
Inhibiting C-Jun to Retard Cell Proliferation Promoted by AP-2β in
the Breast Cancer
Benyu Yang
School of Pharmacy, University of Nottingham, Nottingham, NG7 2RD, U.K.
Keywords: AP-2β, Breast Cancer, C-Jun.
Abstract: AP-2β is a molecular marker in breast cancer cells without too much attention before. A tight association
between AP-2β overexpression and breast cancer was reported. This study investigates whether c-jun is the
certain down-stream protein of AP-2β in regulating the cell proliferation in breast cancer. The expression of
AP-2β in human cell line will be measure by Western Blot. Tumor size in vivo will be measured by volume.
The possible results include: the tumor growth of positive control is slower than negative control group, the
tumor is increasingly faster growing compared with negative control, or the tumor is growing in the same
speed in both negative and positive control groups. The result will indicate whether C-jun is a key down-
stream target for mediating the tumor-promoting role of AP-2β.
1 INTRODUCTION
The breast cancer is one of the most threatening type
of cancers for women worldwide. The most
significant four subtypes are: Luminal A or
HR+/HER2- (HR-positive/HER2-negative), Luminal
B or HR+/HER2+ (HR-positive/HER2-positive),
Triple-negative or HR-/HER2- (HR/HER2-negative)
and HER2-positive. (American Cancer Society. 2019)
There are many additional molecular factors
included in developing breast cancer. Among them,
the activator protein-2 family of transcription factor
is very common. They have five members: AP-2α, -
2β, -2γ, -2δ, -2ε. They are encoded by separate genes
(TFAP2A, TFAP2B, TFAP2C, TFAP2D, TFAP2E).
They are thought to bond with specific DNA
sequence as an activator or repressor to stimulate or
terminate the growth process of cells. (Turner et al
1998) Regarding breast cancer, most studies focus on
AP-2α and AP-2γ. The expression of AP-2β in breast
cancer has only started to be noticed and studied in
recent years.
The tight association of AP-2β with breast cancer
has been observed. (Raap et al 2018) AP-2β is a new
mammary epithelial differentiation marker and its
over expression leads to cell proliferation in breast
cancer. With knockdown of AP-2β , the cell
proliferation is downregulated. But at the same time,
in the previous experiment, proteins included in
developing the breast cancer also get down-regulated
expression such as p75, MMP-2, MMP-9, C-Jun, p-
ERK and STAT3. The expression levels of p75,
MMP-2, MMP-9, C-Jun, p-ERK and STAT3 show
obvious upregulation following overexpression of
AP-2β. (Li, Xu, Luo, Hao, Zhao, Yu et al 2018) To
investigate more about AP-2β underlying mechanism
in developing breast cancer, I hypothesize the c-jun is
a key downstream protein of AP-2β in regulating cell
proliferation. The possible signaling pathway
involving C-Jun is shown in Figure 1. AP-2β is the
transcription factor that contacts the promoter or
enhancer to regulate transcription. C-Jun is supposed
to be a member of downstream pathway in the
hypothesis. The complete downstream pathway is
still not fully understood. AP-2β may be regulate the
process via MEK/ERK/c-jun pathway indicated by
previous study, or maybe through PI3K/Akt pathway
which is also indicated in previous study, or actually
via various pathways together (See Figure 2).
(MAKOTO, Toru, Masaki, Koichi 2011) The
receptors that are responsible for transducing signals
are still under investigation. The estrogen-receptor is
already proven to be independent from expression of
AP-2β. (Raap et al 2018), However, other receptors
included in breast cancer, such as progesterone
receptors, HER2 receptors and EGFR receptors are
still being studied to confirm whether they are
associated with the expression of AP-2β.
302
Yang, B.
Inhibiting C-Jun to Retard Cell Proliferation Promoted by AP-2 in the Breast Cancer.
DOI: 10.5220/0011361100003438
In Proceedings of the 1st International Conference on Health Big Data and Intelligent Healthcare (ICHIH 2022), pages 302-307
ISBN: 978-989-758-596-8
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
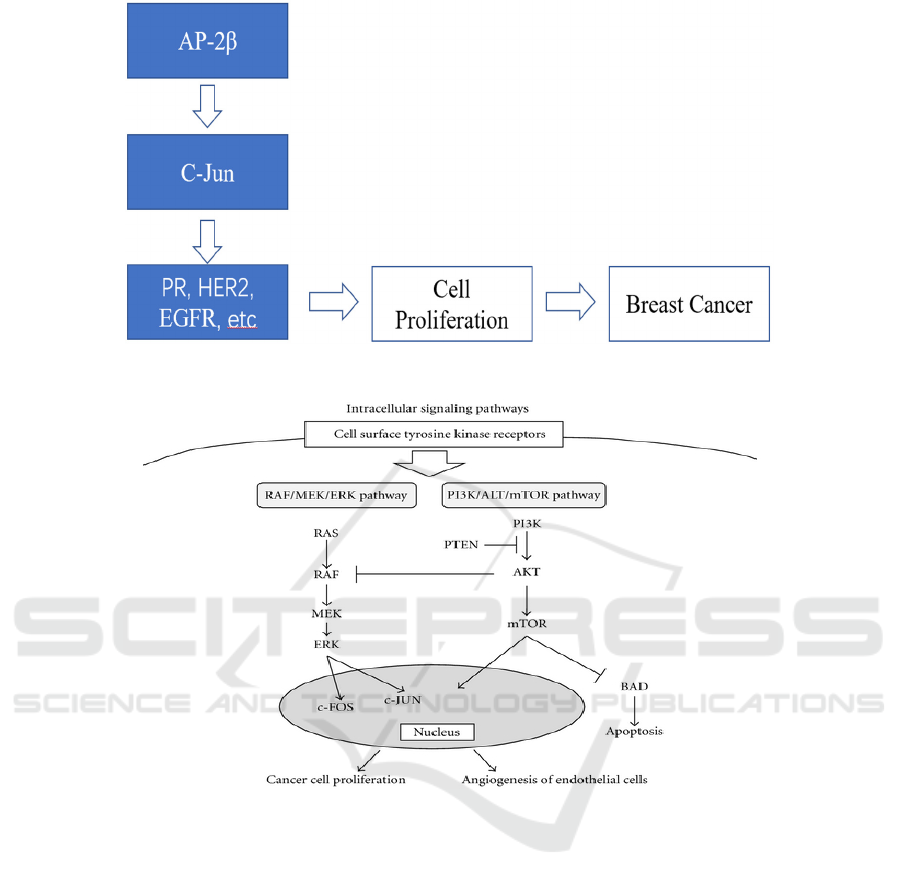
Figure 1: C-Jun Pathway. This figure shows the position and activities of C-Jun in the AP-2β oncogenic pathway.
Figure 2: RAF/MEK/ERK signalling pathway is shown. Growth factors promoting cell proliferation activate the
RAF/MEK/ERK pathway. MEK regulates the intermediate signalling by phosphorylation and activation of the downstream
ERK molecule. ERK regulates cellular activity, indirect inducers of gene expression and transcription factors of the AP-1
family, such as c-JUN and c-FOS. (MAKOTO, Toru, Masaki, Koichi 2011)
2 METHODS
2.1 Materials
Human breast cancer cell line MDA-MB-231
Xenograft mouse models
C-jun inhibitor JNK-JN-8 (Ebelt ND et al 2017)
2.2 In vitro Cell Culture
The cell line is cultured in RPMI-1640 medium
supplemented with 10% fetal calf serum, 10 μg/ml
bovine insulin, 2.5 g/l glucose, 1 mM sodium
pyruvate, 2 mM glutamine, and 10 mM HEPES, in a
water-saturated atmosphere containing 5% CO2 at
37.5°C. (Raap et al 2018) MDA-MB-231 cells will be
divided into two groups: negative control and positive
control. In the negative control group, the cells will
be injected with saline. In the positive control group,
the cells will be injected with c-jun inhibitor (JNK-
IN-8). The results will be analyzed by observing the
cell proliferation.
2.3 Flow Cytometry
The cell proliferation will be measured through flow
cytometry system by ThermoFisher Scientific. The
Inhibiting C-Jun to Retard Cell Proliferation Promoted by AP-2 in the Breast Cancer
303

cell line sample will be measured every 2 days after
injecting JUN-JN-8.
2.4 AP-2β Western Blot
Total cellular proteins are separated by 12% SDS-
PAGE and transferred to nitrocellulose membranes.
Membranes are probed with anti-AP-2β (H-87,
1:1000, Santa Cruz Biotechnology) (Raap et al 2018).
2.5 Animal Model
The xenograft mice will be divided into two groups
of 4: negative control and positive control. For the
mice in the negative control group, the mice will be
injected with saline. For the positive control group,
MDA-MB-231 cells will be injected subcutaneously
into the left flank of each mouse in the same volume
as the saline injected in the mice in negative control
group. The tumor growth will be monitored every
four days after the tenth day of injecting MDA-MB-
231 cells by measuring the tumor size. The tumor’s
size will be measured in the terms of volume and the
calculation equation is V= (width × length) /2. The
tumors will be weighed after death. (Li, Xu, Luo,
Hao, Zhao, Yu et al 2018)
2.6 Statistics
The western blot and immunohistochemistry will be
repeated three times for each group. To compare data
obtained in the positive and negative control groups,
a student t test will be displayed and P ≤0.05 is
considered significant. (Li, Xu, Luo, Hao, Zhao, Yu
et al 2018)
3 RESULTS (OVERVIEW SHOWN
IN TABLE 1)
3.1 Possible Results 1: Applying
JUN-JN-8 Inhibits the Breast
Cancer Cell Proliferation in
MDA-MB-231 Cell Line and the
Tumor Cells in Mouse Model
Inhibiting c-jun leads to significantly slower cell
proliferation in the breast cancer cells and the stop or
much slower growth of the tumor in the mice of
positive control group.
3.2 Possible Result 2: Applying
JUN-JN-8 Inhibits the Breast
Cancer Cell Proliferation in
MDA-MB-231 Cell Line, But Not
the Tumor Cells in Mouse Model
Inhibiting c-jun leads to no effect on tumor growth
although the cell proliferation is reduced in the In
Vitro cell culture experiment.
3.3 Possible Result 3: Applying
JUN-JN-8 Inhibits the Breast
Cancer Cell Proliferation in
MDA-MB-231 Cell Line, but
Promote the Breast Cancer Cell
Proliferation in Mouse Model
Inhibiting c-jun leads to confusingly much quicker
enlargement of the tumor in the condition that the cell
proliferation is inhibited in the breast cancer cells of
In Vitro conditions.
3.4 Possible Result 4: Applying
JUN-JN-8 Inhibits the Breast
Cancer Cell Proliferation in Mouse
Model, But Not the MDA-MB-231
Cell Line
Injecting c-jun inhibitor (JNK-IN-8) do not show
inhibiting effect on breast cancer cell proliferation in
the cell line. However, when MDA-MB-231 cells that
are injected with JNK-IN-8 are injected into the
mouse, the tumor growth speed is slowing down.
3.5 Possible Result 5: Applying JUN-
JN-K Does Not Inhibit the Breast
Cancer Cell Proliferation in Both
MDA-MB-231 Cell Line and the
Mouse Model
Injecting Both In vitro and In vivo experiments show
that injecting c-jun inhibitor has no effect on
inhibiting the breast cancer cell proliferation and
tumor growth.
ICHIH 2022 - International Conference on Health Big Data and Intelligent Healthcare
304
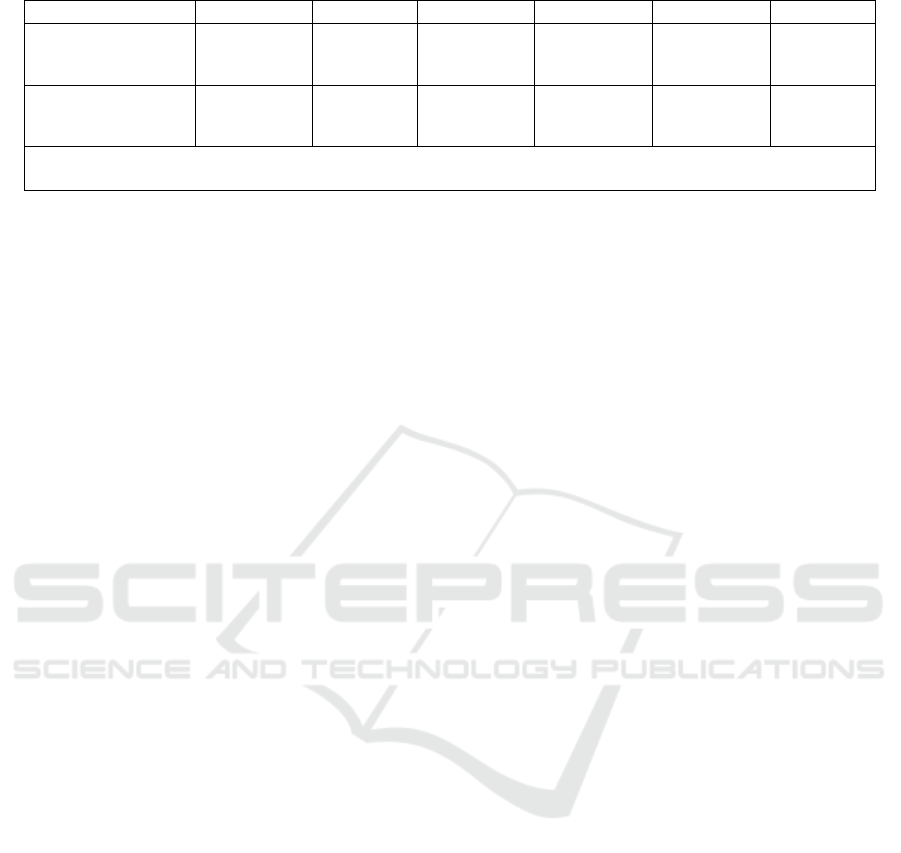
Table 1: Possible Results.
Cell lines Result 1 Result 2 Result 3 Result 4 Result 5 Result 6
In vivo Model + - ? + - ?
MDA-MB-231 + + + - - -
Note. "+" represents a a significant decrease in cell proliferation/tumor growth speed of mouse. "-" represents
not significantly different from negative control.
.
3.6 Possible Result 6: Applying
JUN-JN-8 Does Not Inhibit the
Breast Cancer Cell Proliferation in
MDA-MB-231 and Promote the
Breast Cancer Cell Proliferation in
Mouse Model
In the condition of no difference shown in the
inhibiting cell proliferation compared with negative
control group in the MDA-MB-231, the mouse shows
quicker tumor growth.
4 DISCUSSION
Previous studies have found that silencing AP-2β
leads to downregulation of the expression of a
number of proteins, including c-jun. Possible
downstream pathways are MEK-ERK-c-jun and
MEK/STAT3/MMPs. It is therefore reasonable to
assume that c-jun may be a direct downstream protein
of AP-2β or one of these direct proteins.
Possible Result 1 is consistent with previous
studies that downregulation of AP-2β led to a
downregulation of c-jun protein levels. Previous
studies have shown that the amount of c-jun protein
is influenced by AP-2β. C-jun regulation by AP-2β
most likely promotes tumor cell proliferation through
the MEK/ERK/c-jun signalling pathway, indicated by
previous experiment.
Possible Result 2 contradicts the hypothesis. C-
jun is inhibited by JUN-JN-8 in in vitro human breast
cancer cell line and cell proliferation is also inhibited,
suggesting that c-jun is one of the potential direct
downstream proteins. However, tumor cell
proliferation is not inhibited in the mouse model,
suggesting that there are other signalling pathways at
work in the mouse that disable the inhibition of c-jun
and thus do not inhibit the regulation of AP-2B, or
that there are other AP-2B downstream proteins at
work in the mouse that counteract the proliferative
effects of c-jun inhibition.
Possible Result 3 contradicts the hypothesis. The
inhibition of the cell proliferation process can only
suggest that c-jun can play a role in mediating cell
proliferation, but cannot be characterized as a direct
downstream protein of AP-2β. As there are other
potential direct downstream proteins that mediate the
tumor-promoting effects of AP-2β, the underlying
mechanisms are unclear. In previous studies, when
AP-2β was downregulated, the number of many other
proteins was also reduced, such as MMP9, MMP2
and p75 shown in Figure 3. (Li, Xu, Luo, Hao, Zhao,
Yu et al 2018)
Possible Result 4 are contradictory to the
hypothesis, as they do not provide clear evidence to
identify c-jun as a direct downstream target of AP-2β.
Tumor growth in mice is shown to be inhibited when
no inhibition is shown on MDA-MB-231. This may
demonstrate that c-jun has no direct role in mediating
the cell proliferation pathway, but that it can signal to
other downstream proteins that promote the tumor
pathway of AP-2β.
Possible Result 5 strongly suggests that c-jun is
not a direct downstream protein of AP-2β. and that it
has no therapeutic effect on breast cancer.
Possible Result 6 shows an unlikely result, but it
is also possible that it will emerge in future
experiments. Cell proliferation will not be inhibited
when c-jun inhibitors are injected into breast cancer
cell lines. This is not evidence to determine whether
c-jun is a direct downstream protein of AP-2β, which
may have multiple signalling pathways. However, in
mice injected with c-jun inhibitor-treated MDA-MB-
231, tumor growth will become more pronounced, a
possible outcome that is very strange and if it occurs
more research is needed to explore the mechanisms
involved.
Inhibiting C-Jun to Retard Cell Proliferation Promoted by AP-2 in the Breast Cancer
305
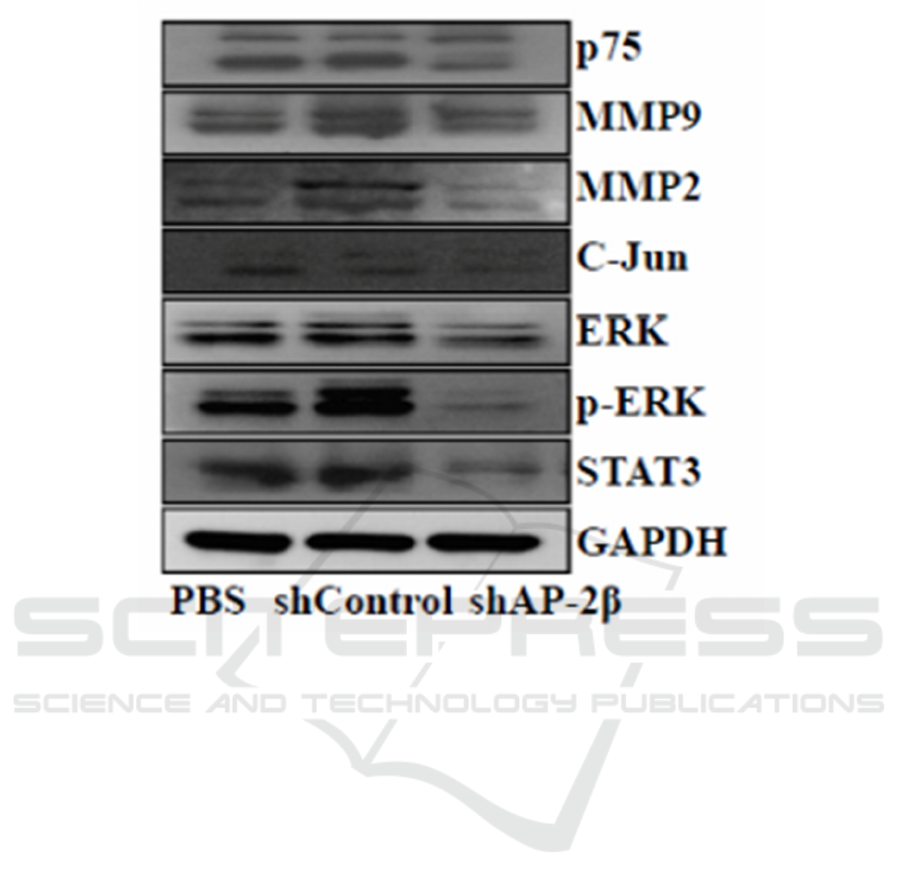
Figure 3: Expression levels of P75, MMP2, MMP9, C-Jun, STAT3 measured by Western blot in MDA-MB-231 cells
following AP-2β knockdown. (Li, Xu, Luo, Hao, Zhao, Yu et al 2018)
5 CONCLUSIONS
C-jun may be an important protein in the downstream
pathway of AP-2β during tumour growth in breast
cancer as experiments have shown that c-jun
expression is closely associated with AP-2β. Overall,
this article discusses the downstream pathway
component of AP-2β in the mechanisms underlying
the promotion of breast cancer cell proliferation and
uses in vitro cell lines and mouse models to determine
whether c-jun is one of the important downstream
proteins. Our findings will indicate whether c-jun
could be a potential target for blocking breast cancer
tumour growth. Results that contradict the hypothesis
would also indicate the existence of other
downstream protein proteins regulated by AP-2β, in
both respects a confirmation of potential therapeutic
targets in breast cancer in the future. As no previous
attention has been paid to the role of AP-2β in breast
cancer, research into its complete mechanism in
breast cancer progression is limited. More research on
how AP-2β regulates cell proliferation still needs to
be clearly detailed in order to find more effective
targets for the treatment of breast cancer.
REFERENCES
American Cancer Society. (2019) Breast Cancer Facts &
Figures 2019-2020. Atlanta: American Cancer Society,
Inc. 2019. https://www.cancer.org/content/dam/cancer-
org/research/cancer-facts-and-statistics/breast-cancer-
facts-and-figures/breast-cancer-facts-and-figures-
2019-2020.pdf
Ebelt ND, Kaoud TS, Edupuganti R, Van Ravenstein S,
Dalby KN, Van Den Berg CL. A c-Jun N-terminal
kinase inhibitor, JNK-IN-8, sensitizes triple negative
breast cancer cells to lapatinib. Oncotarget.
2017;8(62):104894.
Li Z, Xu X, Luo M, Hao J, Zhao S, Yu W, et al. Activator
protein-2β promotes tumor growth and predicts poor
prognosis in breast cancer. Cellular Physiology and
Biochemistry. 2018;47(5):1925-35.
MAKOTO M, Toru M, Masaki K, Koichi H. The Molecular
Pathogenesis and Clinical Implications of
ICHIH 2022 - International Conference on Health Big Data and Intelligent Healthcare
306

Hepatocellular Carcinoma. International Journal of
Hepatology 2011(2):818672.
Raap M, Gronewold M, Christgen H, Glage S, Bentires-Alj
M, Koren S, et al. Lobular carcinoma in situ and
invasive lobular breast cancer are characterized by
enhanced expression of transcription factor AP-2beta.
Lab Invest. 2018;98(1):117-29.
Turner BC, Zhang J, Gumbs AA, Maher MG, Kaplan L,
Carter D, et al. Expression of AP-2 transcription factors
in human breast cancer correlates with the regulation of
multiple growth factor signalling pathways. Cancer
research. 1998;58(23):5466-72.
Inhibiting C-Jun to Retard Cell Proliferation Promoted by AP-2 in the Breast Cancer
307
