
The Genetic Basis of AD Incidence and Treatment
Yiying Zhu
BSc Biomedical Sciences, Division of Biosciences, University College London, Gower Street, London, WC1E 6BT, U.K.
Keywords: Neurosciences, Alzheimer’s Disease, Transgenic Mouse, Gene Therapy.
Abstract: Alzheimer’s disease (AD) is caused by neuron death and is one of the diseases that cannot be cured under
current medical technology. This review provides a summary of the relationship between the expression of
three common AD-related gene variants (APP, PSEN1 and PSEN2) and the formation of abnormal Aβ and
tau proteins aggregations (plaques and tangles). The possibility of using gene therapy to cure AD is also stated
in this review. During research, several recent papers about gene-related AD are viewed. In those papers,
researchers did experiment on transgenic mice and matched the results with markers of human AD to confirm
that investigated gene variants are AD-related. However, mouse models cannot represent whole human AD
characteristics, symptoms like language deficits cannot be investigated on mouse models. Furthermore,
researches show that gene therapy such as overexpression of PGC-1α can eliminate AD-like symptoms in
transgenic mouse models. It illustrates the potential of treating AD by using this type of gene therapy.
Importantly, genetic technology is still under exploration before the safe application of gene therapy on
humans due to possible unknown consequences of editing human genes.
1 INTRODUCTION
Among aged people, AD is a common
neurodegenerative disease. It is mainly represented
by cognitive impairment and memory loss led by
death of nerve cells and diminished synapses. In order
to reduce the suffering of both AD patients and their
families, lots of researchers are trying to know more
about the pathogenesis of AD and find out effective
therapies. According to known information, carriers
of some mutant genes are more possible to develop
AD (Jeong 2017). Reviewing previous papers aims to
list the most common gene variants, which were
confirmed to be able to enhance AD incidence, and
show the pathways they use. For example, gene
mutations might cause abnormal proteins production
to disturb both transmission and survival of neurons
(Thal, Fändrich 2015). During investigation on
curing AD, it is nonnegligible that the commonly
used AD therapies are only for AD symptoms
attenuation but do not have real therapeutic effects.
However, as gene mutations can cause AD, editing
specific gene is possible to treat AD and it has been
proofed feasible on mouse models (Katsouri, Lim,
Blondrath, Eleftheriadou, Lombardero, Birch,
Mirzaei, Irvine, Mazarakis, Sastre 2016). The
therapeutic effects on mouse models show that it is
worth to keep an eye on how gene therapy can cure
human AD without causing unwanted side effects. As
there are too many differences between mice and
humans, future investigations can focus more on
other primates which are closer to humans than
rodents.
2 BASIC INFORMATION ABOUT
ALZHEIMER’S DISEASE
The degeneration of neurons and their connections in
the AD brain is mostly due to accumulation of two
misfolded proteins in the brain: amyloid β-peptide
(Aβ) and tau-protein (an accessory protein of
microtubule). In AD brains, Aβ aggregate to form
intercellular plaques; tau-proteins which do not
correctly attach to microtubule make intracellular
twisted fibres (tangles) (Thal, Fändrich 2015). Aβ
oligomers (intermediate before forming fibril from
Aβ peptides) disturb synaptic plasticity so they can
cause long-term depression and further synapse loss
(Jeong 2017). In addition, the extracellular Aβ
plaques reduce diffusion among cells, therefore result
in decayed neurons communication (Gendron,
Petrucelli 2009). Tau filaments inside cells cause
neurons death because they can displace the location
Zhu, Y.
The Genetic Basis of AD Incidence and Treatment.
DOI: 10.5220/0011368900003438
In Proceedings of the 1st International Conference on Health Big Data and Intelligent Healthcare (ICHIH 2022), pages 325-331
ISBN: 978-989-758-596-8
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
325
and reduce the number of organelles, disturb cellular
homeostasis and impair microtubule dynamics (Thal,
Fändrich 2015). Except for the two proteins, neuron
degeneration can also arise from several other factors,
for instance, chronic inflammation by dysfunctional
glial cells and reduced cerebral vascular blood flow
(https://www.nia.nih.gov/health/what-happens-
brain-alzheimers-disease).
Brain shrinking caused by neurodegeneration
with a specific pathway is an important characteristic
of AD. The entorhinal cortex and the hippocampus,
which are significant brain regions about memory,
are usually the starting points of it. Then, it slowly
spreads into medial parietal, lateral temporal and
frontal regions. Finally, the whole cerebral cortex can
be affected by atrophy, the patients cannot handle
daily tasks (Fjell, McEvoy, Holland, Dale, Walhovd
2014).
Researchers believe that there are various risk
factors that are responsible for the pathogenesis of
AD, such as, age, genetic inheritance, exposure to
aluminium, traumatic brain injury, vascular diseases
(A Armstrong 2019). Although family history is not
the most prominent risk factor of AD, understanding
the mechanisms of the gene mutations that are related
to the neurons degeneration and finding out the
suitable genetic treatments can be effective in
delaying and curing AD.
3 GENETIC EFFECT ON
INCIDENCE OFAD
3.1 Early-onset Familial AD
Early-onset familial AD(EOFAD) usually happens
under 65-year-old and has high heredity, which is
about 92-100%. EOFADs that follow Mendelian
inheritance occupy about 10-15% of all EOFADs and
are confirmed to be associated with mutations on
APP (Amyloid protein precursor), PSEN1
(Presenilin-1) and PSEN2 (Presenilin-2) genes
(duplications and missense) (Ayodele, Rogaeva,
Kurup, Beecham, Reitz, 2021). These mutations have
a similar effect, which is increased Aβ42 to Aβ40
ratio. Because Aβ42 is more prone to aggregation,
plaques are more possible to be formed (Tanzi 2012).
APP is the gene that is mapped into chromosome
21, which codes for amyloid precursor protein (APP)
(Tanzi 2012). Both 40 and 42 amino acids long Aβs
(Aβ40 and Aβ42) can be produced by proteolytic
processing of APP by β-secretase and γ-secretase
(Jeong 2017). In Nilsson and colleagues’ APP knock-
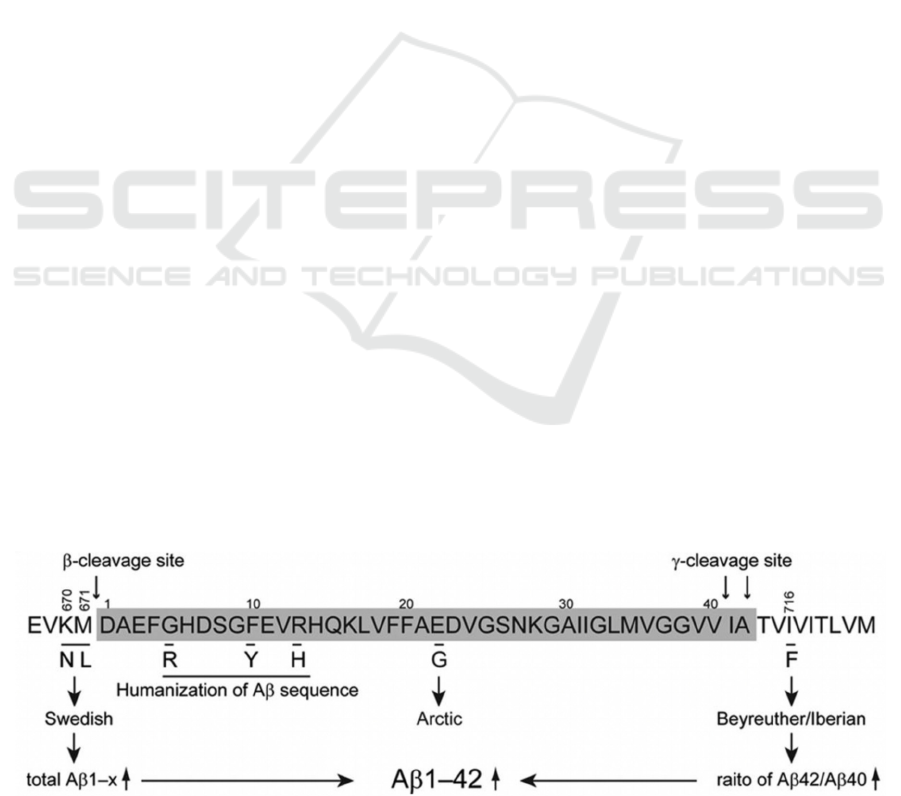
in mouse models, as figure 1 shows, 2 clinical
mutations are introduced to mice APP genes. The
Swedish mutation (KM670/671NL) increases β-
cleavage so enhance total Aβ production and the
Beyreuther/Iberian (I716F) mutation increases γ-
cleavage to increase Aβ42/Aβ40 ratio. Observation
shows formation of plaques that mainly contained
Aβ42 appears at the age of 6 months. In addition,
microglia and astrocytes also accumulated near the
plaques. These observations in APP mutant mice are
consistent with the pathology that can be found in the
human AD brains. The abnormal Aβ accumulation
later reduced synaptic plasticity and led to memory
impairment in the 18 months old transgenic mice,
which also match the symptom of AD (Nilsson, Saito,
Saido 2014).
PSEN1 gene locates on chromosome 14 and
codes PS1 protein, which comprises the γ-secretase
catalytic site (Tanzi 2012, Sasaguri, Nagata,
Sekiguchi, Fujioka, Matsuba, Hashimoto, Sato,
Kurup, Yokota, Saido 2018). In the analysis of
PSEN1-P436S and PSEN1-P117L transgenic mice,
results show that these mutations induce abnormal
cleavage activity of γ-secretase and increase
Aβ42/Aβ40 ratio (Sasaguri, Nagata, Sekiguchi,
Fujioka, Matsuba, Hashimoto, Sato, Kurup, Yokota,
Saido 2018). The reason is that changes in PS1
protein can affect the conformation of catalytic
component of γ-secretase and eventually change its
activity.
Only a single PSEN1 mutation might be
Figure 1: Different mutations and their effects (Nilsson, Saito, Saido 2014).
ICHIH 2022 - International Conference on Health Big Data and Intelligent Healthcare
326
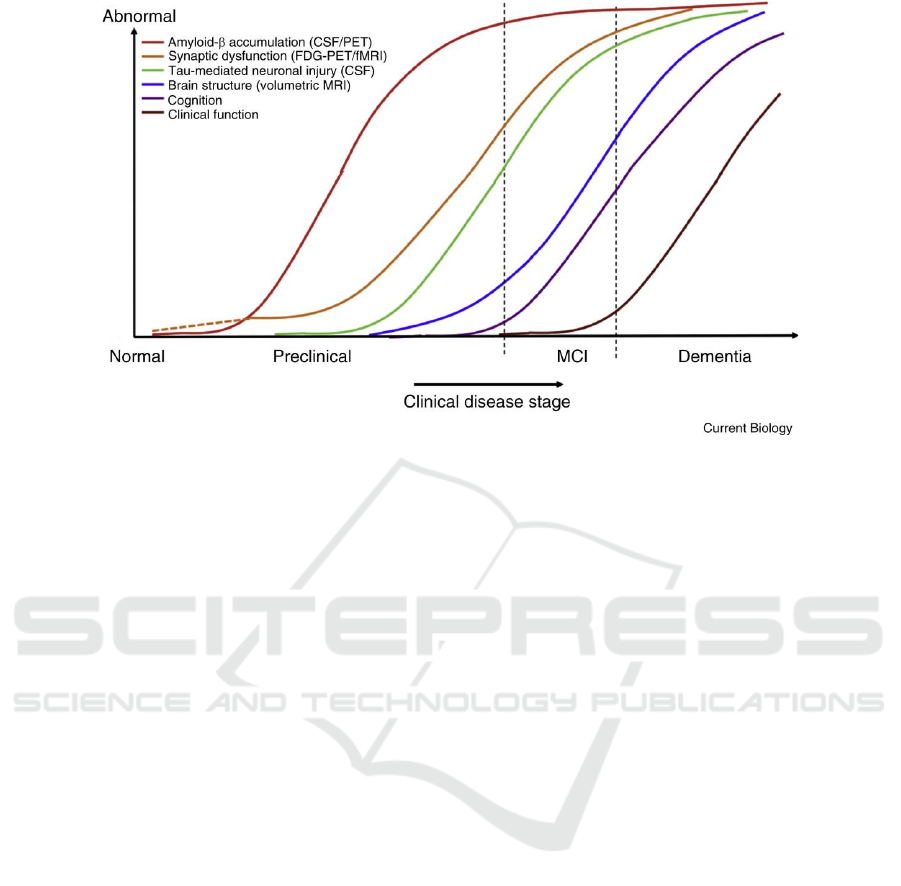
Figure 2. Sequential changes of biomarkers of AD over time (Grøntvedt, Schröder, Sando, White, Bråthen, Doeller 2018)
insufficient to observe all AD characteristics such as
change in behaviors on transgenic mice (Sasaguri,
Nagata, Sekiguchi, Fujioka, Matsuba, Hashimoto,
Sato, Kurup, Yokota, Saido 2018). Nevertheless,
figure 2 illustrates that the abnormal rising Aβ level
is an important biomarker for AD which shows up
first (Grøntvedt, Schröder, Sando, White, Bråthen,
Doeller 2018). The observation on PSEN1 gene
mutant mice is consistent with the biomarker so
PSEN1 mutations can be proofed to be AD-related.
PSEN2 is mapped on chromosome 1 (Tanzi
2012). Similar to PSEN1 mutations, mutant PSEN2
gene also raises Aβ42 level by altering γ-secretase
activity (Giau, Bagyinszky, Youn, An SSA, Kim
2019). It might be even harder to investigate the
influence of mutant PSEN2 individually because its
expression is about 10 folds lower than that of PSEN1
in the brain (Ayodele, Rogaeva, Kurup, Beecham,
Reitz 2021). However, researches show that PSEN2
mutation can accelerate Aβ accumulation and
memory impairment in transgenic mice with APP
mutation. In Fedeli and colleagues’ experiment, to
introduce PSEN2 mutation into APP mutant mouse,
they cross PSEN2 mutant female (N1411) with
Swedish APP mutant male which expresses
humanized Aβ sequence (Tg2576). The double
mutant mouse shows early Aβ aggregation at 2-3
months of age ( 6 months in Tg2576 mice), and also
early impaired learning and memory function at 4-5
months of age (7-8 months in Tg2576 mice) (Toda,
Noda, Ito, Maeda, Shimizu 2011). The observations
fit human AD biomarker and symptoms respectively.
The rest 85-90% EOFADs do not follow
Mendelian inheritance and are caused by other
unknown gene mutations. They are believed to be
induced by undentified or mixed genetic variants
(Ayodele, Rogaeva, Kurup, Beecham, Reitz 2021). It
might be helpful in developing gene therapy of AD if
more AD-related gene variants can be discovered in
the future.
3.2 Late-onset Familial AD
Late-onset sporadic AD (LOSAD) patients are
usually more than 65 years old. Unlike EOFAD,
LOSAD does not have a particular mode of
transmission so weaker familial clustering (Tanzi
2012). APOE gene on chromosome 17 is popular
while studying AD since it is considered the most
common gene that is related to AD (Safieh, Korczyn,
Michaelson 2019). It codes for apolipoprotein E
(apoE protein), a lipid binding and transporting
carrier protein, which is important for the cholesterol
transport in and out the central nervous system
(CNS), Aβ binding, clearance and synaptic function
in the brain (Theendakara, Peters-Libeu, Bredesen,
Rao 2018, Dorey, Chang, Liu, Yang, Zhang 2014).
There are 3 types of alleles of APOE gene, APOE ε4,
APOE ε3 and APOE ε2 (sequence from high to low
risk of developing AD) (Theendakara, Peters-Libeu,
Bredesen, Rao 2018). APOE4 allele is considered as
a significant risk factor of LOSAD (Jeong 2017).
First, APOE4 can increase Aβ deposition in the
brain. In Youmans and colleagues’ mouse models,
The Genetic Basis of AD Incidence and Treatment
327
They cross female mice, which have five familial
AD-related gene muations, with male homozygous
APOE2-, APOE3- and APOE4- mice to produce
EFAD mice. The results indicate that E4FAD mice
have higher Aβ42 levels and plaque deposition than
both E2FAD and E3FAD mice (Youmans, Tai,
Nwabuisi-Heath, Jungbauer, Kanekiyo, Gan, Kim,
Eimer, Estus, Rebeck, Weeber, Bu, Yu, Ladu 2012).
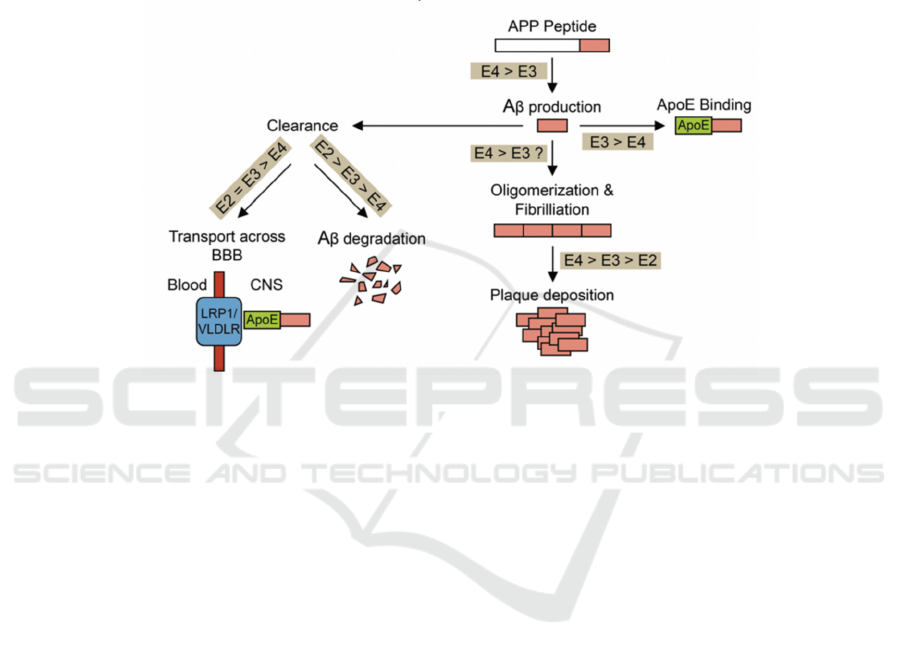
According to figure 3, APOE4 gene have these effects
by increasing production and reduce loss of Aβ
(Dorey, Chang, Liu, Yang, Zhang 2014). APOE4
protein alters γ-cleavage on APP gene to enhance
Aβ42 production, therefore following plaque
deposition as well. Additionally, APOE4 carriers
have reduced elimination of Aβ because: It impairs
degradation of Aβ; it can not cross the blood brain
barrier effectively; APOE3 can form complex with
Aβ to stop fibrillation but APOE4 cannot (Safieh,
Korczyn, Michaelson 2019).
Figure 3. comparison of interaction between APOE gene alleles and Aβ (Dorey, Chang, Liu, Yang, Zhang, 2014)
In addition, APOE4 gene can interact with tau-
protein to increase risk of AD as well (Safieh,
Korczyn, Michaelson 2019). In Shi and colleagues’
investigation, tau transgenic mice (P301S) are treated
by human APOE knock-in or APOE knock out. The
comparison among observations in P301S/E2,
P301S/E3, P301S/E4 and P301S/KO mice shows that
P301S/E4 mice have higher tau level in the brain,
which might results from weak autophagy-mediated
tau clearance caused by APOE4 (Shi 2017).
Additionally, APOE protein can affect the
hyperphosphorylation of tau. APOE3 protein can
bind tau effectively to prevent accumulation while
APOE4 protein cannot; APOE4 protein is stronger in
escaping secretion so it can stay in cytoplasm to
phosphorylate tau to greater extent through both
direct and indirect interaction (Safieh, Korczyn,
Michaelson 2019). The P301S/E4 mice are shown to
have greater hyperphosphorylated tau (ptau) covered
area (Shi 2017). Since neurofibrillary tangles are
mainly constituted by hyperphosphorylated tau and
can directly lead to neurodegeneration, APOE4
significantly raises the risk of developing AD.
There are still problems with AD-related gene
mutations investigations. As scientists cannot do
transgenic experiments on human, and also AD
patients might be affected by various other factors
like different lifestyles, the variables cannot be
controlled strictly while investigating relationship
between mutant genes and the incidence of AD. In
addition, although mouse models are a quite useful
tool, it is impossible to present AD symptoms on
functions beyond rodents’ memory system including
language and episodic memory on them. It is also
important to know that human AD is not only decided
by a single gene mutation, it might comes from the
combination of several mutations and even some
environmental factors. In this case, it is difficult to
present all of these on mouse models. Nevertheless,
the experimental results are still meaningful after
bringing the biomarkers for human AD and other AD-
like symptoms on the transgenic mice together.
Hence, the screening of AD-related genes can be a
reference while evaluating the AD onset possibility of
a person, and also help to make a judgment on
whether early treatment is needed or not. People
should also understand that having AD-related genes
does not mean that they will develop AD for sure,
those genes only means their possibility of having AD
is higher.
ICHIH 2022 - International Conference on Health Big Data and Intelligent Healthcare
328

4 TREATMENT OF AD
The treatment of AD is usually a hot topic among
researchers who are interested in AD. Nowadays, a
common drug for AD patients is Tacrine. Because
acetylcholine is an important neurotransmitters in the
brain and it is broken down by acetylcholinesterase.
These drugs can increase acetylcholine level in
synapses by inhibiting acetylcholinesterase activity.
Therefore the loss of neurons in AD brains can be
offset by the increased activity of survived neurons.
However, these drugs can only attenuate the
symptoms of AD but have no curative effect since
they are not reducing the plaques and tangles, neurons
keep degenerating and AD keeps getting worse.
In order to cure AD, the death of neurons should
be stopped, so the plaques and tangles which cause
this must be eliminated. Hence, both reducing the
formation and increasing the clearance of plaques and
tangles should be considered. In this case, gene
therapy might be the most efficient way to achieve the
aim because proteins are coded by DNA. By editing
patients’ DNA, increasing the expression of proteins
which can inhibit the formation or promote the
clearance of key AD proteins, might be able to
decrease or even remove the plaques and tangles in
AD brain. Gene therapy of AD is proofed feasible on
mouse models. Katsouri and colleagues found that
overexpression of PPARγ coactivator-1α (PGC-1α)
gene can reduce the secreted level of insoluble Aβ by
reducing the transcription of β-secretase through co-
activating nuclear peroxisome proliferator activated
receptor-γ (PPARγ) and other transcription factors
(Katsouri, Parr, Bogdanovic, Willem, Sastre, 2011).
To investigate the therapeutic effect of PGC-1α gene
on AD patients, they inject human PGC-1α (hPGC-
1α) gene to hippocampus and cortex of APP23
transgenic mice. The result shows that selectively
inducing hPGC-1α gene to specific brain regions can
reduce Aβ aggregation, β-secretase expression and
neuroinflammation; improve the spatial and
recognition memory of these mice; provide some
neuroprotective effects. However, this therapy has no
effect on wild-type mice (Katsouri, Lim, Blondrath,
Eleftheriadou, Lombardero, Birch, Mirzaei, Irvine,
Mazarakis, Sastre 2016).
To provide the treatment on humans, there is still
such a long way to go because there might be
unpredictable consequences on editing human gene.
Nevertheless, the PGC-1α gene therapy can be an
inspiration for future research direction since it
effectively reduces neurons degeneration in mouse
models, which shows the potential of gene therapy to
be applied in early AD treatment. Unfortunately, for
late AD patients who have great extent of neurons
degeneration, nearly fully impaired memory and
cognition, gene therapy like PGC-1α overexpression
is not useful because it aims to stop neurons
degeneration but not generating new neurons.
Producing new neurons is another aspect of treating
AD, which might be related to stem cell therapy and
still need lots of effort on the methods to unfrozen the
differentiation of stem cells.
5 CONCLUSIONS
For now, what people know is that AD is an
irreversible disease, which means patients’ symptoms
worsen gradually until they die as recent medical
technology is not able to stop AD effectively.
Therefore, more researches on both the pathogenesis
and treatment of AD are necessary for finding
effective precautions and therapies of AD. This
review only talks about AD pathogenesis which is
related to genes but there are lots of other non-
negligible factors can cause AD such as age and
lifestyle. The reason of focusing on gene is that gene
screening can help early prevention or even early
treatment if already diagnosed as AD. Except for the
four most common genes discussed, there are still lots
of other suspectable gene mutations might affect AD
pathogenesis but they appear fewer and are less
understood. If more AD-related genetic variants can
be confirmed, there can be more target for AD gene
therapy researches no matter direct or indirect.
Currently, gene therapy is still in the laboratory stage
and cannot be applied on humans. Because the gap
between researches on animals and humans is large,
and also the knowledge about human gene is still
insufficient. People are not capable to take the risk of
developing possible unknown side effects by editing
human gene nowadays. Importantly, ethic problems
on genetic therapy should not be neglected as well.
Hence, scientists still have loads of tasks like
discovery and elimination of unwanted side effects
before actual application of gene therapy on human.
To achieve the goal, it is more helpful to do
experiments on other primates such as monkeys
because their brain structures are much more similar
to human brains than rodents. This might be the
general direction of researching gene therapy in the
future. After all, gene therapy has the potential to stop
and even cure AD, people will understand more about
human gene and finally apply safe and effective gene
therapy on AD treatment in the future.
The Genetic Basis of AD Incidence and Treatment
329

ACKNOWLEDGMENTS
Thanks Prof. Kate Jeffery of University College
London and Gongting Wang as the teaching assistant
very much for passing on their knowledge of
neurosciences patiently to me. Their lessons gave me
the chance to learn basic knowledge about brain
science and understand common useful experimental
methods. My curiosity and interest on Alzheimer’s
diseases come from their lessons as well. They
provided me general direction of writing this review
and removed my doubts with patience. Also thanks to
Min Han for guiding me during writing this review,
her advice helps a lot in making this review clearer.
REFERENCES
A Armstrong R. Risk factors for Alzheimer's disease. Folia
Neuropathol. 2019;57(2):87-105. doi:
10.5114/fn.2019.85929. PMID: 31556570.
Ayodele T, Rogaeva E, Kurup JT, Beecham G, Reitz C.
Early-Onset Alzheimer's Disease: What Is Missing in
Research? Curr Neurol Neurosci Rep. 2021 Jan
19;21(2):4. doi: 10.1007/s11910-020-01090-y. PMID:
33464407; PMCID: PMC7815616.
Dorey E, Chang N, Liu QY, Yang Z, Zhang W.
Apolipoprotein E, amyloid-beta, and
neuroinflammation in Alzheimer's disease. Neurosci
Bull. 2014 Apr;30(2):317-30. doi: 10.1007/s12264-
013-1422-z. Epub 2014 Mar 20. PMID: 24652457;
PMCID: PMC5562666.
Fjell AM, McEvoy L, Holland D, Dale AM, Walhovd KB;
Alzheimer's Disease Neuroimaging Initiative. What is
normal in normal aging? Effects of aging, amyloid and
Alzheimer's disease on the cerebral cortex and the
hippocampus. Prog Neurobiol. 2014 Jun;117:20-40.
doi: 10.1016/j.pneurobio.2014.02.004. Epub 2014 Feb
16. PMID: 24548606; PMCID: PMC4343307.
Gendron TF, Petrucelli L. The role of tau in
neurodegeneration. Mol Neurodegener. 2009 Mar
11;4:13. doi: 10.1186/1750-1326-4-13. PMID:
19284597; PMCID: PMC2663562.
Giau VV, Bagyinszky E, Youn YC, An SSA, Kim S. APP,
PSEN1, and PSEN2 Mutations in Asian Patients with
Early-Onset Alzheimer Disease. Int J Mol Sci. 2019
Sep 25;20(19):4757. doi: 10.3390/ijms20194757.
PMID: 31557888; PMCID: PMC6801447.
Grøntvedt GR, Schröder TN, Sando SB, White L, Bråthen
G, Doeller CF. Alzheimer's disease. Curr Biol. 2018
Jun 4;28(11): R645-R649. doi:
10.1016/j.cub.2018.04.080. PMID: 29870699.
https://www.nia.nih.gov/health/what-happens-brain-
alzheimers-disease
Jeong S. Molecular and Cellular Basis of
Neurodegeneration in Alzheimer's Disease. Mol Cells.
2017 Sep 30;40(9):613-620. doi:
10.14348/molcells.2017.0096. Epub 2017 Sep 20.
PMID: 28927263; PMCID: PMC5638769.
Katsouri L, Lim YM, Blondrath K, Eleftheriadou I,
Lombardero L, Birch AM, Mirzaei N, Irvine EE,
Mazarakis ND, Sastre M. PPARγ-coactivator-1α gene
transfer reduces neuronal loss and amyloid-β
generation by reducing β-secretase in an Alzheimer's
disease model. Proc Natl Acad Sci U S A. 2016 Oct
25;113(43):12292-12297. doi:
10.1073/pnas.1606171113. Epub 2016 Oct 10. PMID:
27791018; PMCID: PMC5087021.
Katsouri L, Parr C, Bogdanovic N, Willem M, Sastre M.
PPARγ co-activator-1α (PGC-1α) reduces amyloid-β
generation through a PPARγ-dependent mechanism. J
Alzheimers Dis. 2011;25(1):151-62. doi:
10.3233/JAD-2011-101356. PMID: 21358044.
Nilsson P, Saito T, Saido TC. New mouse model of
Alzheimer's. ACS Chem Neurosci. 2014 Jul
16;5(7):499-502. doi: 10.1021/cn500105p. Epub 2014
May 22. PMID: 24852598; PMCID: PMC4102956.
Safieh M, Korczyn AD, Michaelson DM. ApoE4: an
emerging therapeutic target for Alzheimer's disease.
BMC Med. 2019 Mar 20;17(1):64. doi:
10.1186/s12916-019-1299-4. PMID: 30890171;
PMCID: PMC6425600.
Sasaguri H, Nagata K, Sekiguchi M, Fujioka R, Matsuba Y,
Hashimoto S, Sato K, Kurup D, Yokota T, Saido TC.
Introduction of pathogenic mutations into the mouse
Psen1 gene by Base Editor and Target-AID. Nat
Commun. 2018 Jul 24;9(1):2892. doi: 10.1038/s41467-
018-05262-w. PMID: 30042426; PMCID:
PMC6057936.
Shi Y, Yamada K, Liddelow SA, Smith ST, Zhao L, Luo
W, Tsai RM, Spina S, Grinberg LT, Rojas JC, Gallardo
G, Wang K, Roh J, Robinson G, Finn MB, Jiang H,
Sullivan PM, Baufeld C, Wood MW, Sutphen C,
McCue L, Xiong C, Del-Aguila JL, Morris JC,
Cruchaga C; Alzheimer’s Disease Neuroimaging
Initiative, Fagan AM, Miller BL, Boxer AL, Seeley
WW, Butovsky O, Barres BA, Paul SM, Holtzman DM.
ApoE4 markedly exacerbates tau-mediated
neurodegeneration in a mouse model of tauopathy.
Nature. 2017 Sep 28;549(7673):523-527. doi:
10.1038/nature24016. Epub 2017 Sep 20. PMID:
28959956; PMCID: PMC5641217.
Tanzi RE. The genetics of Alzheimer disease. Cold Spring
Harb Perspect Med. 2012 Oct 1;2(10):a006296. doi:
10.1101/cshperspect.a006296. PMID: 23028126;
PMCID: PMC3475404.
Thal DR, Fändrich M. Protein aggregation in Alzheimer's
disease: Aβ and τ and their potential roles in the
pathogenesis of AD. Acta Neuropathol. 2015
Feb;129(2):163-5. doi: 10.1007/s00401-015-1387-2.
PMID: 25600324.
Thal DR, Fändrich M. Protein aggregation in Alzheimer's
disease: Aβ and τ and their potential roles in the
pathogenesis of AD. Acta Neuropathol. 2015
Feb;129(2):163-5. doi: 10.1007/s00401-015-1387-2.
PMID: 25600324.
ICHIH 2022 - International Conference on Health Big Data and Intelligent Healthcare
330

Theendakara V, Peters-Libeu CA, Bredesen DE, Rao RV.
Transcriptional Effects of ApoE4: Relevance to
Alzheimer's Disease. Mol Neurobiol. 2018
Jun;55(6):5243-5254. doi: 10.1007/s12035-017-0757-
2. Epub 2017 Sep 6. PMID: 28879423.
Toda T, Noda Y, Ito G, Maeda M, Shimizu T. Presenilin-2
mutation causes early amyloid accumulation and
memory impairment in a transgenic mouse model of
Alzheimer's disease. J Biomed Biotechnol.
2011;2011:617974. doi: 10.1155/2011/617974. Epub
2010 Dec 29. PMID: 21234330; PMCID:
PMC3018662.
Youmans KL, Tai LM, Nwabuisi-Heath E, Jungbauer L,
Kanekiyo T, Gan M, Kim J, Eimer WA, Estus S,
Rebeck GW, Weeber EJ, Bu G, Yu C, Ladu MJ.
APOE4-specific changes in Aβ accumulation in a new
transgenic mouse model of Alzheimer disease. J Biol
Chem. 2012 Dec 7;287(50):41774-86. doi:
10.1074/jbc.M112.407957. Epub 2012 Oct 11. PMID:
23060451; PMCID: PMC3516726.
The Genetic Basis of AD Incidence and Treatment
331
