
Analysis on Recent Artificial Retina in Electrochemical and
Mechanical Approaches
Xinrui Zhang
Faulty of Art and Science, University of Toronto, ON L5L 1C6, Toronto, Canada
Keywords: Retinal Prosthesis, Retinal Degenerative Disease, Artificial Vision.
Abstract: Outer retinal degeneration diseases, such as retinitis pigmentosa and age-related macular degeneration, are
the main eye diseases that cause blindness. Although the molecular genetics of retinitis pigmentosa has made
great progress in recent years, no obvious breakthrough has been made in the treatment. The use of retinal
pigment epithelial cell transplantation, or the transplantation of retinal slices including photoreceptors, has
major problems in case selection, immune rejection, efficacy and safety. If a certain method is adopted to
generate the perception of light and the corresponding electric current or release neurotransmitters, the inner
retina, i.e., the inner nuclear layer and ganglion cells are activated, and nerve impulses are generated and
transmitted to the visual cortex. Vision, this device that can activate the inner retina is called an artificial retina
(retinal prosthesis). Activation of the inner retina by means of retinal prosthesis (artificial retina) will be a
promising approach for treatment of outer retina degenerative diseases. Great efforts have been paid to evolve
special new devices in recent years.
1 INTRODUCTION
The design principle of the retinal implanted
electrode system is to replace the damaged
photoreceptor function, that is, to effectively capture
the visual image of the surrounding environment,
convert the visual signal into a neuroelectric signal,
and activate the inner retina to form vision (Humayun
et al. 1994). The production of vision depends on
three major tissues and organs: the eyeball (mainly
the retina), the optic nerve, and the visual cortex. So,
it is necessary for developing prostheses that can
replace these three tissues, namely retinal prostheses,
optic nerve prostheses, and visual cortex prostheses
in order to restore vision. The current approaches are
mainly facing obstacles in the artificial retina implant
location, materials selection and how to efficiently
encode and decode chemical and electrical signals,
Therefore, this article will review recent studies and
try to promote the development of artificial retina in
achieving the capacities of wide field of view, high
resolution and low aberration sensitivity.
The three types of visual prostheses have different
stimulation sites. The visual cortex prosthesis uses
microelectrodes to directly stimulate the primary
visual cortex, which can produce light perception, but
cannot form an image. Optic nerve prosthesis
stimulates nerve bundles and does not require a
complete retinal structure. However, it has low
resolution, difficult surgery, and high risks. It is still
in the basic research stage. The retinal prosthesis is to
implant microelectrodes or photoelectric arrays at
specific locations on the retina, convert additional
video and image information into electrical impulses,
stimulate specific nerve cells, then transmit them to
the visual cortex and brain center through neural
pathways (Rizzo et al. 2014).
The main function of the retinal implanted
electrode system is to replace the damaged
photoreceptor layer, accept and convert the light
signal of the environment. The key to its successful
vision depends on the biological activity of the inner
retinal neurons. Electrode pulses can affect many
inner and outer layers of the retina. At present, it is
generally believed that the ganglion cell body, axon
and proximal segment are the first targets of
extracellular stimulation (Weiland et al. 1999). Based
on the principle that the inner retinal nerve
conduction is only limited to the electrical stimulation
area, only the axons of the retinal ganglion cells in the
electrode stimulation area are activated. This lays the
theoretical foundation for retinal positioning
electrical stimulation and makes it possible to form
Zhang, X.
Analysis on Recent Artificial Retina in Electrochemical and Mechanical Approaches.
DOI: 10.5220/0011370400003438
In Proceedings of the 1st International Conference on Health Big Data and Intelligent Healthcare (ICHIH 2022) , pages 373-378
ISBN: 978-989-758-596-8
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
373
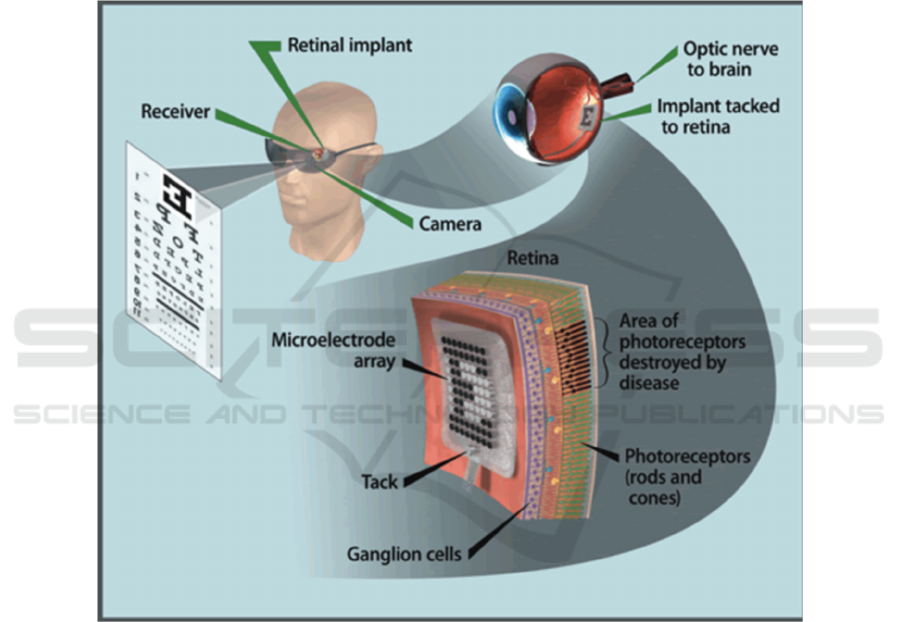
geometric images with simultaneous multi-point
electrical stimulation.
Researchers used bullfrog, rabbit, and mouse
animal models to study the number, size, and current
threshold of electrodes needed to produce effective
vision. It is found that the current value of the 200μm
diameter distribution with an interval of 200μm is
within the safe current value range of long-term
retinal stimulation (Weiland et al. 1999). Electric
pulses below the lower limit of nerve stimulation
cannot produce light perception. Higher than the
upper limit of nerve stimulation may cause tissue
damage. The electrical stimulation threshold will be
manually adjusted after retinal chip implantation until
the patient has light perception. After the patient is
familiar with the device, the electrical stimulation
threshold will change. The adaptation program needs
to be re-adjusted (Humayun et al. 1999). The
adaptation program converts the grayscale value of
the image to a specific value. Then it can be projected
onto the retinal electrode chip through the visual
processing unit to generate the corresponding
electrical stimulation.
Figure 1: The working principle of artificial retina
2 TYPES OF ARTIFICIAL
RETINA
There are three kinds of artificial retina devices which
are placed on the inner surface of the retina, placed on
the under the retina and chemical prosthesis. The
recent progress in three approaches to artificial retina
implementation: epiretinal prosthesis, subretinal
prosthesis and chemical prosthesis is reviewed.
2.1 Epiretinal Prosthesis
Epiretinal prosthesis is to arrange the electrodes for
electrical stimulation close to the inner limiting
membrane of the retina, without damaging the
structure of the eyeball other than the fixed point of
the electrode arrangement. The non-photosensitive
area on the device directly receives electrical signals
containing image information, and the electrodes
directly stimulate the axons of ganglion cells. If the
electronic device needs to be placed outside the
ICHIH 2022 - International Conference on Health Big Data and Intelligent Healthcare
374

eyeball, a wire needs to be led out through the flat part
of the ciliary body. The current surface artificial
retina requires an external system for image
acquisition, image processing, data and power
conversion to the implant, so this information
conversion is more easily controlled by the outside
world. The composition of this device is a very small
field sensor like a camera, which is fixed outside the
eyeball or inside a plastic lens implanted in cataract
surgery. Moreover, a platinum-wrapped metal wire
connects it to the electrode arrangement on the top of
the inner retina. stand up. The implant on the retina is
first a readout chip, which receives the image
information from the sensor and the electrical signal
from the processing unit to generate electrical
impulses, which stimulate the axons of the ganglion
cells and pass into the brain through the axons.
A clinical trial of a retinal surface stimulator
approved by the FDA has recently begun. An
exclusive clinical trial is also underway at the Doheny
Retina Institute at the University of Southern
California, and 2 patients have received implants.
Studies have confirmed that microelectronic devices
have enough energy to directly stimulate retinal
neurons, so that patients who are completely blind can
feel the light consistent with the stimulation pulse.
Each electrode is controllable. Under the control of a
microelectronic device, when each electrode is
activated, it can cause light perception. In Germany,
the researchers temporarily placed a stimulating
device on the retina of a test subject (before eye
surgery, with normal photoreceptors, not blind). The
results showed that there was light under a very low
stimulation current. These results supports the results
of previous preclinical trials of multiple research
groups. What is more, in the case of outer retinal
degeneration, the retina requires higher current
stimulation, which is more difficult to stimulate than
the healthy retina without loss of photoreceptors
(Zhou et al. 2007).
2.2 Subretinal Prosthesis
Subretinal prosthesis is implanted between the retinal
pigment epithelium and the sensory layer of the
retina. The advantage is that the implanted electrodes
are close to the retinal bipolar cells, the stimulation
current required is small. Some devices are designed
to directly generate stimulus current from light
without an external power source; while others are
designed with an external power source to amplify the
electrical signal generated by the light. This device is
composed of thousands of microelectrodes,
containing photosensitive micro photodiodes,
integrated on a very thin board (thickness 50-100μm,
diameter 2-3mm). The photodiode is irradiated by
light and converted into a tiny current on each
microelectrode. The current is "injected" into the
remaining neurons of the retina, and the middle and
inner layers of the retina serve as the processing part
of visual information. In addition, coating
glycoproteins (such as laminin) on the surface of the
micro-photodiode can increase biocompatibility.
Besides, optobionics has been approved by the FDA
for clinical trials using artificial silicon retinas in
recent years (Hauer et al. 2007). This device is an
array of micro-photodiodes, implanted in the
subretinal space, and powered by light only. In June
2000 and July 2001, 3 people received implants on 2
occasions. It was reported at the ARVO meeting in
2002 that the implant still had electrical function and
remained in place, which could indirectly improve
visual function, but under normal light conditions, the
device itself could not directly activate the retina.
Researchers such as the University of Tubingen in
Germany have also developed a device. They
confirmed that in the long-term loss of photoreceptors
in the retina, passive light reception cannot generate
enough current to induce a direct response of retinal
neurons. In order to solve this problem, they used
external infrared energy to provide greater energy to
the implant to generate electrical stimulation pulses.
In addition to the energy supply device, the micro
photodiode located under the retina can receive light
and transmit stimulation pulses to the stimulation
electrodes under the retina. Recently, a research
group at the University of Houston used ceramic
optoelectronic materials to make implants. Japan's
Nidek Co., Ltd. produced a subretinal electrode array
with a wire connected to the electronic device in the
vitreous cavity through the retina (shown in Figure 2)
(Gu et al. 2020). Chemical prosthesis method can
release neurotransmitters in the target area of the
retina has been proposed, but its feasibility is still at
the stage of demonstration. The Kresge Institute of
Ophthalmology at Wayne State University has
proposed a microfluidic device that can be used to
stimulate the cortex and retina which can stimulate
neurons.
Analysis on Recent Artificial Retina in Electrochemical and Mechanical Approaches
375

Figure 2: Electrochemical eye with a hemispherical retina.
2.3 Chemical Prosthesis
Retinal implanted electrodes are the most successful
artificial vision implant system so far. The reasons are
as follows: (1) The complicated fatality rate of
intracranial optic cortex and optic nerve implanted
electrodes is high. With the continuous improvement
of vitreoretinal surgery technology, retinal implanted
electrode surgery. The risk of continuous reduction in
the incidence of surgical complications has gradually
been accepted by authoritative institutions. (2)
Retinal implanted electrodes avoid the complex
processing and transmission of visual signals in the
subretinal, midbrain and visual cortex. Stimulate at
the beginning of the visual pathway It is easier to
produce effective vision. (3) Due to the fusion of
multiple photoreceptor cells in the peripheral area of
the retina during the signal processing of the visual
pathway, and a bipolar cell, it is further fused and
connected to the retinal ganglion cells to transmit
visual signals. The ratio of photoreceptor cells,
bipolar cells and retinal ganglion cells is 1:1:1.
Therefore, placing a multi-electrode stimulation chip
in the macula area is more likely to produce Holmes
retinal topological vision.
3 LATEST RESEARCH AND
DISCUSSION
At present, the research of artificial retina has reached
an important stage, that is, implanting a device in the
blind to replace the lost function (Wang et al. 2020).
There are often robots with artificial eyes in science
fiction novels, and bionic eyes that are connected to
the human brain to restore the vision of the blind.
Scientists have spent a lot of energy to develop such
devices. However, making spherical human eyes-
especially hemispherical retinas-is a huge challenge,
severely limiting the functions of artificial and bionic
eyes. The team of Fan Zhiyong, the member of which
are from the Hong Kong University of Science and
Technology, the University of California at Berkeley
and Lawrence Berkeley National Laboratory reported
an innovative, concave hemispherical retina, which
consists of a series of nano-level light sensors
(photoreceptors) that mimic the human retina
Photoreceptor cells in Researchers apply this kind of
retina to electrochemical eyes, which have multiple
functions equivalent to human eyes and can complete
the basic functions of acquiring image patterns. The
retina of the human eye is hemispherical. In addition,
its optical layout is more sophisticated than the flat
image sensor in the camera: the dome shape of the
retina naturally reduces the light transmission through
the lens, thereby making the focus sharper. The core
component of the bionic electrochemical eye is an
array of high-density photosensitive elements as the
retina. Besides, the photosensitive element is formed
directly in the pores of the alumina hemispherical
film. Thin flexible wires made of liquid metal are
sealed in a soft rubber tube to transmit the signal from
the nanowire light sensor to an external circuit for
signal processing. These wires simulate the nerve
fibers that connect the human eye and brain. The most
impressive is the high-resolution imaging of this
artificial retina, which is due to the high density of the
ICHIH 2022 - International Conference on Health Big Data and Intelligent Healthcare
376

nanowire array. In previous artificial retinas,
photoreceptors were first fabricated on a flat and rigid
substrate; later, they were either transferred to a
curved support surface or folded into a curved
substrate. This limits the density of imager units
because there must be space between them for
transport or folding. In contrast, the nanowires in this
new device are formed directly on the curved surface,
allowing them to be more closely bound together. In
fact, the density of nanowires is much higher than the
photoreceptors on the human retina. The signal from
each nanowire can be obtained separately, but the
pixels in current devices are composed of three or
four nanowires. The overall performance of artificial
eyeballs represents a leap forward for such devices.
Nevertherless, there is still a lot of work to be done.
Firstly, the photoelectric sensor array is only 10×10
pixels size large, and the gap between the pixels is
about 200-µm, which means the light detection area
is only 2 mm wide. In addition, the manufacturing
process involves some expensive and low-throughput
steps. For example, researchers used an expensive
process called focused ion beam etching to prepare
holes for the formation of each nanowire. In the
future, high-throughput manufacturing methods must
be developed to significantly reduce costs to produce
larger arrays of photosensitive elements.
Nonetheless, this work adds a strong touch to the
breakthroughs made in the past few decades. This
breakthrough was achieved by imitating camera-like
eyes and imitating insects-like eyes. Realized by
compound eyes. In view of these developments. It
seems possible for us to witness the widespread
application of artificial and bionic eyes in daily life in
the next decade (Stiles et al. 2010).
What is more, Chinese researcher Feng Miao’s
team proposed that a brain-like visual sensor based on
the vertical heterojunction of two-dimensional
materials can be built through the "atomic Lego"
method. These vertical structures can not only
naturally imitate the vertical layered structure of the
retina, but also contain the differences in the
heterojunction. Two-dimensional materials can be
used to simulate the functions of different cells in the
retina. And the number of electrodes in the artificial
retina system is an urgent hardware system difficulty
that needs to be overcome at present. Realize the
activation of independent electrodes corresponding to
independent retinal ganglion cells and improve the
visual resolution.
At the same time, researchers the United States
designed and constructed a spherical anterior retinal
chip that conforms to the macular curvature to expand
the retinal stimulation area and reduce the electrode-
retinal distance. Research on other hardware systems,
such as the development of intraocular cameras, is
used to replace external Glasses and cameras can be
set to improve the patient’s perception of spatial
positioning, and it also has huge development
potential. Studies have shown that it is a feasible new
method to elicit visual cortex action potentials
through retinal implants, but it is not certain whether
this device can achieve a certain degree of
independence for the blind. There are still many
problems to be solved. For example, it is necessary to
know whether the sense of orientation, motor
perception, and feature positioning are in the visual
cortex, how to achieve the long-term stability of the
implant, whether the retinal neurons can withstand
long-term stimulation without changes in their own
shape and function, and blind people receive
implants. The type of image that can be felt after
entering.
4 CONCLUSIONS
As the first way for humans to obtain and process
information, the human visual system has a
physiological mechanism that is significantly better
than that of optical systems. For the research of the
human visual system, people can achieve a wide
range of applications in biomedicine, machine
intelligence, and visual simulation. It is a research
topic with far-reaching prospects. The final goal of
artificial retina research is to use microelectrode
arrays to directly stimulate internal nerve cells to
replace the diseased retina, thereby restoring the
patient's vision. People need to study the biological
structure of the retina and the information processing
mechanism. Furthermore, people also need to make
microelectrodes that can be integrated with biological
nerves in terms of electronics. Although artificial
retina chips have been successfully developed, they
have been transplanted into human eyes for
experiments and have achieved certain results. There
is still a certain distance from practicality and
economy. And there is no unified model in the basic
theory.
ACKNOWLEDGMENTS
Many thanks to my professors and teachers, without
their help, this paper could not be completed.
Analysis on Recent Artificial Retina in Electrochemical and Mechanical Approaches
377

REFERENCES
Gu L L, Poddar S, Lin Y J, et al. (2020). A biomimetic eye
with a hemispherical perovskitenanowire array retina,
Nature, 581: 278.
Hauer, M. C., Tanguay, A. R., Nasiatka, P. J., & Stiles, N.
R. B. (2007) Intraocular camera for retinal prostheses.
Humayun, M., Propst, R., Juan, E. D., Mccormick, K., & D
Hickingbotham. (1994). Bipolar surface electrical
stimulation of the vertebrate retina. Archives of
Ophthalmology, 112(1), pp.110-116.
Humayun MS, de Juan E Jr, Weiland JD, Dagnelie G,
Katona S, Greenberg R, Suzuki S. (1999). Pattern
electrical stimulation of the human retina. Vision Res.
Jul;39 (15):2569-76.
Luo, H. L., & Cruz, L. D. (2016). The argus ii retinal
prosthesis system. Progress in Retinal & Eye Research,
50.
Rizzo, S., Belting, C., Cinelli, L., Allegrini, L., Genovesi-
Ebert, F., & Barca, F., et al. (2014). The argus ii retinal
prosthesis: 12-month outcomes from a single-study
center. American Journal of Ophthalmology, 157(6),
pp.1282-1290.
Sekirnjak, & C. (2006). Electrical stimulation of
mammalian retinal ganglion cells with multielectrode
arrays. Journal of Neurophysiology, 95(6), pp.3311-
3327.
Stiles, N., Mcintosh, B. P., Nasiatka, P. J., Hauer, M. C., &
Tanguay, A. (2010). An Intraocular Camera for Retinal
Prostheses: Restoring Sight to the Blind.
U.S. Department of Energy Office of Science. (2018). How
the Artificial Retina Works. Retrieved from
https://artificialretina.energy.gov/howartificialretinawo
rks.shtml
Wang, C. Y., Liang, S. J., Wang, S., Wang, P., Li, Z., &
Wang, Z., et al. (2020). Gate-tunable van der waals
heterostructure for reconfigurable neural network
vision sensor. arXiv.
Weiland, J. D., Humayun, M. S., Dagnelie, G. , Juan, E. D.,
Greenberg, R. J., & Iliff, N. T. (1999). Understanding
the origin of visual percepts elicited by electrical
stimulation of the human retina. Graefes Archive for
Clinical & Experimental Ophthalmology, 237(12),
pp.1007-1013.
Zhou, D. & Greenberg, R. (2005). Microsensors and
microbiosensors for retinal implants. Frontiers in
bioscience: a journal and virtual library. 10. 166-79.
10.2741/1518.
ICHIH 2022 - International Conference on Health Big Data and Intelligent Healthcare
378
