Molecular Mechanisms of Osteoporosis: A Road Map for
Osteoporosis Therapeutics
Chao Dai
Zhejiang University-University of Edinburgh Institute and Zhejiang University School of Medicine, Zhejiang University,
310000, Hangzhou, China
Keywords: Osteoporosis, Molecular Mechanisms, Treatment, Mesenchymal Stem Cells.
Abstract: Osteoporosis is widely spread throughout the world and becomes a serious public health problem. It is mainly
caused by the imbalance of the bone remodeling process, that bone resorption (led by osteoclasts) overwhelms
bone formation (led by osteoblasts). This review summarizes several important molecular mechanisms in
osteoporosis and their corresponding treatment. Among them, drug therapy and cell therapy are two major
therapies that are commonly used. Drug therapy is clinically mature, but there are some side effects that cannot
be used for a long time. Cell therapy can cure osteoporosis, and it does little harm to the human body.
However, cell therapy is clinically immature, and there may be ethical issues. More detailed molecular
mechanisms require further investigation and could provide a promising direction for osteoporosis treatment
and prevention.
1 INTRODUCTION
Osteoporosis has become a common public health
concern in human society, especially in aged people.
It is a chronic metabolic bone disease defined by low
bone mineral density (BMD), which could further
cause increased bone fragility and risk of bone
fracture. More than 200 million people are affected
by osteoporosis and the number would continuously
ascend because of an aging population and prolonged
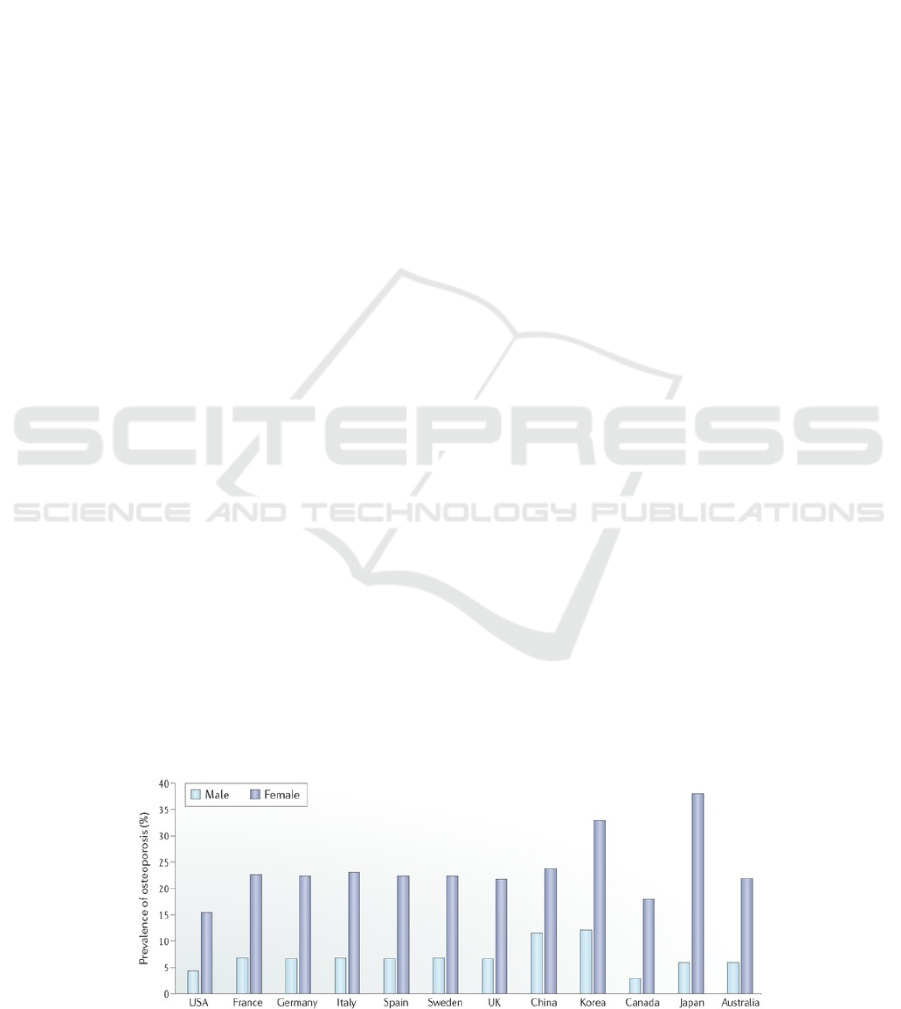
life in human society (Macias 2020). Figure 1 shows
the prevalence of osteoporosis in aged populations in
several countries, indicating a huge number of
distribution of the disorder. Currently, drug therapies
are the most useful clinical intervention strategies for
osteoporosis patients. However, medicine would still
have various adverse effects that may be harmful to
people's health. More effective therapies are therefore
needed to treat osteoporosis. Molecular mechanisms
could specifically indicate the target for the cause of
diseases. Numerous intervention strategies developed
from molecular mechanisms have shown significant
effect in clinic. Molecular factors play a key role in
the study of osteoporosis, but few were clearly
understood. Figuring out the mechanisms of
osteoporosis development would provide promising
therapeutic directions and molecular mechanisms
exhibit their great significance. This review aims to
overview several molecular mechanisms of
osteoporosis, as well as discuss their current and
further therapeutic approaches accordingly.
Figure 1: (derived from Yang, T.L., et al.) Prevalence of osteoporosis in populations of age 50 years and older in selected
countries.
500
Dai, C.
Molecular Mechanisms of Osteoporosis: A Road Map for Osteoporosis Therapeutics.
DOI: 10.5220/0011372900003438
In Proceedings of the 1st International Conference on Health Big Data and Intelligent Healthcare (ICHIH 2022), pages 500-507
ISBN: 978-989-758-596-8
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All r ights reserved
2 OVERVIEW
Osteoporosis occurs due to the imbalance of bone
homeostasis. Osteoclasts clear away bone tissue (bone
resorption) while osteoblasts form bone tissue (bone
formation) to maintain the homeostasis process.
Increased bone resorption or reduced bone formation
would lead to osteoporosis (Yang, et al. 2020). A large
number of molecules could take part in osteoporosis
development and therefore, lead to various molecular
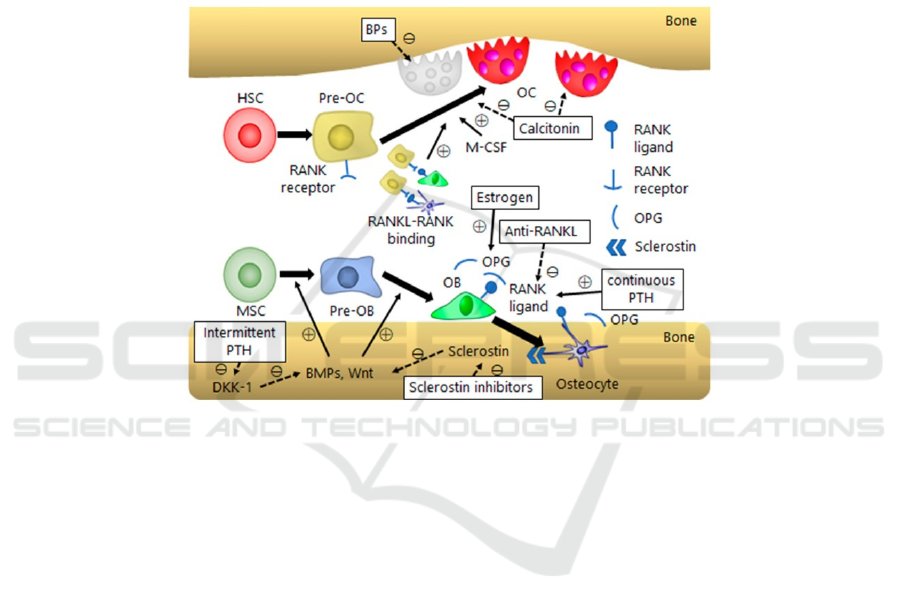
mechanisms. Figure 2 shows a schematic diagram of
bone homeostasis and molecular components
involved, including mesenchymal stem cell (MSC),
parathyroid hormones (PTH), calcitonin and estrogen.
Nevertheless, most molecular mechanisms focus on
the bone homeostasis process, affecting bone
resorption or bone formation to cause osteoporosis.
For instance, MSC, PTH and estrogen have important
roles in modulating osteoblasts, while calcitonin is
revealed to have inhibitory function in osteoclasts.
Consequently, here the author will detailly review
molecular mechanisms that are related to the bone
homeostasis.
Figure 2: (derived from Ukon, Y., et al.) Schematic summary of bone homeostasis and related molecular mechanisms. BP,
bisphosphonate; DKK, dickkopf; M-CSF, monocyte/macrophage colony-stimulating factor; HSC, hematopoietic stem cell;
OB, osteoblast; OC, osteoclast.
2.1 Mesenchymal Stem Cells (MSCs) in
Osteoporosis
2.1.1 Mechanism MSCs Are Progenitors of
Adipocytes and Osteoblasts
They were primarily found in the bone marrow and
therefore, considered to be involved in bone
regeneration. Similar to bone homeostasis, the
differentiation of MSCs to adipocytes and osteoblasts
is also a balanced process. Ascending adipocytes
differentiation could significantly suppress
osteoblasts differentiation. Subsequently, fewer
osteoblasts generation could induce osteoporosis
development. Three main molecular mechanisms
regulate the differentiation of MCSs: signaling
pathways, microRNAs and transcription factors (Hu,
et al. 2018). Two signaling pathways have been
demonstrated and are well-understood in past
decades. The bone morphogenic protein (BMP)
signaling pathway could induce specific complex
formation with other molecules, translocating into the
nucleus and promoting the osteogenic gene
expression. A previous study also showed that a high
concentration of BMP could cause osteoblasts
differentiation while a low concentration of BMP
could lead to adipocytes differentiation. Another
important signaling pathway of MSCs differentiation
is the Wnt signaling. Like BMP signaling, Wnt
signaling also has positive effects on the osteogenic
process. Wnt signaling could inhibit the
phosphorylation of β-catenin. Unphosphorylated β-
catenin in the cell nucleus could facilitate osteoblasts'
differentiation to form bone tissue.
MicroRNAs (miRNAs) are short non-coding
RNA sequences but are widely involved in molecular
and cellular activities. In the MSC differentiation
process, they are found to be anti-osteogenic. Some
miRNAs could indirectly regulate transcription
factors to control MSC differentiation while some
Molecular Mechanisms of Osteoporosis: A Road Map for Osteoporosis Therapeutics
501
miRNAs could directly suppress osteogenic gene
expression. Notably, in different types of cells
miRNAs exhibited opposite regulatory effects on
MSC differentiation, and most detailed mechanisms
of miRNAs remain unexplored.Distinct from two
former molecular mechanisms, transcription factors
are revealed to directly modulate osteogenic or
adipogenic gene expression with specified
mechanisms. As their name suggests, transcription
factors are series of molecular factors that regulate
gene expression via regulating the transcription
process. Runt-related transcription factor 2 (runx2)
and osterix significantly participate in osteogenic
gene expression. In runx2-deficient cells and osterix-
deficient mice, MSCs could not differentiate into
osteoblasts and express adipogenic phenotypes. In
contrast, peroxisome proliferation-activated receptor
γ (PPARγ) could facilitate adipogenic differentiation
in MSCs, indicating that both positive and negative
transcription modulators play key roles in MSC
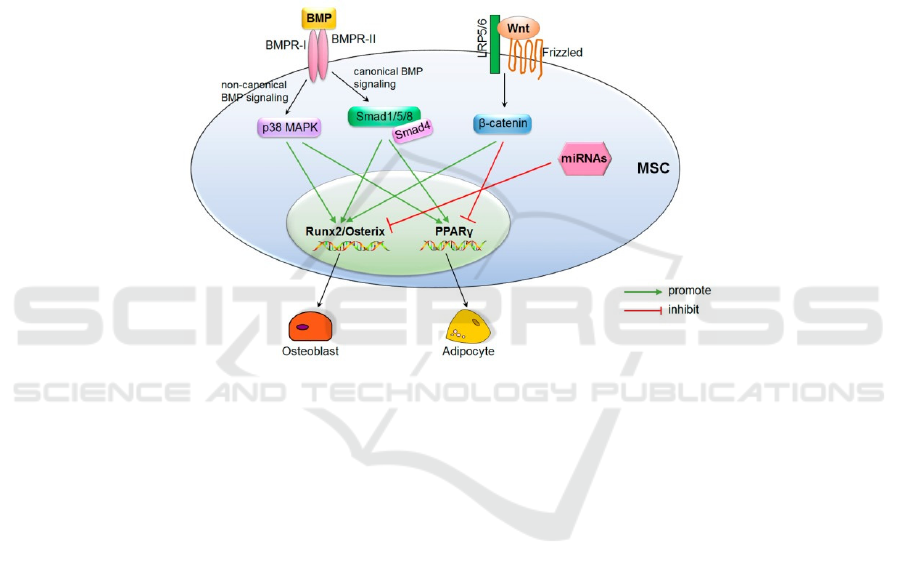
differentiation. Taken together, molecular
mechanisms of MSCs are briefly summarized in
figure 3. Both signaling pathways and microRNAs
are upstream of transcription factors. Involved with
other molecules, all three mechanisms could
significantly regulate the differentiation of MSC.
Figure 3: (derived from Hu, L., et al.) Schematic summary of molecular mechanisms in MSC differentiation.
2.1.2 Current and Future Therapeutic
Applications
The differentiation of MSCs to adipocytes instead of
osteoblast is important in osteoporosis development.
Based on molecular mechanisms of MSC
differentiation, current therapeutic strategies are
mainly focusing on inducing the osteogenic
differentiation process of MSCs.
Two types of MSCs are now conducted in clinical
trials. They are derived from bone marrow tissue and
adipose tissue, respectively. Both of them prefer to
differentiating into osteoblasts, confirmed by
transplantation experiments in previous animal
model research (including mice and rabbits). Though
these MSCs have promising therapeutic potential in
vivo, their applications in clinical trials have not
come up with positive results yet. This may be due to
some ethical problems because some clinical trials
ended up halfway without any reported results.
Moreover, the differences (such as microenvironment
and cellular interaction) between the human body and
animals may also hinder the normal function of
transplanted MSCs.
For future therapies, according to the molecular
mechanisms of the MSC differentiation process in
osteoporosis, more available treatment could be
performed even though there is no reliable
experimental evidence yet. For instance, specific
drugs could be developed to promote the level of
transcription factors in the human body. Besides, by
gene-editing methods, osteogenic-inducing miRNA
sequences could be added to the MSC genome. Such
engineered cells may supply the loss of osteoblasts in
osteoporosis patients.
2.2 Calcium and Parathyroid
Hormones (PTH) in Osteoporosis
2.2.1 Mechanism Calcium Absorption is
Critical in Preventing Osteoporosis
A reduced level of calcium in serum could trigger an
increased secretion level of parathyroid hormones
ICHIH 2022 - International Conference on Health Big Data and Intelligent Healthcare
502

(PTH), which would induce bone resorption. In other
words, if serum has less calcium than normal level, it
will try to gather more calcium from bone tissue by
PTH. Then increased bone resorption induces
osteoclasts differentiation and suppresses osteoblasts
differentiation (bone formation), leading to
osteoporosis.
2.2.2 Current and Future Therapeutic
Applications
Teriparatide, which consists of part of amino acids
sequence of PTH, exhibited positive function in bone
marrow density increase. It is an anti-osteoporosis
drug. In clinical trials teriparatide significantly
decreased the risk of bone fracture. However, its
underlying mechanism which accounts for bone
homeostasis remains unknown. Genetic studies via
mouse model suggested that various molecules
include Fos, Runx2, and insulin-like growth factor
are important in bone formation and closely related to
the action of PTH. Also, in PTH-induced bone
regeneration, the SOST gene and its protein product
sclerostin are revealed to mainly express in
osteocytes. Additionally, another PTH-related
peptide abaloparatide is used to treat osteoporosis in
the US, with the function of increasing BMD and
decreasing osteoporotic fractures (Tanaka 2019).
Similarly, the future direction of osteoporosis
therapies could be based on exploring more PTH-
related peptides isoforms, since several of them have
been proved to be effective in treating osteoporosis.
Other methods to increase the calcium absorption in
serum could also help to decrease bone resorption and
therefore, avoid osteoporosis.
2.3 Calcitonin in Osteoporosis
2.3.1 Mechanism Calcitonin
Mechanism Calcitonin is a type of hormone secreted
by the thyroid gland. It could bind to the receptors on
osteoclasts' membrane, inhibiting osteoclasts'
capacity of bone resorption and their maturity. Thus,
it maintains bone tissue to prevent or treat
osteoporosis. Moreover, calcitonin was demonstrated
to have capacity for pain relief, via modulating
serotonergic systems, sodium channel and alleviation
peripheral circulatory disturbance.
2.3.2 Current and Future Therapeutic
Applications
The function of eel calcitonin was investigated in
several clinical experiments. Besides increasing
BMD, inhibiting bone absorption and decreasing
fractures, eel calcitonin was also demonstrated to
significantly reduce osteoporosis patients’ bone pain
and improve their life quality. Calcitonin could be a
preferred option of acute osteoporotic fractures
(Ukon, et al. 2019).
Future therapeutic efforts could be performed to
explore calcitonin from other animal sources. Eel
calcitonin is effective but still has various adverse
effects, especially it may have risk in oncogenesis
(though experiments only showed weak correlation).
2.4 Estrogen in Osteoporosis
2.4.1 Mechanism Postmenopausal
women were originally reported to have a higher risk
of osteoporosis, which may be due to their reduced
level of estrogen. In vivo experiments suggested that
after removing the estrogen receptors in osteoclasts of
female mice, the phenotype of osteoporosis was
observed. Also, estrogen was found to directly
regulate the survival of mature osteoclasts. It
suppresses osteoclastic bone resorption. Two
cytokines named M-CSF and RANKL could activate
osteoclasts. Estrogen could therefore inhibit
osteoclasts via reducing the expression of those
cytokines which produced in marrow cells and
osteoblasts. Though its detailed molecular
mechanisms are not well-understood, several
signaling pathways (including Wnt signaling) are
regulated or involved with estrogen. In general,
estrogen might control BMD in a positive manner
(Chen 2019).
2.4.2 Current and Future Therapeutic
Applications
Estrogen could prevent or treat postmenopausal
osteoporosis. However, long-term use of estrogen
could induce serious negative effects, such as cancer
and cardiovascular disease. Estrogen may also have
potential impact on the endometrium for women with
an intact uterus. Thus, estrogen is seldom directly
used in the clinic and other estrogen-like hormones
are used instead. These hormones show similar
therapeutic effects as estrogen with mild adverse
effects. Still, most of them could only be used for
prevention or relief, rather than treatment.
Even estrogen-like hormones could have
numerous side effects, new directions may target
estrogen's mechanism in osteoporosis. The estrogen
receptors in osteoclasts are taking a critical role in it.
Specific drugs or molecules could be injected into
bone tissue to increase the affinity (activity) level of
Molecular Mechanisms of Osteoporosis: A Road Map for Osteoporosis Therapeutics
503

those receptors. Moreover, engineering osteoclasts to
express more receptors on their membrane may also
help to reduce bone resorption.
2.5 Bisphosphonates in Osteoporosis
2.5.1 Mechanism Bisphosphonates (BPs)
BPs are the most commonly used drugs nowadays.
BPs exhibit a high binding affinity to bones
(hydroxyapatite). The interaction between BPs and
osteoclasts could suppress bone resorption. Initially,
non-nitrogen-containing BPs were used to induce
osteoclasts apoptosis, while later nitrogen-containing
BPs with stronger anti-resorption ability replaced
former BPs to inhibit the function of osteoclasts
(Langdahl 2021).
2.5.2 Current and Future Therapeutic
Applications
BP's family has various types. Different types may
show different aspects of treating or preventing
abilities. For example, zoledronate was characterized
to prevent vertebral fractures while risedronate was
characterized to prevent non-vertebral fractures.
Alendronate is one of the most popular
bisphosphonates for treating and preventing
postmenopausal osteoporosis, approved by FDA. It
shows a remarkable decrease of vertebral fracture in
clinical patients. Furthermore, as long-term use of BP
may cause adverse effects, a group of patients with
low risk of bone structure were asked to stop using
alendronate for five years. Meanwhile, their key bone
parameters remain normal, indicating that stop
alendronate therapy in a proper period would have
little effect on bones. Scientists also used
nanoparticles to deliver BPs. Nanoparticles
predominantly increased the targeting efficiency and
did not induce an immune response. Nevertheless,
from its investigation to application, there is still a
long way to go.
Taking BP medicine via digestion system could
have side effects on gastrointestinal diseases.
Therefore, delivery methods could be improved.
Except for nanoparticles, injection and inhalation
may be effective, but the dose usage must be carefully
conducted. In addition, BPs could be coated by some
molecules that will not be digested in the
gastrointestinal tract. Only in serum or cellular
environment would those molecules be degraded and
BPs could perform their function.
3 DISCUSSION
Nowadays, drug therapies are still mostly used in the
clinic. Due to different molecular mechanisms of
osteoporosis, drug therapy obtains various types and
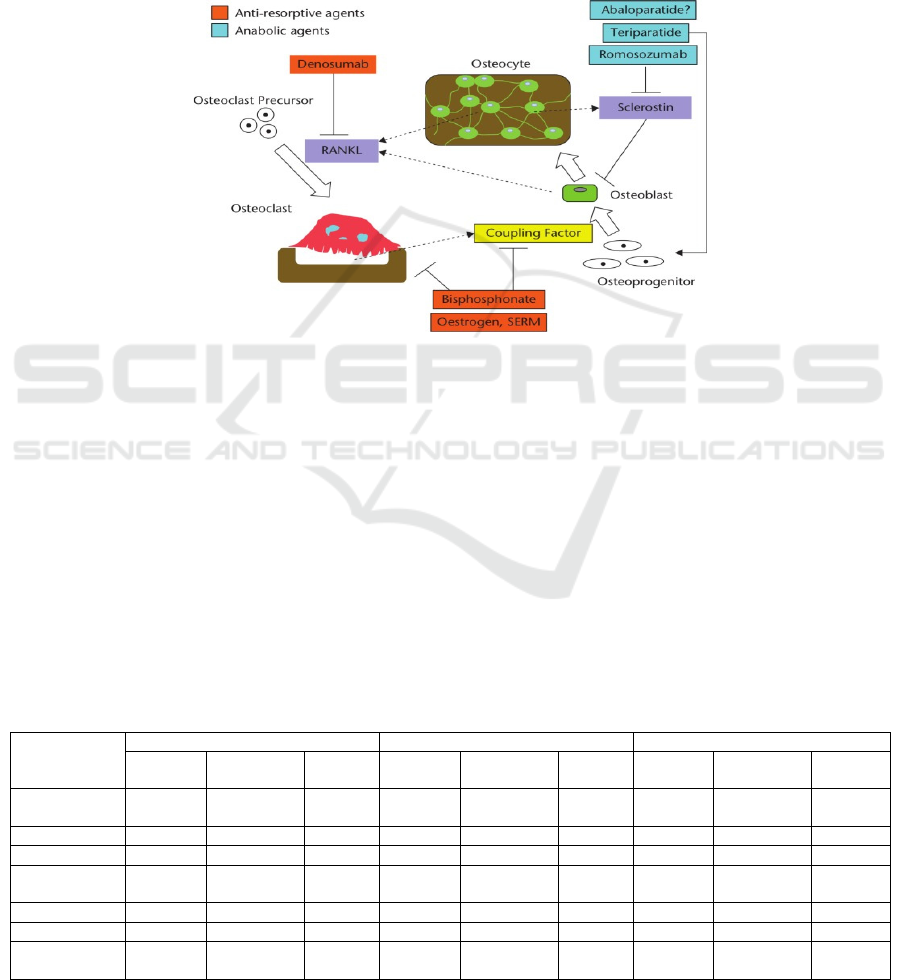
targets. During different stages of bone remodeling,
drug therapies interact with numerous molecules
(figure 4). For example, denosumab and some
cytokines (RANK and RNAKL) contribute to the
activation of osteoclast precursor. Bisphosphonates
could decrease bone resorption. In bone formation,
calcium and phosphate are mainly involved.
Moreover, with further investigation of other cell-
related molecular mechanisms, cell therapy could
also be a novel option for patients. These two major
therapies (as two examples) both have advantages
and limitations in distinct aspects, respectively.
Figure 4: (derived from Langdahl, B.L.) Different molecular treatments in distinct stages of bone remodeling.
ICHIH 2022 - International Conference on Health Big Data and Intelligent Healthcare
504
3.1 Drug Therapy
The advantages of drug therapy have been widely
identified according to clinical trials in the past
decades. Drug therapies are quite effective and have
mature application guidance, including dose usage,
delivery methods and applicable population.
Different drugs or molecules could also be mixed to
perform on the same patient. Clinical results
suggested that combining different drugs could
significantly increase therapeutic effectiveness and
decrease adverse effects. Up to now, several chemical
anti-osteoporotic drugs have been produced to
regulate bone metabolism (figure 5).
Bisphosphonate, oestrogen and selective estrogen
receptor modulator (SERM) could inhibit osteoclast
development as well as the coupling factors. Besides,
denosumab, teriparatide and romosozumab mainly
suppress osteoblast development. Further
investigation based on current drugs may provide
novel therapeutic strategies.
Figure 5: (derived from Tanaka, S.). Regulation of bone metabolism and mechanisms of anti-osteoporotic drugs.
However, adverse effects are still an unavoidable
problem that is extensively existing in drug therapies
(Vandenbroucke 2017). Most drugs would exhibit
more than one adverse event (AE), and those AEs
have various types, distributing in different drugs.
Even worse, drugs have risk in causing withdrawals
and death. (Table 1) Some drugs could only be used
for short-term or even prevention rather than
treatment, just to reduce the harmful effects that
medicine brings to the human body. Some drugs are
gender-limited such as estrogen, which is not suitable
to apply to males. Besides, the delivery efficiency of
medicine requires to be improved. Since most drugs
have to go through the digestion system, serum,
cellular environment and finally to specific tissues, a
high dose of drugs would damage other parts of the
human body while a low dose would have
unsatisfying efficacy. Besides, the molecular
mechanisms of some drug therapies have not been
clearly understood yet. These drug therapies are only
known to be effective in clinic and may interact with
some molecules. Identifying the mechanisms would
contribute to find out more novel therapeutic
strategies.
Table 1: (derived from Vandenbroucke, A., et al.) Summary of several most relevant adverse events from the currently
available osteoporosis treatments (risedronate, zoledronic acid and teriparatide) in very elderly women.
AE
Risedronate Zoledronic acid Teriparatide
Placebo Risedronate P-value Placebo Risedronate P-value Placebo Risedronate P-value
≥1 adverse
even
t
89.7% 90.9% 91.8% 92.6% 0.34 91% 83%
Nausea 8.3% 9.4% 5.9% 7.5% 0.05 9% 8%
Dyspepsia 6.8% 6.8% 5% 4%
Abdominal
pain
7.7% 8.2% 13% 6%
Diarrhea 5.6% 6.8% 0.11 3% 10%
Death 7.1% 5.7% 0.276 7.5% 7.0% 0.58
Withdrawals
due to AEs
20.3% 20.6% 0.947
Molecular Mechanisms of Osteoporosis: A Road Map for Osteoporosis Therapeutics
505
3.2 Cell Therapy
Cell therapy could prevent most of the disadvantages
of drugs. Mature cell therapies exhibit a relatively
low level of side effects in the clinic. Specific stem
cells could be engineered to suit every single patient.
As cells are generally transplanted or injected into the
human body, the delivery process is significantly
reduced when molecules try to reach a specific target.
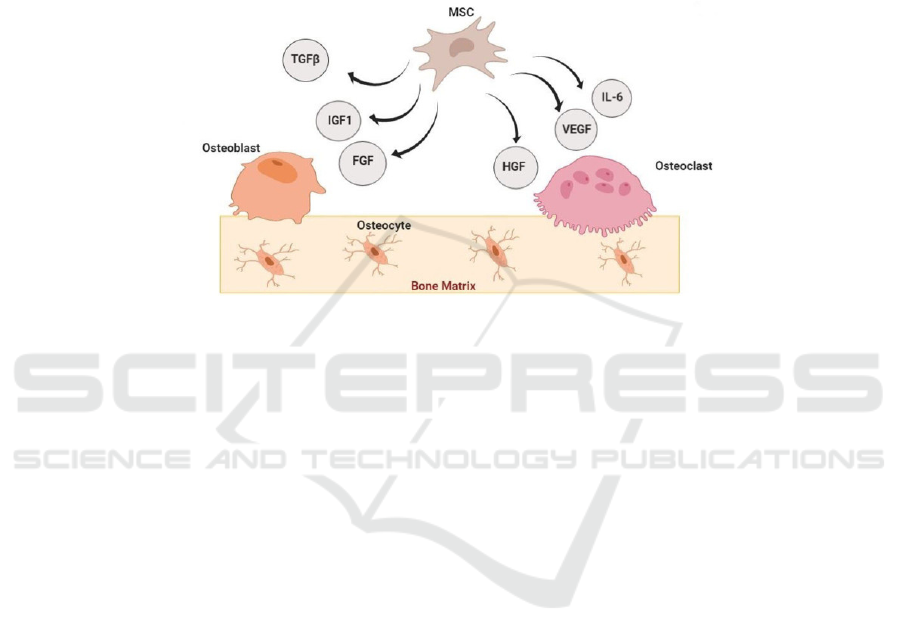
Besides, as mentioned above, MSC could be a
potential target for cell therapies due to its important
mechanisms in osteoporosis. By manipulating its
paracrine secretion of molecules (mainly composed
of several growth factors), both osteoblasts and
osteoclasts could be specifically regulated or
generated (figure 6). More importantly, instead of
taking drugs for a long term, cell therapy could be
performed only once to cure patients because it
exactly targets on defective cells and fix or replace
them to function normally (
Arjmand 2020).
Figure 6: (derived from Arjmand, B., et al.) Paracrine effects of MSCs in bone regeneration. IGF-1: insulin-like growth factor;
TGF-β: transforming growth factor β; VEGF: vascular endothelial growth factor; HGF: hepatocyte growth factor; IL-6:
interleukin−6; FGF: fibroblast growth factor.
Despite those attractive advantages in cell
therapy, limitations are also disturbing scientists.
Ethical problems are often mentioned when applying
stem cells from different donors. Cells from other
donors could trigger an immune response in patients
as well, causing worse situations. Further, most cell
therapies are not mature enough to treat patients.
Some therapeutic strategies have not been accepted to
be used in the clinic yet. Overall, drug therapy and
cell therapy are promising treatments, and they
should be continuously developed to deal with
osteoporosis.
4 CONCLUSIONS
In conclusion, this review summarizes several
molecular mechanisms that are significantly involved
in osteoporosis, as well as discusses the current and
future therapies. Drug therapy and cell therapy are
taken as two major examples to compare their
advantages and limitations.
Based on molecular mechanisms, therapies have
an effective function in the clinic but still require
improvement. Researchers can further study more
new treatment methods. It is worth noting that some
detailed mechanisms (such as calcitonin) of the
interaction between molecules and cells are still
unknown. All these require in-depth exploration by
researchers.
ACKNOWLEDGMENTS
Particularly, I would like to thank my parents that
they offered me strong support. They gave me the
best condition to study. Besides, they encouraged me
to take public health course.
Without attending the course, I cannot come up
with this review topic. Also, I would like to thank my
tutors. They gave me advice on how to construct my
review and indeed taught me a lot in the class. At last,
I would like to thank my friends for their suggestions
during my review writing.
REFERENCES
Arjmand, B., et al, (2020). Prospect of Stem Cell Therapy
and Regenerative Medicine in Osteoporosis. Front
Endocrinol (Lausanne), 11: p. 430.
ICHIH 2022 - International Conference on Health Big Data and Intelligent Healthcare
506

Chen, L.R., Ko, N.Y., and Chen K.H., (2019). Medical
Treatment for Osteoporosis: From Molecular to
Clinical Opinions. Int J Mol Sci, 20(9).
Hu, L., et al., (2018). Mesenchymal Stem Cells: Cell Fate
Decision to Osteoblast or Adipocyte and Application in
Osteoporosis Treatment. Int J Mol Sci, 19(2).
Langdahl, B.L., (2021). Overview of treatment approaches
to osteoporosis. Br J Pharmacol, 178(9): p. 1891-1906.
Macias, I., et al., (2020). Osteoporosis and the Potential of
Cell-Based Therapeutic Strategies. Int J Mol Sci, 21(5).
Tanaka, S., (2019). Molecular understanding of
pharmacological treatment of osteoporosis. EFORT
Open Rev, 4(4): p. 158-164.
Ukon, Y., et al., (2019). Molecular-Based Treatment
Strategies for Osteoporosis: A Literature Review. Int J
Mol Sci, 20(10).
Vandenbroucke, A., et al., (2017). Pharmacological
treatment of osteoporosis in the oldest old. Clin Interv
Aging, 12: p. 1065-1077.
Yang, T.L., et al., (2020). A road map for understanding
molecular and genetic determinants of osteoporosis.
Nat Rev Endocrinol, 16(2): p. 91-103.
Molecular Mechanisms of Osteoporosis: A Road Map for Osteoporosis Therapeutics
507
