
Fear Recognition in Mice based on Neurochat Implantable BCI
Wenbin Qu
a
, Fangcai Mai
b
and Minmin Luo
*c
School of Biomedical Engineering, Southern Medical University, Guangzhou, GuangDong, China
Keywords: Brain-Computer Interface, Neural Signal Acquisition, Fear Response.
Abstract: Establishing the connection between the animal brain and external equipment through the brain-computer
interface and realizing the exchange of information between the brain and the outside world is the basis for
many imaginations of future science and technology. This project improved the production of the brain-
computer interface collection component-Neurochat series, which realized the signal collection from brain
waves, to local field potentials and neuron spikes, and successfully identified the fear response of mice.
1 INTRODUCTION
1.1 Brain-Computer Interface
In 2008, neurobiologists at the University of
Pittsburgh claimed that monkeys could manipulate
mechanical arms to feed themselves by using brain
computer interface (BCI) (Velliste 2008). In April
2021, Neuralink, a BCI company owned by Elon
Musk, showed the world their practical brain-
computer interface technology and automatic
implantation of surgical equipment so that a monkey
can play video games with his mind. This also
provides unlimited possibilities for the future of BCI
(Vourvopoulos 2019, Wu 2020, Shi 2018, Tomislav
2018, Chai 2017). Through interdisciplinary
research such as neuroscience, signal detection and
machine learning, it is popular in the medical and
entertainment industries, especially in the field of
virtual manipulation (Patil 2008).
At present, the methods of obtaining information
by brain computer interface include invasive and non-
invasive. Non-invasive is safe for humans and
animals, but the acquired EEG signals are not
accurate. Invasive type damages the animal brain, but
the potential of a single brain cell can be accurately
recorded. We improved and fabricated a set of
invasive brain computer interface elements neurochat
series, which were implanted into the superior
colliculus (SC) nucleus of mouse brain through
a
https://orcid.org/0000-0002-8364-1289
b
https://orcid.org/0000-0002-6907-4580
c
https://orcid.org/0000-0003-2971-2311
electrodes. In the experimental environment, sound
and visual stimuli were used to verify and stably
trigger the animal's instinctive defense behavior.
According to the collected EEG signals, we can judge
whether the mice have instinctive fear. The
experimental results prove the effectiveness of the
brain-computer interface system made in this project,
and provide a basis for the next theoretical research
and practical application.
1.2 Fear and Instinctive Defensive
Response
In nature, in order to survive, animals have an innate
fear of danger signals from the external environment
and induce them to make innate behaviors. The
generation of this instinctive fear defense depends on
the animal's sensory nervous system basically, such
as using smell to perceive predator's scent
information, using vision to observe the predator's
figure, and using the auditory system to perceive
predator's sound information.
The instinctive defense response of animals has
three main manifestations: Startle, Flight and
Freezing. Startle is a short-term startle response
caused by high-intensity sound, which is mainly
regulated by the cochlear nucleus (CN) located in the
lower brainstem and the specific loop is CN-pontine.
Flight can be directly induced by noise or light
stimulation in the awake state. Freezing mainly
490
Qu, W., Mai, F. and Luo, M.
Fear Recognition in Mice based on Neurochat Implantable BCI.
DOI: 10.5220/0011372700003438
In Proceedings of the 1st International Conference on Health Big Data and Intelligent Healthcare (ICHIH 2022), pages 490-495
ISBN: 978-989-758-596-8
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
occurs under the condition of auditory fear. It is rare
for mice to induce freezing behavior by simple sound
stimulation. The superior colliculus has many
advanced functions and is one of the important nuclei
in the midbrain. However, for SC research, most of
the predecessors focused on vision-related fields.
Through the Neurochat brain-computer interface
system, we conducted a preliminary study on the role
of SC in the fear and instinctive defense response
loops in terms of vision and hearing.
2 MATERIALS AND METHOD
2.1 Neurochat BCI System
The overall system architecture diagram is shown in
Figure 1.
Figure 1: Overall system design diagram.
This project uses NeuroCollector to complete the
signal collection with a single fine-tuning electrode.
The structure diagram is shown in Figure 2 and the
electrode driving operation logic is shown in Figure
3. We will describe the design and production of the
Neurochat overall system separately.
Figure 2: Single fine-tuning electrode structure diagram.
Figure 3: Single fine-tuning electrode driving operation
logic.
The recording system we chose is a self-built
system based on RHD2000 (Intan, USA).It is a multi-
channel recording system and the Intan Technologies
RHD2000 Interface software is used for signal
acquisition and control. Its software supports
monitoring signals from any channel and transfers the
collected data to the front-end PC in binary format.
Finally, the data file is imported into MATLAB
(MathWorks, USA) for subsequent processing and
analysis.
2.2 Auditory Stimulation Method
The mouse is placed in a behavior box containing two
chambers as shown in Figure 4 and the mouse is free
to explore in the behavior boxes on both sides. Two
cameras are used to capture the behavior information
of the mouse at the same time. When the mice moved
freely to the central area of the chamber, they were
randomly and non-repeatedly given sound
stimulation with an intensity of 30-80db each time,
and the behavioral results were recorded and
analysed.
Figure 4: Stimulating behavior paradigm.
2.3 Visual Stimulation Method
For visual stimuli, we adopt the same behavioral
paradigm. When the mice move freely to the central
area of the chamber, randomly and non-repetitively
give looming stimuli with a contrast of 25%-100%
each time, record and analyze the behavioral results.
2.4 Surgery
We choose mouse with a body weight of about 20 g,
normal hearing, and good condition for operation
preparation. Mouse was prepared for surgery within
3-5 days before the experiment. The animal is
prepared for surgery as follows: anesthetize with the
equipped 1.5% sodium pentobarbital solution or
isoflurane. After anesthesia, the mouse head is
Fear Recognition in Mice based on Neurochat Implantable BCI
491

depilated with a shaving device and shaving cream,
then wiped with alcohol and then smeared with
iodophor for disinfection. Use sterile scissors to cut
the scalp to an appropriate size to expose the bregma
of the mouse. Use a stereotaxic instrument for
leveling to ensure that the front and rear fontanelles
of the mouse are in a horizontal state. Four skull nails
are symmetrically implanted on the surface of the
mouse skull for fixation and then locate the SC.
Figure 5: Mouse implanted with NeuroCollector
The SC location coordinates of the mouse are 2.8-
4.5mm behind the bregma, and the maximum
distance beside the midline is 1.75mm. After
marking, the skull is drilled to open the window, the
dura mater and pia mater are removed and stop
bleeding. After the exposed area is clean and free of
blood clots, the electrode is slowly lowered at a speed
of 10 micrometers per second and inserted into the
nucleus of SC. Use biological silica gel and white
wax to seal the skull window, and then use dental
cement to paste a shallow circle on the skull nail and
the surface of the skull. After drying, the whole
adjustable electrode device NeuroDrive or the single
adjustable electrode device NeuroCollector is pasted
and fixed on the mouse skull with dental cement to
ensure that the device can remain stable and does not
produce relative displacement with the skull due to
the free movement of the mouse. Figure 5 shows a
mouse successfully implanted with NeuroCollector.
3 RESULTS
3.1 Auditory Response Verification
Experiment
Since the SC receives a large number of axon inputs
from the auditory nucleus, we first verified the
mouse's auditory stimulus response. In a sound-
shielded room, we perform sound stimulation on
mice: give different frequencies of pure tones or
noises, and record neurophysiological activities at the
same time
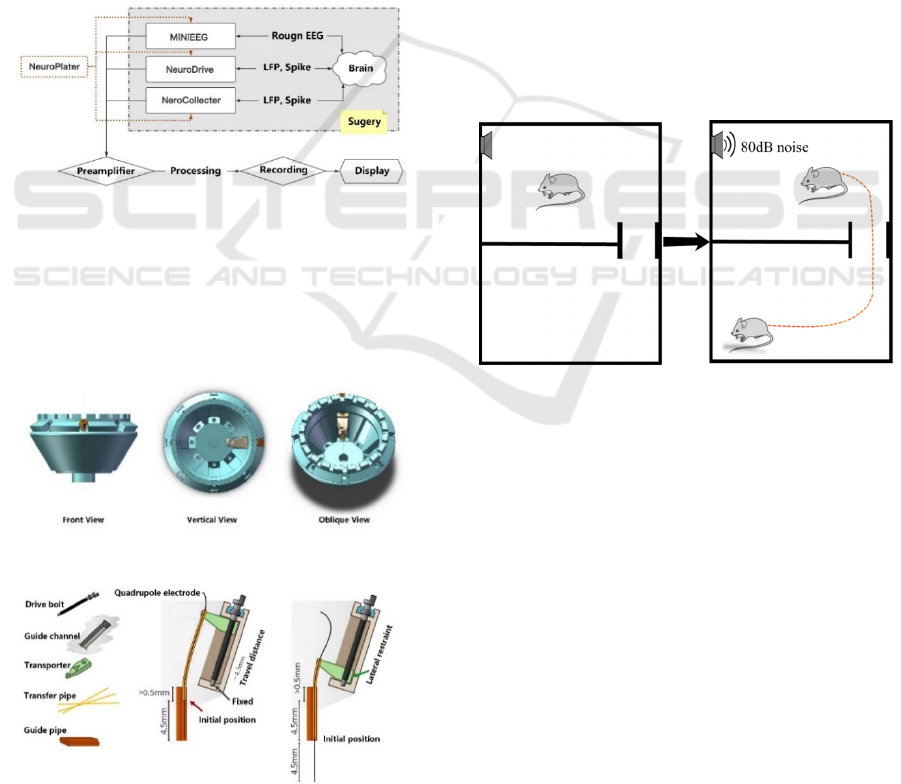
As shown in Figure 6, A is the recorded real-time
data, which reflects the action potentials evoked by
10 auditory stimuli. B is the sum of action potentials
accumulated by all stimuli (PSTH diagram:
histogram of time distribution after stimulation). C is
a scatter plot, the horizontal axis is the time and the
vertical axis is the length of the stimulus sound, and
the corresponding scatter plot of the complete sound
sequence stimulus is drawn.
3.2 Auditory Response Verification
Experiment
We found that high-intensity noise could very stably
induce the instinctive defense behavior flight of
awake mice. When the mouse is out exploring, giving
80db and 70db sound stimulation can stably induce
the mouse's flight behavior. When the sound intensity
is 60db, the probability of flight is 67.5%; when the
sound intensity is 50db and 40db, the proportion of
flight is 37.5%; when the sound intensity is 30db, the
mouse will not show the defensive behavior of flight,
but maintain the state of free movement.
At the same time, as the stimulus intensity
decreases, the maximum speed of the mouse in the
process of generating a flight also decreases in a
stepwise manner. This indicates that as the intensity
of the stimulus increases, the mice show stronger and
stronger defensive behaviors, and auditory
stimulation is more likely to induce the mouse to
produce flight. Figure 7 is an analysis diagram of the
trajectory and speed of the mouse after hearing 80db
sound stimulation. In subsequent experiments, we
used 80db sound intensity for stimulation.
ICHIH 2022 - International Conference on Health Big Data and Intelligent Healthcare
492
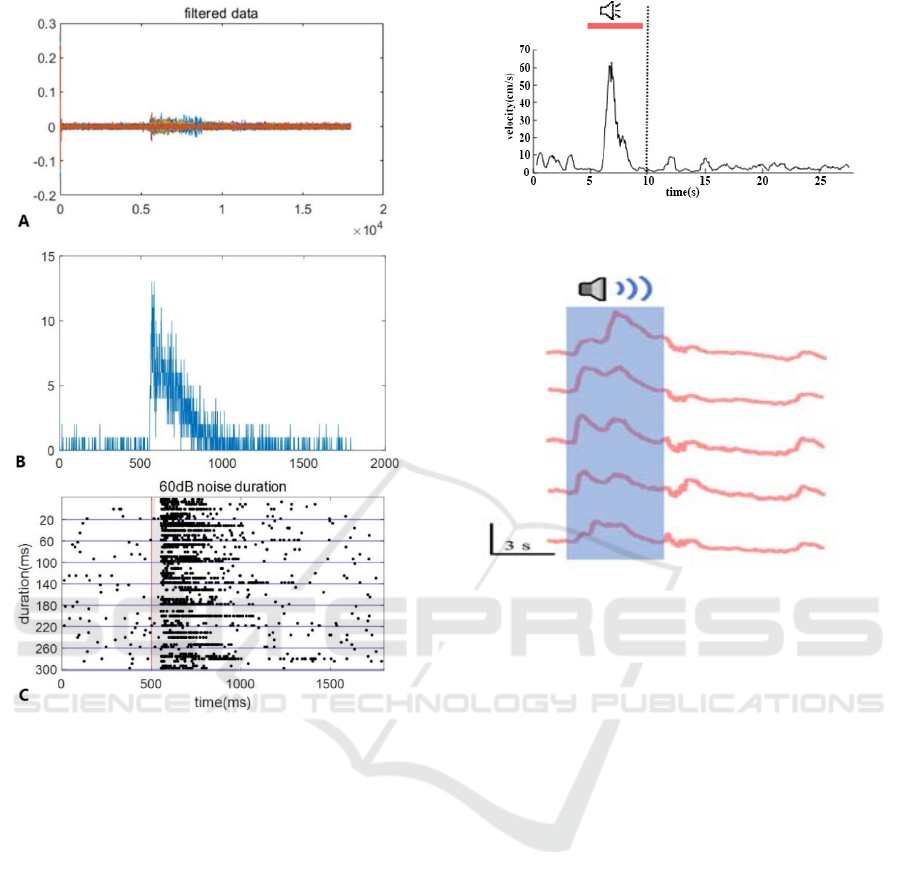
Figure 6: An example of data analysis of neuron response
to noise in SC.
We record the changes of SC neuron activity
signals after mouse received auditory stimulation and
count the number of action potentials within 3ms, as
shown in Figure 8. During the recording process, the
intensity of the sound stimulation we gave is 80db and
the duration is 5s. In the sound interval, the mice
developed instinctive fear, which caused defensive
behaviors, and a large number of neurons were fired.
After the sound was over, the mouse completed their
defense. The calcium signal quickly weakened and
returned to the baseline level, that is, when the mouse
heard noise stimulation and produced defensive
behaviors, SC neurons fired in large numbers, which
was related to the instinctive fear emotion.
Figure 7: The speed analysis graph of the mouse after
hearing 80db sound stimulation.
Figure 8: Cell firing and change rate in SC after sound
stimulation to mouse.
3.3 Visual Instinct Fear Experiment
For the visual system, giving different speeds and
contrasts of looming (visual approximation) will also
induce the flight and freezing behavior of mice. When
a mouse is out exploring, no matter what the contrast
of the looming stimulus is given, it will make the
mouse have flight behavior. When the mouse is in a
corner, given the looming stimulus, there is a 60%
probability that the mouse will have a freezing
behavior, 40% of mice will have flight behavior. At
the same time, as the intensity of the stimulus
decreases, the maximum speed during which the
mouse generates a flight also linearly decreases. This
shows that as the stimulation intensity increases, the
mice show stronger and stronger defensive behaviors,
among which the flight behavior tendency is more
pronounced. Figure 9 is an analysis diagram of the
trajectory and speed of the mouse after being
stimulated by looming with a contrast of 100%. In
subsequent experiments, we gave a looming stimulus
with a contrast of 75%.
Fear Recognition in Mice based on Neurochat Implantable BCI
493
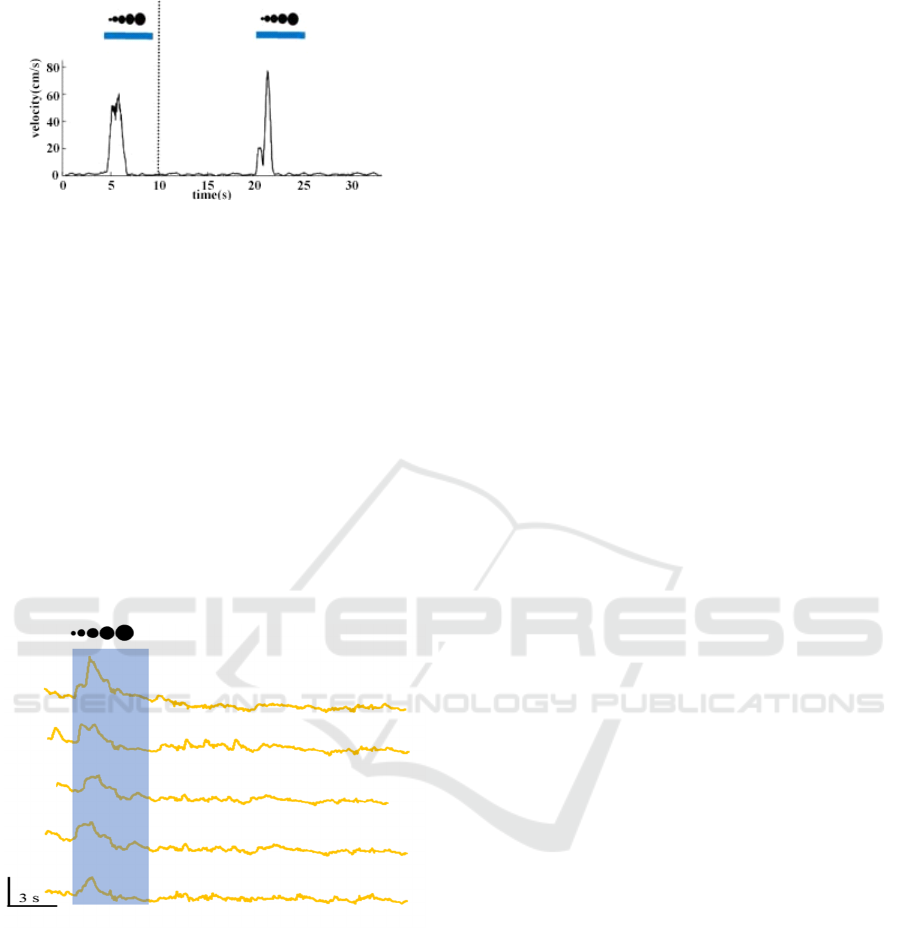
Figure 9: Analysis of the trajectory and speed of the mouse
after being stimulated by looming with a contrast of 100%.
Next, we recorded the changes in the SC neuron
activity signals after the mouse is stimulated by visual
looming and the results are shown in Figure 10.
During the recording process, we give looming
stimulation with a contrast of 75%, similar to the
result of auditory stimulation. In the stimulation
interval, the mouse produced flight/freezing defense
behavior, and the signal rose rapidly. After the
stimulation, the mouse completed their defense. In
behavior, the calcium signal quickly weakened and
returned to the baseline level. That means, when the
mouse felt visual stimulation and produced defensive
behavior, SC neurons were fired in large numbers,
which indicates that SC is related to visually evoked
defensive behaviour.
Figure 10: Cell fire and change rate in SC after looming
stimulation to mouse.
In the next step, we will establish a Support Vector
Machine (SVM) model, take neuro-
electrophysiological signals as the input, and take
whether the mouse produces defense response as a
criterion for fear, trains SVM and analyzes the
mouse’s fear emotions. Using f1 score as the
standard, evaluate the analytical effect of the model,
complete the two-classification problem, and realize
whether the mouse has fear or not and predict the
subsequent response.
4 CONCLUSIONS
Through the self-improved brain-computer interface
Neurochat, this project realizes the signal acquisition
requirements of brain computer interface from EEG
to local field potential and then to neuron spike
potential, and successfully analyzes the instinctive
fear of mice. It is proved that our system scheme is
feasible and effective.
The various methods integrated by the project
system have mature theoretical foundations, and there
is a huge market space for applications in the fields of
neurocognitive science, electrophysiology, and brain-
computer interface. The field of brain-computer
interface is known as the highway for communication
between the human brain and the outside world
(
Belwafi 2018)
. It is the key core technology of the
latest human-computer interaction and human-
computer hybrid intelligence, and its application
prospects are unlimited. Using Neurochat series can
provide experimental evidence in a multi-faceted,
multi-layered, and humanized manner, and provide
help for the further application of brain-computer
interfaces (Lee 2010, Gao 2020).
ACKNOWLEDGEMENTS
Thanks to the Neuroinformation Engineering
Laboratory of the School of Biomedical Engineering,
Southern Medical University.
REFERENCES
Belwafi, K., Romain, O., Gannouni, S., Ghaffari, F.,
Djemal, R., & Ouni, B. (2018). An embedded
implementation based on adaptive filter bank for brain-
computer interface systems.J. Journal of Neuroscience
Methods, S016502701830116X.
Chai, R., Naik, G. R., Ling, S. H., & Nguyen, H. T. (2017).
Hybrid brain–computer interface for biomedical cyber-
physical system application using wireless embedded
eeg systems. J. Biomedical Engineering Online, 16(1),
5.
Gao, Z., Y Li, Y Yang, Dong, N., Yang, X., & Grebogi, C.
(2020). A coincidence-filtering-based approach for
cnns in eeg-based recognition. J. IEEE Transactions on
Industrial Informatics, 16(11), 7159-7167.
Lee, P. L. , Sie, J. J. , Liu, Y. J. , Wu, C. H. , & Shyu, K. K. .
(2010). An ssvep-actuated brain computer interface
using phase-tagged flickering sequences: a cursor
system. J. Annals of Biomedical Engineering, 38(7),
2383-2397.
ICHIH 2022 - International Conference on Health Big Data and Intelligent Healthcare
494

Patil, P. G., & Turner, D. A. (2008). The development of
brain-machine interface neuroprosthetic devices. J.
Neurotherapeutics, 5(1), 137-146.
Shi, M. H. , Zhou, C. L. , Xie, J. , Shao-Zi, L. I. , Hong, Q.
Y. , & Jiang, M. , et al. (2018). Electroencephalogram-
based brain-computer interface for the chinese spelling
system: a survey. J. Front Inform Tech El, 19(3), 423-
436.
Tomislav, M., Sarma, A. A., Daniel, B., Simeral, J. D., Jad,
S., & Chethan, P., et al. (2018). Stable long-term bci-
enabled communication in als and locked-in syndrome
using lfp signals. J. Journal of
Neurophysiology, 120(1), 343-360.
Velliste, M., Perel, S., Spalding, M. C., Whitford, A. S., &
Schwartz, A. B. (2008). Cortical control of a prosthetic
arm for self-feeding. J. Nature, 453(7198), 1098-1101.
Vourvopoulos, A., Jorge, C., Abreu, R., Figueiredo, P.,
Fernandes, J. C., & Badia, S. (2019). Efficacy and brain
imaging correlates of an immersive motor imagery bci-
driven vr system for upper limb motor rehabilitation: a
clinical case report. J. Frontiers in Human
Neuroscience, 13.
Wu, Q., Yue, Z., Ge, Y., Ma, D., & Wang, J. (2020). Brain
functional networks study of subacute stroke patients
with upper limb dysfunction after comprehensive
rehabilitation including bci training. J. Frontiers in
Neurology, 10.
Fear Recognition in Mice based on Neurochat Implantable BCI
495
