
Functional Oligosaccharides: The Preparation Methods and Therapy
Mechanism Related to Inflammatory Bowel Disease
Kangjia Jiang
a
, Minghan Zhao
b
, Yuting Han
c
, Le Su
d
, Xinli Liu
e
, Qiulin Yue
f
,
Song Zhang
*g
, and Lin Zhao
*h
School of Bioengineering Qilu University of Technology Shandong Academy of Sciences, Jinan, 250353, China
yueqiulin88@163.com, zhangsrz@163.com, iahb205@163.com
Keywords: Ulcerative Colitis, Oligosaccharides, Preparation, Mechanism, Inflammatory Bowel Disease.
Abstract: The incidence of inflammatory bowel disease has increased substantially in recent decades. Some studies
have found that oligosaccharides have anti-inflammatory, antioxidant and other physiological activities. This
article summarizes the research progress in the preparation of oligosaccharides and the treatment of colitis in
recent years. The preparation methods of oligosaccharides mainly focus on enzymatic degradation and acid
degradation. In addition, this article summarized the mechanisms of oligosaccharides in IBD, including the
regulation of inflammatory factors, regulation of oxidative stress, regulation of intestinal microbes, and
influence on inflammatory signal pathways. In summary, oligosaccharides have the potential to treat
inflammatory bowel disease, which provides new ideas for the clinical treatment of IBD.
1 INTRODUCTION
Crohn’s disease (CD) and ulcerative colitis (UC) are
two dominant forms of Inflammatory bowel disease
(IBD). In UC, the inflammatory process affects only
the mucosa and extends continuously from the rectum.
The typical symptom of colitis is bloody diarrhea,
which may be accompanied by abdominal pain or
fever(Besednova, Zaporozhets et al. (2020). The
prevalence of IBD is increasing every year worldwide.
Epidemiological studies show that in North America
more than 1.2 million people and 2 million people in
Europe suffer from IBD, and it exceeds 0.3% of the
population in many countries in Oceania, North
America and Europe. It is predicted that in 2025, the
total number of patients with IBD in the world would
be equal to the total population of Western countries,
and the treatment of IBD is imminent (Zhang, Huang
et al. (2019).
a
https://orcid.org/0000-0003-4117-3226
b
https://orcid.org/0000-0002-3396-4776
c
https://orcid.org/0000-0002-4545-0529
d
https://orcid.org/0000-0003-4794-6138
e
https://orcid.org/0000-0002-8416-7865
f
https://orcid.org/0000-0003-3904-3270
g
https://orcid.org/0000-0001-5160-8321
h
https://orcid.org/0000-0002-4016-3176
From the current point of view, IBD is thought to
be the result of the interaction of a number of factors.
NF-κB is an important signaling pathway in
inflammatory response, regulation of inflammatory
cytokine expression. Activation by NF-κB induces
the production of immune mediators. In intestinal
immunity in IBD, cytokines are important mediators
between activated cells and non-immune cells.
The intestinal barrier facilitates the separation of
substances and prevents the invasion of pathogenic
antigens(Dong, Li et al. 2020). Intestinal microbe is a
kind of microorganisms that live on the surface of
intestinal mucosa and intestinal lumen for a long time.
Patients with colitis were found to have less diversity
and a change in their gut flora composition. Intestinal
microorganisms can induce regulatory and protective
immune responses(Belkaid and Harrison 2017).
Studies have shown that reactive oxygen and
nitrogen are significantly associated with
Jiang, K., Zhao, M., Han, Y., Su, L., Liu, X., Yue, Q., Zhang, S. and Zhao, L.
Functional Oligosaccharides: The Preparation Methods and Therapy Mechanism Related to Inflammatory Bowel Disease.
DOI: 10.5220/0011369100003438
In Proceedings of the 1st International Conference on Health Big Data and Intelligent Healthcare (ICHIH 2022), pages 343-349
ISBN: 978-989-758-596-8
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
343

inflammatory bowel disease as they regulate the
associated oxidative stress and redox(Korenaga,
Takesue et al. 2002). Oxidative stress in IBD not only
produces excessive ROS/RNS, damages cell lipids
and other components, but also leads to mucosal
damage, dysfunction and inflammation, directly
leading to intestinal injury, and redox signaling
disorders, promoting the overexpression of
inflammatory factors and adhesion
molecules(Grisham and Granger 1988).
Currently, the drugs used in clinical treatment of
IBD include glucocorticoids, immunosuppressants
and biological agents. The main purpose is to relieve
the acute onset of inflammation, but personalized
treatment plans are still adopted in clinical
practice(Ramos de Mattos, Gracindo Garcia et al.
2015). However, their use is often harmful and has
side effects. Amino Salicylates can cause patients to
experience nausea and vomiting or other intestinal
side effects. Glucocorticoids can lead to osteoporosis,
hypertension, obesity, type 2 diabetes.
Glucocorticoids should not be used as long-term
treatment(Ramos de Mattos, Gracindo Garcia et al.
2015). Methotrexate in the treatment of IBD showed
a good anti-inflammatory effect, but nausea, vomiting,
and certain renal toxicity. Some heart conditions,
allergic or infectious complications and skin lesions
are side effects of anti-TNF-alpha drugs(Nielsen
2014).
Because of the severe side effects of traditional
drug therapy, an important way to improve the
clinical symptoms of IBD is to find new sources of
drugs. Oligosaccharides are attracting more and more
attention as prebiotics functional food ingredients.
Methods of obtaining include extraction from various
biological sources, or obtaining by enzymatic and
acid digestion of polysaccharides, or synthesis by
enzymatic transfer reactions using simple
oligosaccharides(Rastall 2010). Recently, other
physiological functions of oligosaccharides have
been found. For example, short-chain fatty acids, a
metabolite that provides energy to cells in the
intestine, can also accelerate cell renewal(Courtois
2009).
Many oligosaccharides in recent studies,
including fructo-oligosaccharides(Winkler, Butler et
al. 2007) and lacto-oligosaccharides(Algieri,
Rodriguez-Nogales et al. 2014), can alleviate damage
to the intestinal barrier. This article summarizes the
possibility of using oligosaccharides extracted from
natural substances or degraded by polysaccharides as
drugs or functional food products to prevent and treat
inflammatory bowel disease.
2 TYPES AND PREPARATION
OF OLIGOSACCHARIDES
2.1 Oligosaccharides Derived from
Plants
2.1.1 Oligogalacturonic Acid
Oligogalacturonic acid (OGA) is a polymer
consisting of 2~10 galacturonic acids attached by α-
1, 4-glycosidic bond(Huang, Huang et al. 2018).
Oligomeric isomalturonic acid esters (OGA) from
citrus pectin hydrolysed by microbial pectinases can
be used as food emulsifiers and have shown
antioxidant capacity and the ability to inhibit lipid
oxidation., and had bactericidal effect on foodborne
pathogens(Huang, Lu et al. 2011). The literature
reports that OGA fragments can prevent the
multiplication of human cancer cells (Wu, Li et al.
2014).
2.1.2 Xylo-oligosaccharides
Xyloglucan (XOS) is a common oligosaccharide
consisting of xylose(Seesuriyachan, Kawee-ai et al.
2017). XOS is mainly obtained by physical, chemical
and enzymatic degradation of xylan in corn cob,
bagasse and other agricultural Enzymatic method is
the main method for industrial production of xylo-
oligosaccharide due to its mild reaction conditions,
easy control, high conversion rate and environmental
friendliness(Chapla, Pandit et al. 2012). Adding XOS
to the diet to show the effect of XOS
probiotics(Karlsson, Schmitz et al. 2018). They can
significantly increase beneficial intestinal bacteria
and thus relieving the damage to the intestinal
tract(Christensen, Licht et al. 2014).
2.1.3 Fructose-oligosaccharides
FOS is an indigestible carbohydrate, chemically
composed mainly of a fructose unit chain and a
glucose unit connected by the glycoside bond beta -
(2-1) (Table 1). Their structure is formed by the
repeated combination of disaccharides such as
sucrose(Flores-Maltos, Mussatto et al. 2016). A few
articles have proposed new bioprocesses that can
integrate the production of inulin endosynthesis, FOS
fermentation and impurity removal into a single
reactor, left-handed disaccharide, which can
significantly improve the yield of FOS(Wang, Li et al.
2016). Fructo-oligosaccharides have low calorific
value, help intestinal absorption of ions, reduce lipid
and cholesterol levels, and stimulate Bifidobacteria.
ICHIH 2022 - International Conference on Health Big Data and Intelligent Healthcare
344

Because of its potential health benefits, purified linear
fructose oligomers are added to a variety of
foods(Bali, Panesar et al. 2015).
Table 1: Types and molecular structure of functional
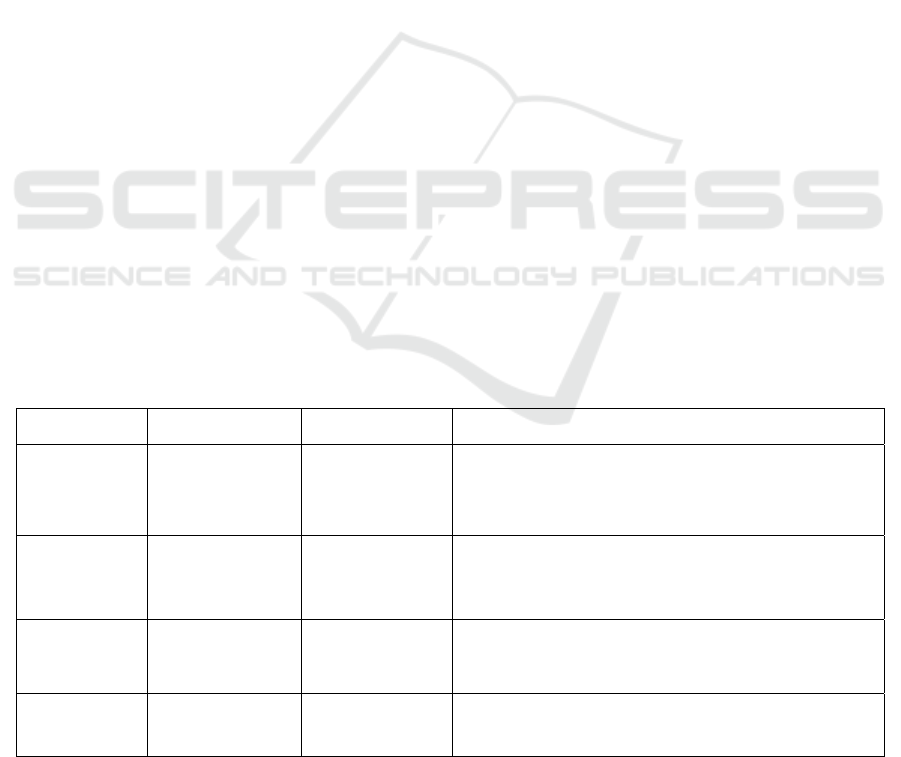
oligosaccharides.
Oligosaccharide Structure
Xylooligosaccharides
(
XOS
)
(Xylβ-1,4)n Xyl; n =1 ~ 4
Fructooligosaccharides
(FOS)
Glcα-1,2(Fruβ-1,2/β-1,6)n
Fru; n =1 ~ 3
Isomaltooligosaccharid
es (IMO)
Glcα-1,6 Glcα-1,6 Glc;
Glcα-1,4 Glcα-1,6 Glc
lactosucrose Galβ-1,4Glcα-1,2Fru
lactulose Galβ-1,4Fru
2.1.4 Oligomeric Mannose
Mannan oligosaccharide (MOS) consists of mannose
residue fragments attached by beta-1,4-mannose, and
is further characterized as galactomannan,
galactomannan, and galactomannan. They are usually
found in the endosperm of legumes(Singh, Singh et
al. 2018). At present, it mainly adopts physical
method, chemical method and enzyme method MOS
was prepared by degradation of mannan(Malgas, van
Dyk et al. 2015).
2.1.5 Oligomeric Maltose
Isomalto Oligosides (IMO) are functional
oligosaccharides with a degree of 2-10, consisting of
glycosylated units with α-1,6-glucosidic
bonds(Zhang, Wang et al. 2019). In general, IMO is
produced by the conversion of starch hydrolysates by
α -glucosidase in conventional industrial
processes(Huang, Li et al. 2018). IMO is a natural
functional oligosaccharide that regulates the
intestinal flora. For example, after using IMO, the
levels of Bifidobacteria and Lactobacilli are
increased(Shi, Hou et al. 2016).
2.1.6 Alginate Oligosaccharides
Alginic acid consisting of alpha-1, 4-glycosidic bonds
between manuronic acid and guluronic acid. AOS is
the depolymerization of alginate by enzymolysis
(Table 2), acid hydrolysis and oxidative degradation.
Alginate lyase is an important tool enzyme in the
production of AOS(Zhu, Ni et al. 2021). Alginate
oligosaccharide treatment can regulate intestinal
microbial community and significantly reduce the
expression of inflammatory markers, which has good
anti-inflammatory effect(Wang, Li et al. 2020).
2.1.7 Agarose Oligosaccharides
Agarose oligosaccharides (AGOs) are produced by
the hydrolysis of agarose. The structure and
biological activity of AGO have been extensively
studied(Chen and Yan 2005). AGOs has repeated
agarose units consisting of non-reducing d-galactose
and reducing 3, 6-dehydrogen-L-galactose
(Higashimura, Naito et al. 2014).
2.2 Oligosaccharides from Animals
2.2.1 Galactose Oligomeric
Galactosyl oligosaccharides (GOS) are formed by 1
to 10 galactosyl units connected to a terminal
glucose(Dai, Lyu et al. 2017), or formed from
galactose-based units only. β-galactosidase can
catalyze galactose acylation to produce galactose
oligosaccharides(You, Zhang et al. 2017). GOS
reduces the incidence of intestinal diseases and a
significant increase in the SCFAs concentration was
found. One study found that GOS has significant
potential to improve intestinal health and body
immunity(Wang, Zhu et al. 2020).
2.2.2 Oligo-chitosan
Chitosan is a polysaccharide with varying degrees of
n-acetylation. It is obtained from the degradation of
chitosan and is the n-deacetylated form of chitin.
Chitosan has been prepared by various enzymatic and
acid hydrolysis methods. In contrast, COS, the
hydrolysis product of chitosan, consisting of beta-1,4-
glycosidic bonds linking 2-amino-2-deoxy-D-
glucopyranose. The short chain of D-glucosamine
units in COS and the small number of free amino
groups make it more soluble under physiological
conditions(Lodhi, Kim et al. 2014).
3 ANTI - INFLAMMATORY
EFFECT AND MECHANISM OF
OLIGOSACCHARIDES
3.1 Effects on Cytokines
Inflammatory bowel disease (IBDs) is a debilitating
condition in which chronic inflammation leads to
intestinal damage. The cytokines produced by the
immune cells are involved in colitis due to the large
number of immune cells that infiltrate the
colon(Francescone, Hou et al. 2015). Tumour
Functional Oligosaccharides: The Preparation Methods and Therapy Mechanism Related to Inflammatory Bowel Disease
345
necrosis factor (TNF) has been reported to influence
cell proliferation, differentiation and apoptosis, and
has also been associated with inflammation and
cancer development(Liu 2005). Different target cells
are triggered by Il-6, to affect pro-inflammatory
functions(Kim, Keku et al. 2008). Studies have
shown that Il-1 β indirectly activates endothelial cells
and angiogenesis by regulating pro-inflammatory and
pro-angiogenic molecules(Carmi, Dotan et al. 2013).
Ferulic acid oligosaccharides can significant
reduction IL-23 and IL-6 in dendritic cells (DCs) in
vivo and in vitro, enhance the secretion of TGF-β1,
and regulate colitis in mice(Xia, Zhu et al. 2019).
Glucosamine oligomers reduced pro-inflammatory
factors in mouse serum and effectively alleviated
clinical signs of colitis in mice(Azuma, Osaki et al.
2015).
3.2 Effects on Inflammatory Signaling
Pathways
3.2.1 Effects on NF-κB Signaling Pathway
One way in which gene expression of inflammatory
cytokines is elevated is through NF-κB signaling
pathway(Atreya, Atreya et al. 2008). In the DSS-
induced mouse colitis model, NF -κB p65 protein
expression was suppressed because of galactose
intervention(Dai, Feng et al. 2018). Sucrose (LS)
significantly reduced the levels of TLR-2 protein and
NF-κB pathway protein. Therefore, LS has potential
as a nutritional intervention for colitis(Zhou, Ruan et
al. 2015).
3.2.2 Influence on MAPK Signaling Pathway
A variety of serine and threonine kinases make up the
mitogen-activated protein kinase (MAPK)(Wang,
Pan et al. 2019). It was found that fructose-
oligosaccharides can down-regulate the expressions
of Jun and JNK proteins in d-galactose induced rat
aging model, suggesting that fructose-
oligosaccharides may improve lung inflammation
and fibrosis in aging rats by inhibiting the activation
of JNK/Jun pathway, and has obvious anti-
inflammatory effect(Yeh, Wu et al. 2014).
3.3 Regulation of Oxidative Stress
Oxidative stress injury was significantly correlated
with the onset and severity of IBD(Rezaie, Parker et
al. 2007). Infiltrates of immune cells, especially
neutrophils, are histologic features of IBD. The
excessive production of ROS in host tissues
exacerbates oxidative damage and may damage the
mucosa of the intestine. Neutrophil-myeloperoxidase
(MPO), a granulocyte enzyme, increases the levels of
more potent ROS. Biomarkers of intestinal mucosal
damage appear significantly increased in patients
(Chami, Martin et al. 2018). Lactulose can prevent
and suppress intestinal inflammation, which can
significantly reduce myeloperoxidase activity, TNF-
α and leucotriene B4 concentration in colon(Algieri,
Rodriguez-Nogales et al. 2014). Hame oxygenase-1
(HO-1) has a significant antioxidant effect. A mouse
macrophage inflammation model (RAW264.7 cells)
was constructed with LPS and HO-1 expression was
upregulated by chitosan (COS) intervention(Hyung,
Ahn et al. 2016).
Table 2: Common preparation methods of oligosaccharides.
Polysaccharide Method Oligosaccharide Mechanism
Xylan Xylanase
Xylo-
oligosaccharides
The endonuclease cleaves the xylan chain at specific
cleavage sites to produce different oligosaccharides
(Karlsson, Schmitz et al. 2018).
Pectin Polygalacturonase
Oligogalacturonic
acid
Polygalacturonase catalyzes the cleavage of pectin
molecule poly-α-(1,4)-polygalacturonic acid and
participates in the degradation of pectin(Li, Coffman et
al. 2015).
Mannan Mannanase
Mannose
oligosaccharides
β-Mannanase randomly cleaves the backbone to produce
shorter beta-1,4-mannan oligomers. It is very important
in the preparation of mannans(Liu, Ning et al. 2020).
Sucrose
α-amylase、α-
glucosidase
Isomalt
oligosaccharide
The receptor reaction catalyzed by glucose glucosidase
can generate branched imo or glucose oligosaccharides
(GOSs) from sucrose(Goffin, Delzenne et al. 2011).
ICHIH 2022 - International Conference on Health Big Data and Intelligent Healthcare
346
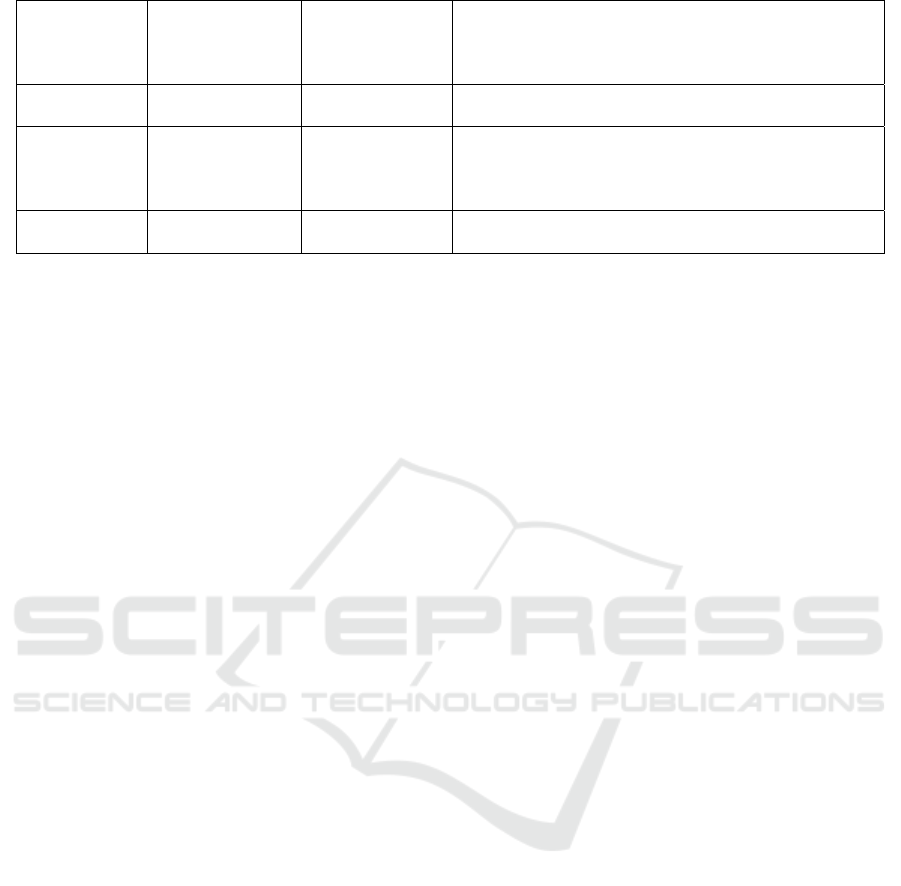
Alginate Alginate lyase
Alginate
oligosaccharide
Glycosidic bonds within alginate polymers are cleaved by
alginate lyase to produce unsaturated oligosaccharides,
while exonuclease can degrade the oligosaccharides
a
g
ain to become monomers
(
Zhu, Ni et al. 2021
)
.
Agar Agarase
Agaro
oligosaccharides
Breakage of the alpha-1,3-glycosidic bond of agarose by
alpha-agarase(Jiang, Cheng et al. 2021).
Lactose β-galactosidase
Galacto
oligosaccharide
Galacto oligosaccharides are produced by the conversion
of lactose through glycosylation and can be catalyzed by
many enzymes. It includes two processes: glycosylation
and de
g
l
y
cos
y
lation
(
Gao, Wu et al. 2019
)
.
Chitosan Chitosanase
Chitosan-
oligosaccharide
Chitosanase can hydrolyze the bata-(1-4)-sugar-silicic
acid bond of chitosan(Liu, Jiang et al. 2009).
3.4 Repair of the Intestinal Barrier
There are many factors that affect intestinal health
and the intestinal barrier is a very significant part. The
composition of the intestinal barrier includes the
mucus layer and the intestinal epithelium. The
mucosal barrier consists of gel formation and
transmembrane mucin. It prevents the entry of
pathogens. Therefore, the intestinal barrier is
implicated in IBD(Johansson, Sjovall et al. 2013). It
was found that in mice with acetic acid-induced
colitis, COS infusion reduced the inflammatory
response and restored intestinal barrier
damage(Yousef, Pichyangkura et al. 2012).
Interestingly, another study showed that COS can
promote T84 cell tight-knit assembly(Muanprasat,
Wongkrasant et al. 2015).
3.5 Influence on Intestine Flora
Intestinal microbe is a kind of microorganisms that
live on the surface of intestinal mucosa and intestinal
lumen for a long time. It was found that altered
intestinal flora diversity in UC patients, and the group
was transformed(Frank, Amand et al. 2007). In the
colon, AG and FOS exhibit different fermentation
properties (FOS in the proximal end, AG in the distal
end). These two fibers can repair the intestinal barrier
and effectively reduce inflammation(Daguet,
Pinheiro et al. 2016). Konjac oligosaccharide can
significantly relieves inflammatory symptoms of
experimental acute colitis induced by TNBS, and
improve intestinal flora structure. Therefore, the
mechanism is at least involved in the improvement of
intestinal microflora structure and anti-inflammatory
effect(Liu, Li et al. 2016).
4 CONCLUSIONS
In recent decades, the research on oligosaccharides
has been the focus of research at home and abroad.
The preparation methods of functional
oligosaccharides mainly include chemical method,
enzymatic method and physical method. Among them,
enzymatic preparation is green, efficient, and the
most promising method for application.
Oligosaccharides have a positive impact on the relief
of other intestinal disorders, but they are effective in
the intervention of oligosaccharides. With further
research into the mechanism of action of
oligosaccharides in reducing inflammation, high-
efficiency and non-toxic oligosaccharide anti-
inflammatory drugs will be screened.
ACKNOWLEDGMENTS
This work was supported by Science Foundation of
China (31501396) and Shandong Taishan leading
talent project (grant number LJNY202015,
tscy20180507).
REFERENCES
Algieri, F., et al. (2014). "Intestinal Anti-inflammatory
Effects of Oligosaccharides Derived from Lactulose in
the Trinitrobenzenesulfonic Acid Model of Rat Colitis."
Journal of Agricultural and Food Chemistry 62(19):
4285-4297.
Atreya, I., et al. (2008). "NF-kappa B in inflammatory
bowel disease." Journal of Internal Medicine 263(6):
591-596.
Azuma, K., et al. (2015). "Anti-inflammatory effects of
orally administered glucosamine oligomer in an
experimental model of inflammatory bowel disease."
Carbohydrate Polymers 115: 448-456.
Bali, V., et al. (2015). "Fructo-oligosaccharides: Production,
Purification and Potential Applications." Critical
Reviews in Food Science and Nutrition 55(11): 1475-
1490.
Belkaid, Y. and O. J. Harrison (2017). "Homeostatic
Immunity and the Microbiota." Immunity 46(4): 562-
Functional Oligosaccharides: The Preparation Methods and Therapy Mechanism Related to Inflammatory Bowel Disease
347

576.
Besednova, N. N., et al. (2020). "Extracts and Marine Algae
Polysaccharides in Therapy and Prevention of
Inflammatory Diseases of the Intestine." Marine Drugs
18(6).
Carmi, Y., et al. (2013). "The Role of IL-1 beta in the Early
Tumor Cell-Induced Angiogenic Response." Journal of
Immunology 190(7): 3500-3509.
Chami, B., et al. (2018). "Myeloperoxidase in the inflamed
colon: A novel target for treating inflammatory bowel
disease." Archives of Biochemistry and Biophysics 645:
61-71.
Chapla, D., et al. (2012). "Production of
xylooligosaccharides from corncob xylan by fungal
xylanase and their utilization by probiotics."
Bioresource Technology 115: 215-221.
Chen, H.-M. and X.-J. Yan (2005). "Antioxidant activities
of agaro-oligosaccharides with different degrees of
polymerization in cell-based system." Biochimica et
biophysica acta 1722(1): 103-111.
Christensen, E. G., et al. (2014). "Dietary xylo-
oligosaccharide stimulates intestinal bifidobacteria and
lactobacilli but has limited effect on intestinal integrity
in rats." BMC research notes 7: 660-660.
Courtois, J. (2009). "Oligosaccharides from land plants and
algae: production and applications in therapeutics and
biotechnology." Current Opinion in Microbiology 12(3):
261-273.
Daguet, D., et al. (2016). "Arabinogalactan and
fructooligosaccharides improve the gut barrier function
in distinct areas of the colon in the Simulator of the
Human Intestinal Microbial Ecosystem." Journal of
Functional Foods 20: 369-379.
Dai, Z. Q., et al. (2018). "Anti-inflammatory effects of
newly synthesized a-galacto-oligosaccharides on
dextran sulfate sodium-induced colitis in C57BL/6J
mice." Food Research International 109: 350-357.
Dai, Z., et al. (2017). "Effects of alpha-
Galactooligosaccharides from Chickpeas on High-Fat-
Diet-Induced Metabolic Syndrome in Mice." Journal of
Agricultural and Food Chemistry 65(15): 3160-3166.
Dong, N., et al. (2020). "Astragalus polysaccharides
alleviates LPS-induced inflammation via the NF-kappa
B/MAPK signaling pathway." Journal of Cellular
Physiology 235(7-8): 5525-5540.
Flores-Maltos, D. A., et al. (2016). "Biotechnological
production and application of fructooligosaccharides."
Critical Reviews in Biotechnology 36(2): 259-267.
Francescone, R., et al. (2015). "Cytokines, IBD, and
Colitis-associated Cancer." Inflammatory Bowel
Diseases 21(2): 409-418.
Frank, D. N., et al. (2007). "Molecular-phylogenetic
characterization of microbial community imbalances in
human inflammatory bowel diseases." Proceedings of
the National Academy of Sciences of the United States
of America 104(34): 13780-13785.
Gao, X., et al. (2019). "Rational design of the beta-
galactosidase from Aspergillus oryzae to improve
galactooligosaccharide production." Food Chemistry
286: 362-367.
Goffin, D., et al. (2011). "Will Isomalto-Oligosaccharides,
a Well-Established Functional Food in Asia, Break
through the European and American Market? The
Status of Knowledge on these Prebiotics." Critical
Reviews in Food Science and Nutrition 51(5): 394-409.
Grisham, M. B. and D. N. Granger (1988). "Neutrophil-
mediated mucosal injury. Role of reactive oxygen
metabolites." Digestive diseases and sciences 33(3
Suppl): 6S-15S.
Higashimura, Y., et al. (2014). "Preventive effect of agaro-
oligosaccharides on non-steroidal anti-inflammatory
drug-induced small intestinal injury in mice." Journal
of Gastroenterology and Hepatology 29(2): 310-317.
Huang, C.-S., et al. (2018). "Synergistic Antitumor Effect
of Oligogalacturonides and Cisplatin on Human Lung
Cancer A549 Cells." International Journal of Molecular
Sciences 19(6).
Huang, P.-H., et al. (2011). "Antioxidant Activity and
Emulsion-Stabilizing Effect of Pectic Enzyme Treated
Pectin in Soy Protein Isolate-Stabilized Oil/Water
Emulsion." Journal of Agricultural and Food Chemistry
59(17): 9623-9628.
Huang, Z., et al. (2018). "Continuous Production of
Isomalto-oligosaccharides by Thermo-inactivated Cells
of Aspergillus niger J2 with Coarse Perlite as an
Immobilizing Material." Applied Biochemistry and
Biotechnology 185(4): 1088-1099.
Hyung, J. H., et al. (2016). "Involvement of Nrf2-mediated
heme oxygenase-1 expression in anti-inflammatory
action of chitosan oligosaccharides through MAPK
activation in murine macrophages." European Journal
of Pharmacology 793: 43-48.
Jiang, C., et al. (2021). "Advances in agaro-
oligosaccharides preparation and bioactivities for
revealing the structure-function relationship." Food
Research International 145.
Johansson, M. E. V., et al. (2013). "The gastrointestinal
mucus system in health and disease." Nature Reviews
Gastroenterology & Hepatology 10(6): 352-361.
Karlsson, E. N., et al. (2018). "Endo-xylanases as tools for
production of substituted xylooligosaccharides with
prebiotic properties." Applied Microbiology and
Biotechnology 102(21): 9081-9088.
Kim, S., et al. (2008). "Circulating levels of inflammatory
cytokines and risk of colorectal adenomas." Cancer
Research 68(1): 323-328.
Korenaga, D., et al. (2002). "Impaired antioxidant defense
system of colonic tissue and cancer development in
dextran sulfate sodium-induced colitis in mice." The
Journal of surgical research 102(2): 144-149.
Li, Q., et al. (2015). "Development of reproducible assays
for polygalacturonase and pectinase." Enzyme and
Microbial Technology 72: 42-48.
Liu, R. X., et al. (2016). "The effects of konjac
oligosaccharide on TNBS-induced colitis in rats."
International Immunopharmacology 40: 385-391.
Liu, Y.-L., et al. (2009). "Recombinant expression of a
chitosanase and its application in chitosan
oligosaccharide production." Carbohydrate Research
344(6): 815-819.
ICHIH 2022 - International Conference on Health Big Data and Intelligent Healthcare
348

Liu, Z. G. (2005). "Molecular mechanism of TNF signaling
and beyond." Cell research 15(1): 24-27.
Liu, Z., et al. (2020). "High-level expression of a
thermophilic and acidophilic beta-mannanase from
Aspergillus kawachii IFO 4308 with significant
potential in mannooligosaccharide preparation."
Bioresource Technology 295.
Lodhi, G., et al. (2014). "Chitooligosaccharide and Its
Derivatives: Preparation and Biological Applications."
Biomed Research International 2014.
Malgas, S., et al. (2015). "A review of the enzymatic
hydrolysis of mannans and synergistic interactions
between beta-mannanase, beta-mannosidase and alpha-
galactosidase." World Journal of Microbiology &
Biotechnology 31(8): 1167-1175.
Muanprasat, C., et al. (2015). "Activation of AMPK by
chitosan oligosaccharide in intestinal epithelial cells:
Mechanism of action and potential applications in
intestinal disorders." Biochemical Pharmacology 96(3):
225-236.
Nielsen, O. H. (2014). "New strategies for treatment of
inflammatory bowel disease." Frontiers in medicine 1:
3-3.
Ramos de Mattos, B. R., et al. (2015). "Inflammatory
Bowel Disease: An Overview of Immune Mechanisms
and Biological Treatments." Mediators of Inflammation
2015.
Rastall, R. A. (2010). Functional Oligosaccharides:
Application and Manufacture. Annual Review of Food
Science and Technology, Vol 1. M. P. Doyle and T. R.
Klaenhammer. 1: 305-339.
Rezaie, A., et al. (2007). "Oxidative stress and pathogenesis
of inflammatory bowel disease: An epiphenomenon or
the cause?" Digestive Diseases and Sciences 52(9):
2015-2021.
Seesuriyachan, P., et al. (2017). "Green and chemical-free
process of enzymatic xylooligosaccharide production
from corncob: Enhancement of the yields using a
strategy of lignocellulosic destructuration by ultra-high
pressure pretreatment." Bioresource Technology 241:
537-544.
Shi, Q., et al. (2016). "Optimization of
Isomaltooligosaccharide Size Distribution by Acceptor
Reaction of Weissella confusa Dextransucrase and
Characterization of Novel alpha-(1 -> 2)-Branched
Isomaltooligosaccharides." Journal of Agricultural and
Food Chemistry 64(16): 3276-3286.
Singh, S., et al. (2018). "Mannans: An overview of
properties and application in food products'."
International Journal of Biological Macromolecules
119: 79-95.
Szade, A., et al. (2009). "The role of heme oxygenase-1 in
the inflammatory bowel diseases." Przeglad
Gastroenterologiczny 4(6): 283-287.
Wang, D., et al. (2016). "A one-step bioprocess for
production of high-content fructo-oligosaccharides
from inulin by yeast." Carbohydrate Polymers 151:
1220-1226.
Wang, G., et al. (2020). "Optimization for
galactooligosaccharides synthesis: A potential
alternative for gut health and immunity." Life Sciences
245.
Wang, J. W., et al. (2019). "Salidroside regulates the
expressions of IL-6 and defensins in LPS-activated
intestinal epithelial cells through NF-kappa B/MAPK
and STAT3 pathways." Iranian Journal of Basic
Medical Sciences 22(1): 31-37.
Wang, Y., et al. (2020). "Alginate oligosaccharide improves
lipid metabolism and inflammation by modulating gut
microbiota in high-fat diet fed mice." Applied
Microbiology and Biotechnology 104(8): 3541-3554.
Winkler, J., et al. (2007). "Fructo-oligosaccharide reduces
inflammation in a dextran sodium sulphate mouse
model of colitis." Digestive Diseases and Sciences
52(1): 52-58.
Wu, M.-C., et al. (2014). "Assessment of
Oligogalacturonide from Citrus Pectin as a Potential
Antibacterial Agent against Foodborne Pathogens."
Journal of Food Science 79(8): M1541-M1544.
Xia, X., et al. (2019). "Feruloylated Oligosaccharides
Alleviate Dextran Sulfate Sodium-Induced Colitis in
Vivo." Journal of Agricultural and Food Chemistry
67(34): 9522-9531.
Yeh, S. L., et al. (2014). "Fructo-oligosaccharide attenuates
the production of pro-inflammatory cytokines and the
activation of JNK/Jun pathway in the lungs of D-
galactose-treated Balb/cJ mice." European Journal of
Nutrition 53(2): 449-456.
You, S., et al. (2017). "Development of a novel integrated
process for co-production of beta-galactosidase and
ethanol using lactose as substrate." Bioresource
Technology 230: 15-23.
Yousef, M., et al. (2012). "Chitosan oligosaccharide as
potential therapy of inflammatory bowel disease:
Therapeutic efficacy and possible mechanisms of
action." Pharmacological Research 66(1): 66-79.
Zhang, F., et al. (2019). "Heterologous Expression of a
Thermostable alpha-Glucosidase from Geobacillus sp.
Strain HTA-462 by Escherichia coli and Its Potential
Application for Isomaltose-Oligosaccharide
Synthesis." Molecules 24(7).
Zhang, L.-J., et al. (2019). "Protective effect of three
glucomannans from different plants against DSS
induced colitis in female BALB/c mice." Food &
Function 10(4): 1928-1939.
Zhou, Y., et al. (2015). "A diet with lactosucrose
supplementation ameliorates trinitrobenzene sulfonic
acid-induced colitis in rats." Food & Function 6(1):
162-172.
Zhu, B., et al. (2021). "Marine oligosaccharides originated
from seaweeds: Source, preparation, structure,
physiological activity and applications." Critical
Reviews in Food Science and Nutrition 61(1): 60-74.
Functional Oligosaccharides: The Preparation Methods and Therapy Mechanism Related to Inflammatory Bowel Disease
349
