests that domestic rabbits has already become a
source of T. gondii infection endemically. Also, in
Japan, serum samples of 337 rabbits were examined
and the seropositivity for T. gondii was 0.89% (3/337)
and 0.29% (1/337) in IgG and IgM ELISA
respectively (Salman et al. 2014). Besides, human
had long been keeping birds as family pet to replace
little mammals for cities’ limitation. To illustrate,
recent research that covers three representative
administrative region in Gansu province suggests the
overall T. gondii seroprevalence was 11.21% (77/687)
(Wang et al. 2014). Therefore, since pet animals have
frequent daily interaction with human beings, both
pet owners and public health workers should develop
more unique and comprehensive prevention plans
against T. gondii.
2.2 Toxoplasmosis in Human
Only occasional inflammatory response in intestinal
system, or temporary fever and muscle soreness are
reported suggest that T. gondii is not a deadly parasite
to most healthy people (Watanabe et al. 2018). Thus,
healthy population generally do not need special
treatment for toxoplasmosis infection. However,
people with immunodeficiency and women during
pregnancy may show some more vital symptoms
which require medical support.
For immunocompromised cohort, especially those
diagnosed with the acquired immunodeficiency
syndrome (AIDS), deficiency of immune system
renders early recognition less quickly and effectively.
Also, current therapy for acute toxoplasmosis is not
applying to clear chronic infection because of the
relatively slow dividing of bradyzoite and
asynchronous growth, which generally result in long–
lived affection (Dunay et al. 2018). After ingesting
bradyzoites, except for possible ileitis and other
severe lesions of digestive system, T. gondii can
spread beyond the gut to deeper tissues through
lymphatics and blood, including spleen, liver, lungs
and gradually reaching the brain which led fatal
infection within CNS (Rinkenberger et al. 2021).
Even though human being can take advantage of a
special mechanism named immune privilege to
protect CNS from infection in most circumstances,
study has shown that to T. gondii and some other
pathogens, this privilege is invalid since they can
across blood-brain barrier (Barragan et al., 2019
Rinkenberger et al. 2021). This explains the high
incidence of focal necrotizing encephalitis due to T.
gondii among patients with AIDS (Ringenberger et
al. 2021). Moreover, not only AIDS patients react this
way to T. gondii. Some clinical cases also suggest that
the clinical findings of immunodeficient patients
without AIDS are similar to those of AIDS, such as
severe combined immunodeficiency (SCID)
Toxoplasma Gondii Infection and Toxoplasmosis in Different Species: a Review
525
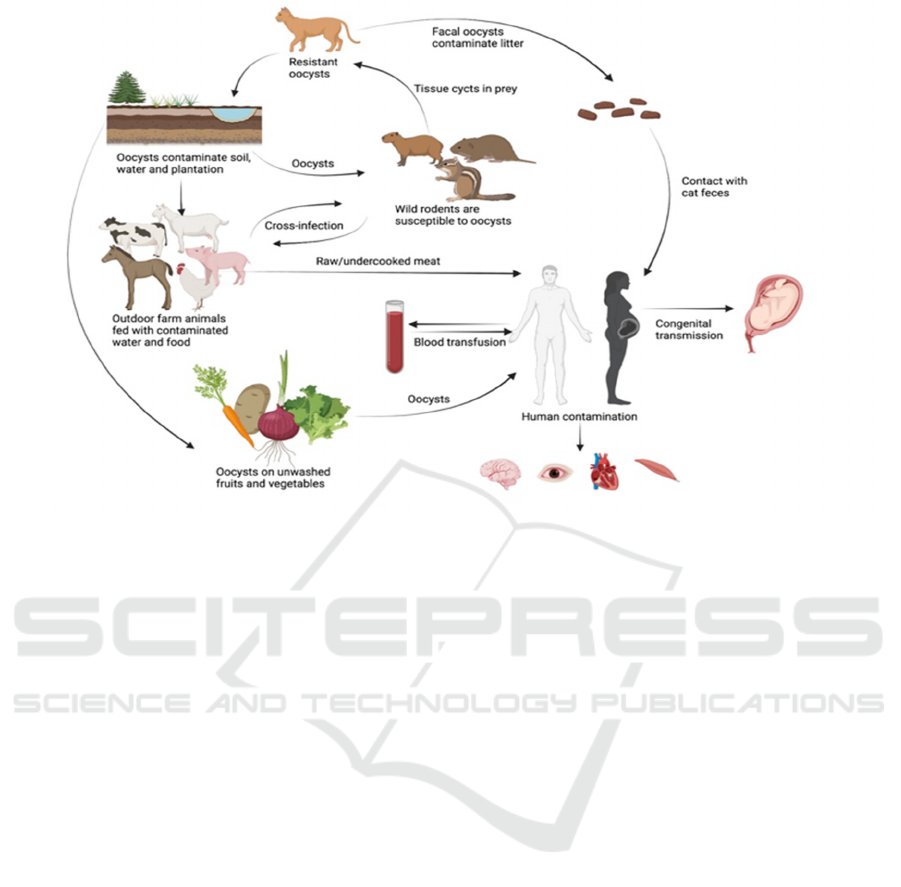
Fig1.
Transmission route of T. gondii
Apart from immunocompromised population,
infection in pregnant women also shows fetal
morbidity and other subclinical neonatal infection
which usually developed into ocular and neurological
sequelae (Fanigliulo et al. 2017). The most commonly
occurred complication of congenital toxoplasmosis
are abortion, stillborn during pregnancy and major
ocular and neurological sequelae, ranging from slight
diminution of vision to retinochoroiditis,
intracerebral calcifications and hydrocephalus after
parturition (Khan et al. 2018). Besides, the risk of
congenital infection and the severity depends on the
gestational age when T. gondii infection occurs – if
the infection occurs in early pregnancy, the chance of
transmission is relatively low. However, in later
phase, the transmission rate is much higher which is
quite common in other laboratory animals. Clinical
cases of chorioretinitis shows that as the gestational
phase develops, the rate of transmission to fetus can
rise from 15 to 65% (Remington et al. 2006).
2.3 Toxoplasmosis in Farm Animals
In addition to felines and human, hazards of
toxoplasmosis to farm animals are observable but
usually overlooked. In outdoor farms, some highly
uncontrollable factors, such as the presence of
infected felines and rodents in farms, as well as the
pollution of the diet, water and soil, may lead to
infection and spread of toxoplasmosis within farm
animals. For example, pork is considered to be a
crucial food resource to human being except for
Muslim area. However, acute toxoplasmosis has long
been reported after ingesting contaminated pig meat.
The seropositive in Estonia is 5.8% (22/382) and the
proportion of seropositive pig in one herd varies
between 0 and 43% (Santoro et al., 2017), the
collection of 89 indoor-reared sows, 128 indoor
finishers and 37 outdoor-reared finisher in Denmark
found that 33.7% sows reared indoors, 3.1% indoor-
reared finishers and 10.8% outdoor reared finishers
were T. gondii seropositive (Kofoed et al. 2017).
Also, many pig producers are unaware that T. gondii
infection in pigs are important, and the public impacts
and risk of T. gondii are uncommon knowledge to
producers which makes the toxoplasmosis spread
more easily (Wagenberg et al. 2020). Overall, both
farm owners and public health workers should
complement specific measure to improve the control
of T. gondii in pigs.
Besides, as an important farm animal, horse meat
and milk are significant food resources in some
region. The prevalence of horse racing also increase
vulnerability to the infection of toxoplasmosis. Even
the clinical toxoplasmosis is very limited now, we still
cannot rule out the possible damage that horses can
rise. The life cycle of T. gondii within horses are
similar to those of other intermediate hosts species –
ICHIH 2022 - International Conference on Health Big Data and Intelligent Healthcare
526

the cyst wall dissolved in horses’ digestive system,
releasing oocyst, and invade intestinal epithelial to
differentiate into tachyzoites. Tachyzoites can form
special vacuoles within host cells, rapidly replicating
tachyzoites multiply cell will lead severe rupture and
possible death. This process generally results in
lesion characterized with necrosis and granuloma.
Besides, this kind of lesion often found in lungs,
livers and spleens of horses, gastrointestinal tract,
central nerve system and heart are also commonly
infected among reported samples (Kimble et al. 2021,
Shaapan et al. 2008). Case study suggest that the
initial infection may happens in caecum, and the
infection of gastric area is limited to slight serositis,
no neurological signs was observed and no lesion was
detected in heart (Kimble et al. 2021). Even though
few clinical horse’s toxoplasmosis has been reported
previously, serological evidence has shown a
worldwide infection of T. gondii among horse.
Except for pigs and horses, sheep and goats are
also common livestock human share high
exposure. Apart from potential danger to infect
human, as worldwide economic livestock providing
functional wool, infection of T. gondii triggers sheep
abortion which is a great loss to sheep owners.
Toxoplasma oocysts picked up from hay or feed tend
be contaminated by fines and cause toxoplasmosis,
and cysts shed by an adolescent cat can infect more
than a thousand ewes (Shaapan et al. 2008).
3 DIAGNOSIS AND TREATMENT
OF TOXOPLASMOSIS
3.1 Diagnosis
Since T. gondii first be discovered in 1908
annamed a year after, its medical and veterinary
importance became widely known in 1939 and
1957 for two famous clinical and veterinary cases
(Cowen et al. 1939). Researchers after that had
strived to figure out the most effective approach to
detect and diagnose this protozoan parasite. Direct
observation of the parasites in stained tissue
sections or other biopsy material is primitive
diagnosis method which is one of the most
favorable detection approaches before modern
molecular biotechnology was invented. But direct
microscopy is used less frequently nowadays
because of the difficulty to obtain specimens. That
is, parasite can only be extract and isolate from
body fluids (e.g. cerebrospinal fluid), which is
complicated and requires considerable labor and
time.
Cellular level detection of Toxoplasma-specific
antibodies is the primary diagnostic method in
laboratories today, for commercially available kits
can tremendously reduce the workload of the
scientists
(Marques et al. 2020). The
immunofluorescence assay (IFA), enzyme-linked
immunosorbent assay (ELISA) tests for IgG and IgM
antibodies are the tests most commonly used today
and had utilized in a large amount of different samples
(Barros et al. 2017)
. The IgG serves as the indicator
of the immune status, while IgM indicates precise
time when infection happens which is particular
important for pregnant women. ELISA’s edge lies on
its hyper-sensitivity, while IFA shows greater
specificity
(Garcia et al. 2006). 300 serum samples
from sheep slaughtered in main abattoir in in Cairo,
Egypt were measured by a comparative serological
examination, the result shows ELISA has high
sensitivity (90.1%) and IFA, which suggest the lowest
sensitivity (80.4%). Conversely, IFA was proved to
have the highest specificity (91.4%), and ELISA
(85.9%)
(Shappan et al. 2008). And the modified
agglutination test (MAT) is uniquely designed to
adapt a large number of different hosts. For example,
When toxoplasmosis abortion storm occurred in a
flock of purebred Suffolk ewes on a farm in Texas,
MAT was functioned to exam the sheep infection
(Edwards et al. 2013). Besides, MAT is also used to
detect peafowls with T. gondii in Yunnan Province
(Tian et al. 2012) and the seroprevalence of domestic
donkeys (Equus asinus) in Durango, Mexico
(Alvarado et al. 2015)
. As well as the seroprevalence
of T. gondii in Harbor Seals (Phaco vitulina) in
Southern Puget Sound, Washington
(Lambourn et al.
2001)
.
In addition, Polymerase Chain Reaction (PCR)
and real-time PCR (qPCR) is another widely used
method to detect T. gondii especially for sampling in
food market, because T. gondii oocysts persist and
remain infective in water and soil for a long time
which result in food contamination extensively
(Marques et al. 2020)
. However, it is noteworthy that
there are imperative but complex preparations before
carrying out PCR or qPCR for identification and
quantification of T. gondii DNA in fruit and
vegetable,
that is, the concentration of oocysts after
washing samples which applies high resolution water
filtration and immunomagnetic separation
(Marchioro er al. 2016)
. qPCR is a reformative
version of traditional PCR, the major advantages of
qPCR is its ability to quantify the infection load of a
clinical specimen, and significantly reduce the chance
for being false positive since traditional direct PCR
Toxoplasma Gondii Infection and Toxoplasmosis in Different Species: a Review
527

have a different accuracy in detecting T. gondii
within different sample sizes regardless of sample
source
(Rani et al. 2020). Besides, for wild animal
and other meat products, PCR analyses shows great
sensitivity and specificity too, manifested by an
excellent discriminating ability for each of the
examined tissues
(Santoro et al. 2019). Heart and
diaphragm sample from wild rabbits in central
Portugal are tested by PCR which separately
amplified the 5′ and 3′ ends of the surface antigen 2
(SAG2) which has been extensively used for
genotyping T. gondii isolates
(Sabaj et al. 2010).
Table 1: Advantages and limitations of diagnosis methods.
Method Advantages Limitations Reference
Microscopy Simple and direct
Slow
Considerable labor
Hard to obtain specimen
28
IFA High sensitivity and
specificity
Labor, time and cost consuming
30, 31
ELISA Low cost and high
sensitivity and specificity
Sensitivity and specificity are highly
dependent on the antigen used
30, 31
MAT Medium sensitivity and
specificity
Low cost
Need well-trained laboratory
technicians
2, 32
PCR and qPCR High sensitivity and
specificity
qPCR reduce PCR’s false
positive specimen
Require concentration of specimen
Possibility of false-possitive
29, 36, 37, 38, 39
LACA Very high specificity in
chicken
Not applicable for all species yet
51
3.2 Treatment
Felines are only definite host of T. gondii and also
very prevailing family pets, it is of greater potential
danger when their owners and other who have access
to them are immunocompromised population or
prepare or during pregnancy. Options for diagnosis in
felines include fecal examination for oocysts and
serologic
testing. It can be difficult to accomplish
eventual diagnosis of toxoplasmosis
(Barrs et al.
2006)
, so if any clinical improvement is not observed
within three days, the diagnosis of toxoplasmosis may
be questioned. Besides, general treatment usually
involves antibiotic treatment, clindamycin is most
commonly used clinically, either alone or in
combination with corticosteroids when severe
inflammation happens in eyes, or even worse, the
central nervous system is involved. Treatment should
ideally be started immediately after diagnosis is made
and continued for several days after signs have
disappeared. Treatment for human toxoplasmosis
especially within immunocompromised population
and congenital transmission route did not achieve
significant breakthrough, while some regular
treatment has become increasingly mature. Infants
with congenital toxoplasmosis, for example, after
maternal seroconversion during the first two
trimesters, spiramycin (9 million IU/d) was
prescribed until birth. In other condition, such as the
third trimester or when the maternal transmission risk
is high, Doctors can prescribe pyrimethamine and
sulfonamide systemically immediately after the
diagnoses are made, and the prescription generally
last around one year
(Kieffer et al. 2008). However,
these drugs are not ideal choices since clinical cases
shows they have various serious side effects: some
hematological abnormalities, bone marrow
suppression etc. and when the parasite encysts in the
tissues, these drugs can hardly eliminate them and
also poorly tolerated
(Antczak et al. 2016). In
addition, the follow-ups were generally limited to two
years the first two years is generally thought to be a
principal end point for diagnosis of a first
retinochoroiditis lesion was decided during this
period. More importantly, the risk for a lesion to
develop in the absence of a previous retinochoroiditis
is weaker after the age of 2 years
(Antczak et al.
2016, Kieffer et al. 2008).
Also, T. gondii infection
is seen as a main pathogen that leads to the death of
immunocompromised people, especially AIDS
ICHIH 2022 - International Conference on Health Big Data and Intelligent Healthcare
528

patients. Because their susceptibility to cancer (e.g.,
lymphoma, leukemia, and myeloma etc.) and the
subsequent regular antitumor treatment make them
have better opportunity to reactivate latent T. gondii
infection. Recent study has shown that sulfonamides,
in conjunction with Pyrimethamine (PYR) are
mainstream in toxoplasmosis treatment nowadays,
but AIDS patients are unable to tolerate this
treatment. Also, there have been several failed reports
on the long-term treatment of toxoplasmosis among
AIDS patients
(Luft et al. 1992). That is, even great
progress has made to understand the pathogenesis of
T. gondii and more effective medicine has been
developed. Medicines we currently prefer use to
against toxoplasmosis still show some side effects,
thus prolonged courses are required, and both effect
and side effect may be fluctuated by differences
among the virulence of T. gondii strains found around
the world
(Alday et al. 2017).
4 DISCUSSION
Apart from regular detection methods, there are
significant number of updated discoveries in
detection method of T. gondii serving as an extension
of traditional means. For example, MAT was found to
have greater sensitivity and specificity compared with
PCR and qPCR. For instance, in the study detecting
the overall T. gondii prevalence from Phillip Island,
Australia, the 95% confidence interval of qPCR and
MAT are 72.6–85.0 and 84.6–95.8, respectively
(Adriaanse et al. 2020)
. Moreover, some targeted
novel detection method has now been studied to
detect T. gondii in certain species. One apt illustration
involves luciferase-linked antibody capture assay
(LACA), LACA is detected to have an unexpected
great sensitivity (90.5%) and specificity (95.4%)
when testing chicken with T. gondii and some other
pathogens, suggesting a better performance in special
species compared with its conventional counterparts
(Duong et al. 2020)
. Closer scrutiny to those
evolutionary detection methods reveals that they are
generally multidisciplinary approaches, and it is this
very character that makes them more productive than
their conventional counterparts. For example, MAT in
conjunction with Bayesian latent class (BLC)
analysis, which is a computationally model in
Statistic, forms a new method to determine sensitivity
and specificity we researchers faced with an absence
of sufficient reference samples
(Adriaanse et al.
2020)
. LACA takes advantage of a novel luciferase-
linked capture antibody platform by using
recombinant nanoluciferase conjugated GRA8
antigen is a perfect example of utilizing molecular
science
(Rezaei et al. 2019). Thus, the prosperity of
interdisciplinary detection of T. gondii should be
developed in the future studies.
Although the life circle and pathogenetic
mechanisms of T. gondii has already been revealed,
we do not have any vaccine for human been licensed
by now
(Rezaei et al. 2019). However, experiment in
model animals suggests great progress in vaccine
development. Some essential antigens have been
discovered: dense granule antigens (GRAs), rhoptry
antigens (ROPs), surface antigens (SAGs) and
microneme antigens (MIC
). Among them, with active
involvement in parasites virulence, survival and
replication processes, serving as major proteins of the
excretory secretory antigens, GRAs are considered as
a predominant vaccine candidate and in recent
studies, common laboratory animals such as ewe,
mouse, pig and sheep were used for a wide variety of
experiments to evaluate humoral responses
(Rezaei
et al. 2019)
. Researchers deem GRA7 the most
competitive candidate for vaccine experiment since
TgGRA7 has been found in almost every infectious
stages of parasite, Tests to evaluate immune response
of different GRAs in little mammals such as sheep
indicates GRA7 present greater IFN
level within
whole experiment process
(Hiszczyńska-Sawicka et
al. 2011)
. ROPs takes part in the cell invasion and the
formation of parasitophorous vacuole (PV) both
process are essential for survival of T. gondii in host
cells
(Boothroyd et al. 2008), recent study in mice
indicates multi-epitope ROP8 DNA vaccine can
induce strong humoral and cellular responses and
extends the survival time
(Foroutan et al. 2020).
Furthermore, it is noteworthy that a nonselective
beta-adrenoceptor antagonist – propranolol as
adjuvant in association with tachyzoite SAG-1 as an
antigen can leads to stronger immune reactions to Th1
and cellular immunity
(Abasi et al. 2019). Since MIC
plays a crucial role in entering host cells and parasites
gliding motion, an increasing number of articles
about MICs suggests their growing
importance as
vaccine candidates that can induce intense immune
responses against toxoplasmosis.
As the improving consumer demand for “animal-
friendly” or “organic” animal products, farm animals
have an increasing opportunity to be infected by
T. gondii’s oocysts because of the better chance of
outdoor activities. More importantly, grazing animals
raised by nomadic people on pasture are directly
threatened by infected wild felines and rodents, at the
same time the uncovered feed and the contaminated
soil and water are dangerous infection source too.
One apt illustration involves horses and sheep: the
Toxoplasma Gondii Infection and Toxoplasmosis in Different Species: a Review
529

overall prevalence of T. gondii in the horses was
5.15% (5/97) for Jilin Province, China, 5.55% (3/54)
for Liaoning Province, China, and 7.50% (6/80) for
Xinjiang Uygur Autonomous Region, China (Ren
2019). And the regions in Qinghai Province, specific
IgG against T. gondii are detected in 21.33% (95%
confidence) (Liu 2015); a cross-sectional study
carried out in 319 random sheep in Northwestern Rio
Grande do Sul State, Brazil shows 70.2% (224/319)
T. gondii detection (Consalter et al. 2019). Although
many farm owners are aware of potential risk and
consequences of T. gondii infection within pigs, the
more profound and extensive risks regarding of
public health are not yet comprehensive knowledge to
all farm owners, even in developed countries, such as
Dutch. Furthermore, farm owners vary in motivation
and capability to control and address T. gondii
infections. We should warn farm owners that they
should not expect some tangible symptoms on
livestock when toxoplasmosis infection happens,
because T. gondii infection do not have sensible
performance in most circumstances. Moreover, we
should suggest farm owners to be more conscientious
about stray cats and rodents living in the farms and
call for their consciousness about farm health
condition as well as breeding method.
5 CONCLUSIONS
T. gondii as a world-widely distributed parasite
disease can severely infect pregnant women and
people with immune deficiency. Since no apparent
symptom in healthy individuals and other
intermediate hosts, which makes T. gondii hard to be
detected. So far, PCR and qPCR are utilized more
commonly in large-scale detection, while
ELISA is
wildly accepted in clinical detection. Other targeted
detecting methods such as LACA for the specific
species are urgently needed. When individual get
infected by T. gondii, the common treatment is
antibiotic treatment to control the proliferative
tachyzoite cycle and no specific medicines for all
stages of T. gondii development. Additionally, the
cases of farm animals infected by T. gondii, such as
pigs, horses, and sheep, are gradually increasing and
causing economic loss. Therefore, the spread of T.
gondii in farm should be paid attention. Since there
are growing number of vulnerable groups and
worldwide distribution infection cases, the infection
of T. gondii is worth heeding.
REFERENCES
Abasi, E., Shahabi, S., Golkar, M., Khezri, P., &
Mohammadzadeh Hajipirloo, H. (2019). Evaluation of
Immunogenic Effect of Toxoplasma gondii
RecombinantSAG-1 Antigen with Propranolol as
Adjuvant in BALB/c Mice. Advanced Pharmaceutical
Bulletin, 9(4)
Abou Elez, R. M., Hassanen, E. A., Tolba, H. M., &
Elsohaby, I. (2017). Seroprevalence and risk factors
associated with Toxoplasma gondii infection in
domestic rabbits and humans. Veterinary Parasitology:
Regional Studies and Reports, 8, 133-137.
Alday, H., & Doggett, J. (2017). Drugs in development for
toxoplasmosis: Advances, challenges, and current
status. Drug Design, Development and Therapy,
Volume11, 273-293.
Alvarado-Esquivel, C., Alvarado-Esquivel, D., & Dubey, J.
P. (2015). Prevalence of toxoplasma gondii antibodies
in domestic donkeys (equus asinus) in Durango,
Mexico slaughtered for human consumption. BMC
Veterinary Research, 11(1), 6.
Antczak, M., Dzitko, K., & Długońska, H. (2016). Human
toxoplasmosis–searching for novel chemotherapeutics.
Biomedicine & Pharmacotherapy, 82, 677-684.
BARRS, V., MARTIN, P., & BEATTY, J. (2006).
Antemortem diagnosis and treatment of toxoplasmosis
in two cats on cyclosporin therapy. Australian
Veterinary Journal, 84(1-2), 30-35.
BARRS, V., MARTIN, P., & BEATTY, J. (2006).
Antemortem diagnosis and treatment of toxoplasmosis
in two cats on cyclosporin therapy. Australian
Veterinary Journal, 84(1-2), 30-35.
Boothroyd, J. C., & Dubremetz, J. (2008). Kiss and spit:
The dual roles of Toxoplasma rhoptries. Nature
Reviews Microbiology, 6(1), 79-88.
Cong, W., Meng, Q., Song, H., Zhou, D., Huang, S., Qian,
A., . . . Zhu, X. (2014). Seroprevalence and genetic
characterization of Toxoplasma gondii in three species
of pet birds in China. Parasites & Vectors, 7(1).
Consalter, A., Frazão-Teixeira, E., Dubey, J. P., Zanella, E.
L., Da Silva, A. F., De Souza, G. N., & Ferreira, A. M.
(2019). Epidemiological investigation of Toxoplasma
gondii infections in commercial sheep flock in an
endemic area for ocular toxoplasmosis in Southern
Brazil. Acta Parasitologica, 64(3), 514-519.
Dubey, J. (1998). Refinement of pepsin digestion method
for isolation of Toxoplasma gondii from infected
tissues. Veterinary Parasitology, 74(1), 75-77.
Dubey, J. P., Ferreira, L. R., Martins, J., & Jones, J. L.
(2011). Sporulation and survival of Toxoplasma gondii
oocysts in different types of commercial cat litter.
Journal of Parasitology,
97(5), 751-754.
Dubey, J., Lindsay, D. S., & Lappin, M. R. (2009).
Toxoplasmosis and other intestinal coccidial infections
in cats and dogs. Veterinary Clinics of North America:
Small Animal Practice, 39(6), 1009-1034.
Dupont, C. D., Christian, D. A., & Hunter, C. A. (2012).
Immune response and immunopathology during
toxoplasmosis. Seminars in Immunopathology, 34(6),
ICHIH 2022 - International Conference on Health Big Data and Intelligent Healthcare
530

793-813.
Edwards, J. F., & Dubey, J. (2013). Toxoplasma gondii
abortion storm in sheep on a Texas farm and isolation
of mouse virulent atypical genotype T. Gondii from an
aborted lamb from a chronically infected ewe.
Veterinary Parasitology, 192(1-3), 129-136.
Fanigliulo, D., Marchi, S., Montomoli, E., & Trombetta, C.
M. (2020). Toxoplasma gondii in women of
childbearing age and during pregnancy: Seroprevalence
Study in central and Southern Italy from 2013 to 2017.
Parasite, 27, 2.
Foroutan, M., Ghaffarifar, F., Sharifi, Z., & Dalimi, A.
(2020). Vaccination with a novel multi-epitope ROP8
DNA vaccine against Acute Toxoplasma gondii
infection induces strong B and T cell responses in mice.
Comparative Immunology, Microbiology and Infectious
Diseases, 69, 101413.
Garcia, J. L., Navarro, I. T., Vidotto, O., Gennari, S. M.,
Machado, R. Z., Da Luz Pereira, A. B., & Sinhorini, I.
L. (2006). Toxoplasma gondii: Comparison of a
rhoptry-elisa with Ifat and mat for antibody detection in
Sera of experimentally infected pigs. Experimental
Parasitology, 113(2), 100-105.
GILBERT, R. E. (2000). Is ocular toxoplasmosis caused by
prenatal or postnatal infection? British Journal of
Ophthalmology, 84(2), 224-226.
Hiszczyńska-Sawicka, E., Olędzka, G., Holec-Gąsior, L.,
Li, H., Xu, J. B., Sedcole, R., . . . Stankiewicz, M.
(2011). Evaluation of immune responses in sheep
induced by DNA immunization with genes encoding
GRA1, GRA4, GRA6 and gra7 antigens of Toxoplasma
gondii. Veterinary Parasitology, 177(3-4), 281-289.
Hosseini, S. A., Amouei, A., Sharif, M., Sarvi, S., Galal, L.,
Javidnia, J., . . . Daryani, A. (2018). Human
toxoplasmosis: A systematic review for genetic
diversity of Toxoplasma gondii in clinical samples.
Epidemiology and Infection, 147.
Khan, K., & Khan, W. (2018). Congenital toxoplasmosis:
An overview of the neurological and ocular
manifestations. Parasitology International, 67(6), 715-
721.
Kieffer, F., Wallon, M., Garcia, P., Thulliez, P., Peyron, F.,
& Franck, J. (2008). Risk factors for retinochoroiditis
during the first 2 years of life in infants with treated
congenital toxoplasmosis. Pediatric Infectious Disease
Journal, 27(1), 27-32.
Kim, K., & Weiss, L. M. (2004). Toxoplasma gondii: The
model apicomplexan. International Journal for
Parasitology, 34(3), 423-432.
Kimble, K. M., Gomez, G., Szule, J. A., Dubey, J. P.,
Buchanan, B., & Porter, B. F. (2021). Systemic
toxoplasmosis in a horse. Journal of Comparative
Pathology, 182, 27-31.
Lambourn, D. M., Jeffries, S. J., & Dubey, J. P. (2001).
Seroprevalence of Toxoplasma gondii in harbor seals
(phoca vitulina) in southern Puget sound, Washington.
The Journal of Parasitology, 87(5), 1196.
Lindsay, D., & Dubey, J. (2007). Toxoplasmosis in wild and
domestic animals. Toxoplasma Gondii, 133-152.
Luft, B. J., & Hafner, R. (1990). Toxoplasmic encephalitis.
AIDS, 4(6), 593-596.
Marchioro, A. A., Tiyo, B. T., Colli, C. M., De Souza, C. Z.,
Garcia, J. L., Gomes, M. L., & Falavigna-Guilherme,
A. L. (2016). First Detection oftoxoplasma gondiidna
in the fresh leafs of vegetables in South America.
Vector-Borne and Zoonotic Diseases, 16(9), 624-626.
Marques, C. S., Sousa, S., Castro, A., & Da Costa, J. M.
(2020). Detection of toxoplasma gondii oocysts in fresh
vegetables and Berry Fruits. Parasites & Vectors, 13(1).
Matta, S. K., Rinkenberger, N., Dunay, I. R., & Sibley, L.
D. (2021). Toxoplasma gondii infection and its
implications within the Central Nervous System.
Nature Reviews Microbiology, 19(7), 467-480.
Montazeri, M., Mikaeili Galeh, T., Moosazadeh, M., Sarvi,
S., Dodangeh, S., Javidnia, J., . . . Daryani, A. (2020).
The global serological prevalence of Toxoplasma
gondii in felids during the last five decades (1967–
2017): A systematic review and meta-analysis.
Parasites & Vectors, 13(1).
Rani, S., & Pradhan, A. K. (2020). Evaluation and meta-
analysis of test accuracy of direct PCR and bioassay
methods for detecting Toxoplasma gondii in meat
samples. LWT, 131, 109666.
Remington, J. S., McLeod, R., Thulliez, P., & Desmonts, G.
(2006). Toxoplasmosis. Infectious Diseases of the Fetus
and Newborn Infant, 947-1091.
Ren, W., Zhang, X., Long, C., Zhao, Q., Cheng, T., Ma,
J., . . . Ni, H. (2019). Molecular detection and genetic
characterization of Toxoplasma gondii from horses in
three provinces of China. Vector-Borne and Zoonotic
Diseases, 19(9), 703-707.
Rezaei, F., Sharif, M., Sarvi, S., Hejazi, S. H., Aghayan, S.,
Pagheh, A. S., . . . Daryani, A. (2019). A systematic
review on the role of gra proteins of Toxoplasma gondii
in host immunization. Journal of Microbiological
Methods, 165, 105696.
Robert-Gangneux, F., & Dardé, M. (2012). Epidemiology
of and diagnostic strategies for toxoplasmosis. Clinical
Microbiology Reviews, 25(3), 583-583.
Sabaj, V., Galindo, M., Silva, D., Sandoval, L., &
Rodríguez, J. C. (2009). Analysis of toxoplasma gondii
surface antigen 2 gene (SAG2). relevance of genotype
I in clinical toxoplasmosis. Molecular Biology Reports,
37(6), 2927-2933.
Salman, D., Oohashi, E., Mohamed, A. E., A. E., Okada, T.,
& Igarashi, M. (2014). Seroprevalences of toxoplasma
gondii and neospora caninum in pet rabbits in Japan.
Journal of Veterinary Medical Science, 76(6), 855-862.
Santoro, A., Tagel, M., Must, K., Laine, M., Lassen, B., &
Jokelainen, P. (2017). Toxoplasma gondii
seroprevalence in breeding pigs in Estonia. Acta
Veterinaria Scandinavica, 59(1).
Santoro, M., Viscardi, M., Sgroi, G., DʼAlessio, N.,
Veneziano, V., Pellicano, R., . . . Fusco, G. (2019). Real-
time PCR detection of Toxoplasma gondii in tissue
samples of wild boars (sus scrofa) from southern Italy
reveals high prevalence and parasite load. Parasites &
Vectors, 12(1).
Schlüter, D., & Barragan, A. (2019). Advances and
challenges in understanding cerebral toxoplasmosis.
Toxoplasma Gondii Infection and Toxoplasmosis in Different Species: a Review
531

Frontiers in Immunology, 10.
Shaapan, R., El-Nawawi, F., & Tawfik, M. (2008).
Sensitivity and specificity of various serological tests
for the detection of Toxoplasma gondii infection in
naturally infected sheep. Veterinary Parasitology,
153(3-4), 359-362.
Tian, Y., Dai, F., Huang, S., Deng, Z., Duan, G., Zhou,
D., . . . Zou, F. (2012). First report of toxoplasma gondii
seroprevalence in peafowls in Yunnan Province,
southwestern China. Parasites & Vectors, 5(1).
Watanabe, P. D., Trevizan, A. R., Silva-Filho, S. E., Góis,
M. B., Garcia, J. L., Cuman, R. K., . . . Nogueira de
Melo, G. D. (2018). Immunocompetent host develops
mild intestinal inflammation in acute infection with
Toxoplasma gondii. PLOS ONE, 13(1).
Wolf, A., Cowen, D., & Paige, B. (1939). Human
toxoplasmosis: Occurrence in infants as an
encephalomyelitis verification by transmission to
animals. Science, 89(2306), 226-227.
ICHIH 2022 - International Conference on Health Big Data and Intelligent Healthcare
532
