
Knockdown of the Cysteine Dioxygenase Gene in Damaged
Mitochondrial Cardiomyocytes May Be Protected by the Effective
Range of Taurine Level
Lifan Zhou
1,*
, Xinyi Zhou
2
, Yanxi Ren
3
, Yuwei Zha
4
and Jiarong Bei
5
1
School of bioscience, University of Nottingham Sutton Bonington Campus, Nottingham, NG7 2RD, U.K.
2
School of Biomedical Science, University of Bristol, Bristol, BS8 1TW, U.K.
3
Shenzhen Middle School, Shenzhen, 518001, China
4
Wuxi Big Bridge Academy, Wuxi, Jiangsu, 214115, China
5
Westover School, Middlebury, 06762, U.S.A.
Keywords: Taurine, CDO Gene, Cardiomyopathy Treatment.
Abstract: Cardiomyopathy is a disease caused by various reasons, including myocardial hypertrophy and autologous
gene defects. Taurine has been used in cardiomyopathy treatment for many years. It is a cytoprotective agent
that can protect the damaged mitochondria of the cardiomyocytes. Previous studies suggest that the high level
of taurine is primarily controlled by the cysteine dioxygenase (CDO) gene. This paper investigates the
optimum value of taurine concentration into CDO knockdown cardiomyocytes in vitro and vivo. In vitro,
taurine solution (ranges from 0μL to 1000μL) was added into the CDO knockdown cell culture medium daily
for every six groups. Then,we use ATP detection and Mitochondria membrane potential (MMP) detection
to investigate the effect of the taurine supplement. In vivo, 50 male mice are divided into ten groups fed with
taurine ranging from 1.0g to 3.0g as 0.2g increments. There is no result since all experiments are conducted
in virtual due to COVID-19. However, this paper is the first that provides the protocol to detect the right
taurine concentration in the case of CDO knockdown in cardiomyocytes. Thus, it may provide some ideas in
treating cardiomyopathy using a suitable amount of taurine in clinical.
1 INTRODUCTION
1.1 Background
Cardiomyopathy is characterized by a collection of
abnormal myocardial conditions including
myocardial mechanical and/or electrical dysfunction,
ventricular hypertrophy, and dilation, which can
eventually lead to cardiac death or progressive heart
failure (Zhuge, Ruiqi et al, 2017, Holmgren, D et al,
2003, Debray, François-Guillaume et al. 2007). And
this disease is often caused by genetic inheritance or
autoimmune disease (like lupus) (Cardiomyopathy
2017). A possible cause of cardiomyopathy is
mitochondria failure (Suzuki, Takeo, et al, 2002).
Cardiomyopathy can cause damage to the
mitochondria of myocardial cells, leading to a greater
possibility of heart failure (Chen, Yu-Han et al., 2019,
Pion et al., 1987, Moise, N S et al., 1991,
Marcinkiewicz, Janusz, Ewa Kontny. 2014).
Taurine (2-aminoethanesulfonic acid) is the most
abundant free amino acid in the human body. It plays
an important role in many important biological
processes, such as bile acid-binding, calcium
maintenance. Homeostasis, osmotic adjustment, and
membrane stability. In addition, the reduction of
apoptosis and its antioxidants. Activity seems to be
essential for cell protection taurine (Marcinkiewicz,
Janusz, Ewa Kontny, 2014, Lambert et al., 2015). In
1985, taurine (2-aminoethanesulphonic acid) was
first used in the treatment of congestive heart failure
in Japan (Azuma, et al., 1985, Azuma, et al., 1983),
and it is now established by numerous contemporary
works of literature that a decrease in the cellular level
of taurine considerably increases the possibility of
mitochondrial diseases, especially cardiomyopathy
(Suzuki, Takeo et al, 2002, Chen, Yu-Han et al., 2019,
Pion et al., 1987,
Marcinkiewicz, Janusz, Ewa
Kontny., 2014). Some studies have shown that taurine
as a cytoprotective agent can protect damaged
cardiomyocyte mitochondria (Marcinkiewicz,
944
Zhou, L., Zhou, X., Ren, Y., Zha, Y. and Bei, J.
Knockdown of the Cysteine Dioxygenase Gene in Damaged Mitochondrial Cardiomyocytes May Be Protected by the Effective Range of Taurine Level.
DOI: 10.5220/0011313400003443
In Proceedings of the 4th International Conference on Biomedical Engineering and Bioinformatics (ICBEB 2022), pages 944-950
ISBN: 978-989-758-595-1
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

Janusz, Ewa Kontny, 2014, Lambert et al., 2015). As
the most abundant free amino acid in excitable tissue,
taurine plays an essential role in several biological
functions including central nervous system
development and membrane stabilization. Studies
have also shown that mammals need to supplement
taurine by eating foods rich in taurine (Lambert, et al.,
2015).
In humans, taurine can be synthesized from other
sulfur-containing amino acids (Polakof, Sergio et al.
2018, Sampath, et al. 2020), one of which is cysteine.
It was long discovered that an enzyme, cysteine
dioxygenase (CDO), primarily controls the high level
of taurine in the human body (Wl, et al., 2019).
Regulating through the oxidation pathway of
cysteine, CDO expression level contributes to the
taurine biosynthesis in multiple human organs,
including the two major contributing organs: liver
and mammal glands (Ueki, Iori, Martha H Stipanuk.
2007). Through the CDO gene synthesis pathway, the
taurine content of taurine-containing plants can be
increased. Controlling the CDO gene can also help
control the content of taurine in the body. (Tevatia,
Rahul, et al. 2019)
Gene knockdown is considered better since it
achieves the same purpose and attains the same
results without directly regulating the genes, like
deletion in gene knockout or addition in gene knock-
in. It only affects the transcription and mostly
translation of a specific gene of interest, with high
accuracy and specificity. Plus, it will be far easier to
conduct knockdown than knockout.
Adenosine triphosphate (ATP) is formed by
connecting adenine, ribose, and 3 phosphate groups.
It releases more energy during hydrolysis and is the
most direct source of energy in the body. ATP release
and autocrine signals through purinergic receptors
promote T cell activation to form the immune synapse
formed by T cells and APC. (Ledderose, Carola et al.
2018) ATP can help human cells to carry out
immunity, and cardiomyopathy can reduce the ATP
produced by autogenous movement in the heart.
(Bloemink, Marieke et al., 2014, Ichihara, Sahoko, et
al., 2017)
1.2 Hypothesis
Therefore, this review will outline the important role
of taurine in mitochondrial cardiomyopathy. We
believe that increasing the content of taurine in the
body to a certain extent can help protect the damaged
mitochondria of cardiomyocytes. Change the original
CDO in the body to control the initial content of
taurine in the body. By changing the content of
taurine in the food used to help the experimenter to
supplement taurine, at the same time can detect the
content of ATP to select the most appropriate taurine
supplement. We believe that there should be a suitable
range for supplementing taurine content, which
should not be too high or too low.
2 EXPERIMENT DESIGN
2.1 Cardiomyocyte Cell Culture
Cardiomyocyte cell culture. Two groups of neonatal
cardiomyocytes are isolated from three-day-old
murine hearts, one from wild-type, the other from
mice with cardiomyopathy. The cells are resuspended
in DMEM supplemented with 10% fetal bovine
serum, 100units ml−1 penicillin, 100μg ml−1
streptomycin. After another 24 h with a regular
culture medium, 20μg ml−1 cytosine β-D-
arabinofuranoside will be added into the medium to
suppress non-interest cells. (Ladeira, et al, 2010)
2.2 CDO Knockdown in
Cardiomyocyte Cell Culture
Short interfering RNA (siRNA) Oligonucleotides.
In order to obtain CDO sequence siRNA
oligonucleotides, the experiment requires siRNA
manufacturer companies to design the required
complementary sequences, select potential target
sites, and then search with NCBI Blast to confirm the
specificity of each CDO exon expression. Since
there is no commercially available or known siRNA
that specifically downregulates the CDO gene in
murine cardiomyocytes, a positive control group
cannot be carried out. For the negative control group,
this work design non-targeting siRNAs that lack the
RNA sequences of interest in the targeting genome to
eliminate the possible experimental material
interference. (Han 2018) Aliquot the resuspended or
annealed siRNA into new tubes and store at −20 °C.
Single-Wall Carbon nanotubes (SWCNTs).
Ladeira et al. (Ladeira, et al, 2010) have already
validified that the covalent conjugation of siRNA to
SWCNTs for RNA interference and gene knockdown
is of high efficiency, especially in cell lines that are
poorly transfected, such as cardiomyocytes. SWCNT
is added into the cell medium with a concentration of
0.0250mg ml−1 for 48-h incubation of
cardiomyocytes. To guarantee the presence of
SWCNTs in the cells, the work use Raman
spectroscopy. The sample cell is excited by a He-Ne
laser (632.8 nm), and an oil objective lens with a
Knockdown of the Cysteine Dioxygenase Gene in Damaged Mitochondrial Cardiomyocytes May Be Protected by the Effective Range of
Taurine Level
945

magnification of 60 times and NA = 1.4 is used to
focus on the sample surface.
Transfection. Prepare a stable short SWCNT
(~200 nm length) aqueous solution using high-purity
short COOH-SWCNT dissolved in MilliQ water.
After centrifugation, 50nM CDO-specific siRNA was
added to 50μL of CNT aqueous solution, sonicated
for 30 minutes, and added to the cell culture medium.
For RNAifect preparation, wash the cells and provide
1 ml of fresh tissue culture medium. 50nM siRNA is
added to 3μL RNAifect, QIAGEN transfection
reagent, and then add 100μL tissue culture medium.
Following a 15-minute incubation at 37°C to allow
for complex formation, the mixture was reconstituted
with 900 mL of tissue culture media and subsequently
poured over the cells dropwise. The cells then are
plated into fibronectin-coated culture dishes at 37°C
in a 5% CO
2
incubator for two days. (Ladeira, et al,
2010)
Reverse Transcription Polymerase Chain
Reaction (RT-PCR) and Gel Electrophoresis. RT-
PCR is used for the evaluation of CDO knockdown
efficacy and the after-treatment reactions in the
negative control group. To purify the CDO mRNA of
interest, add 0.75mL of Trizol-LS® Reagent to
0.25ml of freshly isolated cardiomyocytes. To allow
the full dissociation of nucleoprotein complexes, mix
the cells multiple times with a pipette and then
incubate the lysates for 5 min at room temperature.
Then, centrifuge the lysates at 12,000 g for 10 min at
4°C, and discard the suspended liquid. To conduct
RT-PCR, using the High-Capacity cDNA Reverse
Transcription Kit, follow the instructions, and use
NCBI BLAST to create primers of interest. Then
place the tubes in a thermal-cycler and run RT-PCR
with 10 mins at 25°C, 2 hrs at 37°C, and 5 secs at
85°C. (Guan, Yang 2008) For the ethidium bromide-
stained gel analysis, 10μL each RT-PCR product is
loaded on a 7% polyacrylamide (19:1) gel and run for
3 hours at 120 V. The two groups of results are
compared and analyzed.
Western Blot. For a more detailed and
comprehensive investigation, the protein expression
of CDO is tested through Western Blot. Antibodies
are targeted towards CDO protein, and the protocol is
available online, See reference for more ( “
Antibodies, Proteins, Kits and Reagents for Life
Science.” Abcam).
In vivo siRNA delivery. Purchase C57BL/6 6–8-
week male mice from Beijing University. They are
kept in a 12:12 light/dark condition at 25°C on a chow
diet. Mice are anesthetized with pentobarbital sodium
(50mg/kg) by intraperitoneal injection. As shown in
Figure 1, 3mM of 20μL CDO-specific siRNAs are
administered intramyocardially into the left ventricle
using a 32G needle at roughly five regions around the
beginning section of the left anterior descending
coronary artery.
Figure 1: The injected locations are shown by the injectors.
Intramyocardial injection into the left ventricle (LV) at
roughly five places surrounding the beginning region of the
LAD coronary artery was performed. (The figure is adapted
from (Huang, Kun et al 2016)) .
2.3 Taurine Supplement into
Cardiomyocytes with CDO
Knockdown
a) In Vitro
Taurine Supplement. Inject taurine solution onto the
cell culture medium daily with 200μL as an increment
for each group, ranging from 0μL to 1000μL.
ATP Detection. The Detection Kit named
ab113849- Luminescent ATP Detection Assay Kit is
used to test the concentration of ATP. To extract ATP
in selected cardiomyocytes, PBS is first employed to
wash cells, and then add the cell lysis buffer on the
culture plate, which is then vibrated on the micro-
oscillator for 5 ~ 10min. To obtain the cell suspension
for further tests, first use a cell scraper to scrape the
cells on the culture plate, transfer the cell suspension
into a 1.5ml centrifuge tube, and fully vibrate on a
vortex oscillator for 30 seconds. Next, l dilute
luciferin with ATP assay buffer in the proportion of
1/20 and then add 1/10 volume of luciferase and
ddH
2
O to prepare the required volume of an assay
reagent. After all, the preparation adds the reagent
into the measuring tube and record the luminous unite
of firefly luciferase.
Mitochondria Membrane Potential (MMP)
Detection. The Detection Kit named ab112134-JC-10
Mitochondrial Membrane Potential Assay Kit is used
to test the membrane potential of cardiomyocytes’
mitochondria. For preparation, it is needed to place
cardiomyocytes overnight in a 90μL growth medium
at a 96-well plate. Then mix 50 µL of 100X JC-10 and
5 mL Assay Buffer A, treat cells by adding 10μL of
10X test compounds into PBS. Next incubate the cell
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
946
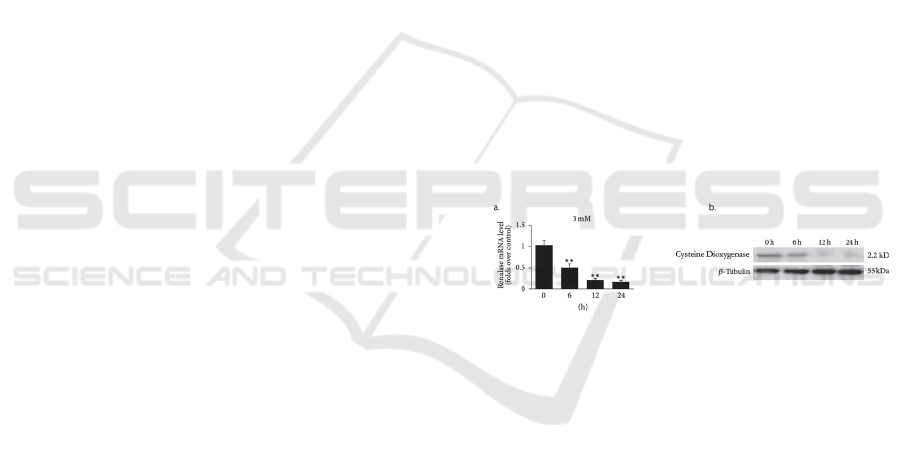
plate in a 37°C, 5% CO2 incubator for 4-6 hours to
induce apoptosis, and then add JC-10 dye-loading
solution into the cell plate. After the addition, another
30-min no-light incubation a 37°C, 5% CO2
incubation is needed. Then add the Assay Buffer B
into the dye loading plate, read the fluorescence
intensity, and monitor the fluorescence intensities at
E
x
/E
m
= 490/525 nm (cut off at 515 nm) and 540/590
nm (cut off at 570 nm) for ratio analysis.
b) In Vivo
Experimental Period Estimation. In each of the
cell culture experiments, when the ATP and MMP
detection data from experimental groups deviate
significantly from the control group, this work will
make a note and regard it due to the ineffectiveness
of siRNAs. These statistics will be comprehensively
considered as an estimation of the efficient period of
our siRNA and be considered during in vivo
experiment conduction.
Taurine Supplement. Mice are chosen to do an
experiment on. Use 12 groups of male mice of the
same species, and each group has five mice. The first
group is all healthy mice without any treatment. The
second group is all mice with cardiomyopathy but
without any other treatment. The third group to the
twelfth group is mice with cardiomyopathy and with
CDO knockdown. These ten groups of mice are fed
with taurine, which is fed with 1.0g, 1.2g, 1.4g, 1.6g,
1.8g, 2.0g, 2.2g, 2.4g, 2.6g, 2.8g, and 3.0g.
Magnetic Resonance Imaging (MRI). The
mouse is under anesthesia and allowed to breathe
freely. First inhaled isoflurane into mice. Isoflurane
inhalation is currently the preferred method for mice
because anesthesia induction and wake-up are fast,
hemodynamic inhibition is minimal, and the depth of
anesthesia is easy to adjust. Then, the mice will
receive 1.0-2.0% isoflurane, 30%-50% oxygen, 50-
70% air. The location of the animals is also a critical
step, and they must be reproducible. Location affects
the quality of data and the degree to which motion
artifacts affect imaging. The mouse should be in the
prone position (fixed to an animal sled or other fixture
with tape or plastic pins). Then, an anesthesia cone is
provided through the nose, a breathing sensor is
usually connected to the abdomen, and the
temperature is measured through a fixed rectum.
After completing the previous step, the expected
gating synchronized with the ECG must be
performed. Finally, obtain cine cardiac imaging based
on multiphase gradient echocardiography.
High-Performance Liquid Chromatography.
After the mice are fed for 12 hours, blood samples
from their tail vein are collected, and each sample is
100μL. Add 5μL 1% heparin solution into each blood
sample and then centrifuge. Then, plasma samples
form. The pre-colum derivation is prepared, which
mixes 100μL blood sample with 50μL derivatization.
Add samples on y-reversed-phase column
chromatography, (125*3 mm, ODS Hypersil 3 m)
(VDS Optilab, Chromatographie Technik, GmbH),
the mobile phase is 27% methanol+73% 0.1 mol/L
Na2HPO4, 0.13 mmol/L EDTA water solution, speed
is 0.8 mL/min, detected volumetrically (ESA
Coulochem II; Bedford, Mass. USA) using three
electrodes, a guard (0.4 V), peroxidation (0.4 V) and
working (0.6 V) electrodes (analytical cell
ESA5011). The concentrations of taurine in mice’s
blood are tested out.
Computerized tomography (CT) and
anatomical observation. Mice euthanasia was
performed and left ventricular myocardial tissue was
extracted and utilized for detection.
3 RESULTS
3.1 Investigating CDO Knockdown in
Vitro
Expect experiment results as in Figure 2.
Figure 2: CDO has been knocked down using RT-PCR (a)
and Western Blot (b) (Mock trials).
3.2 Investigating Effect of Taurine
Levels in Vitro and in Vivo
a) ATP level in vitro using ATP Detection Assay
Kit.
From Figure 3, we expect a positive correlation
between the taurine supplement diet and ATP level.
Knockdown of the Cysteine Dioxygenase Gene in Damaged Mitochondrial Cardiomyocytes May Be Protected by the Effective Range of
Taurine Level
947
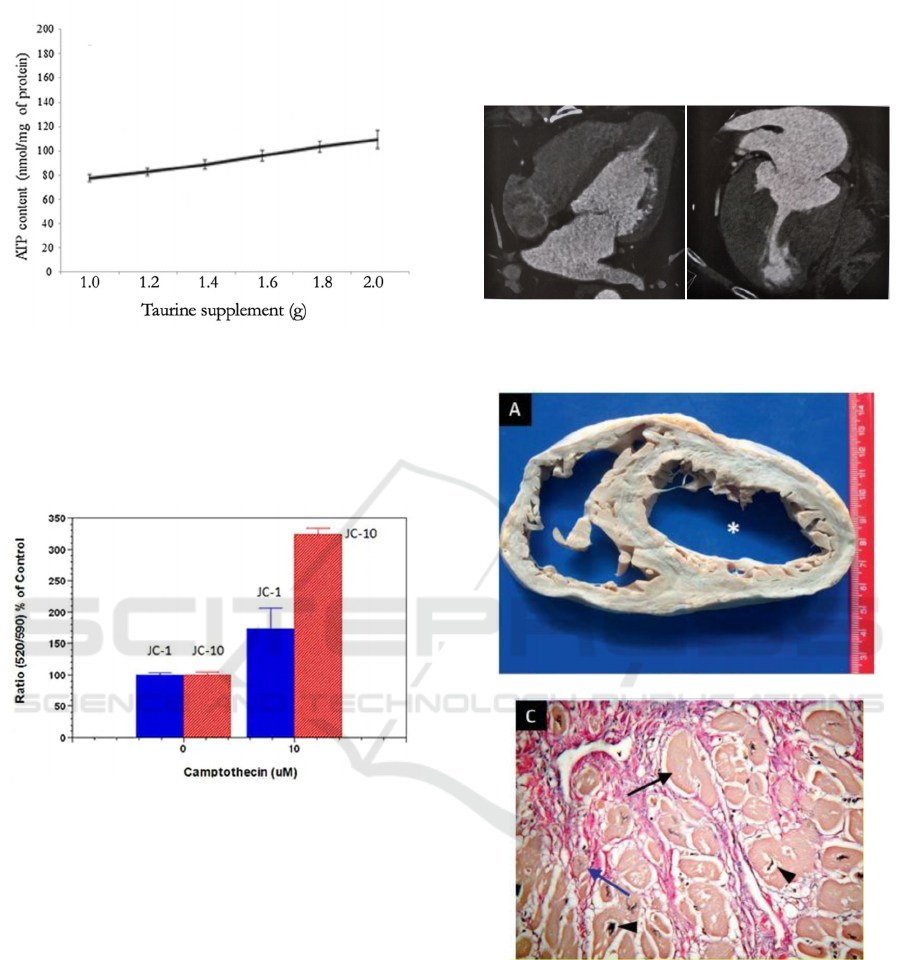
Figure 3: A positive correlation between taurine levels and
ATP levels is expected (Mock trials).
b) Mitochondrial Membrane Potential in vivo.
We expect a proper change in cell membrane
potential (Figure 4).
Figure 4: A proper change is expected in mock trails.
c) Taurine level in mice using MMR.
A typical mouse will contain 2.4 mg/g taurine or
(for better comparison with the other values: 100g
would contain 240mg) 4 ounces of a mouse would
equal over 2400 mg taurine (However, 2500mg is
minimum for a 10-lb cat). Compared with humans
and cats, the mouse exhibits a considerable
biosynthetic capacity for taurine.
d) Computerized tomography (CT) and
anatomical observation
Two symptoms of cardiomyopathy are thinner
myocardial wall and cardiac hypertrophy. CT is used
to supervise the change of these two symptoms of
each mouse (Figure 5). At last, dissect the hearts of
mice to see the condition of hearts (Figure 6). Since
it is a virtual experiment, there is no exact data. The
ideal situation is to find out mice of which taurine
level has a greater condition of the heart through
comparing these ill mice’s hearts with the healthy
ones. From this, the taurine range that can help cure
cardiomyopathy can be found out.
Figure 5: CT shows cardiomyopathy will cause thinner
myocardial wall and cardiac hypertrophy.
Figure 6: Thinner myocardial wall (a), cardiomyocyte
hypertrophy (c).
4 CONCLUSION
Due to the restricted conditions, the results are all
predicted. So, if all results meet the hypothesis, we
can propose that the protection of damaged
mitochondrial of cardiomyocytes with CDO
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
948

knockdown by a taurine supplement. However, there
will be still have some concerns about methods.
In the beginning of the experiment, this work
considered the knockdown CDO gene instead of
knockout it. This has also arisen a problem, which is
knockdown may have some uncertainty and
inaccuracy as we cannot be able to detect whether the
CDO gene was still knockdown during the entire
experiment. Also, further studies can be investigated
by using the knockout CDO gene method if this did
happen in a laboratory. Also, due to Han Haiyong
(Han 2018) mentioned in his paper that the efficient
effect of knockdown should be examined and to
determine the optimal time point for assessing
cellular effects of siRNA knockdown. Therefore, this
work will have some further studies on the monitor
the efficiency of knockdown and thus, used in mice
model. Moreover, in this report cannot ensure taurine
can be absorbed 100% by the human body whether
due to taurine consumption. Therefore, although
there were several repetitions of the experiment, we
cannot be 100% sure that the experiment will not
have the same absorption problems. This report also
considered the detection by high-pressure liquid
chromatography to detect the level of ATP in vitro,
but considering its stability is not as good as the kit,
we chose to use the kit after careful consideration. If
it is not possible to use the kit for an accurate surface
in real experiments, we can also choose to use high-
pressure liquid chromatography.
Apart from that, we are proud of an experiment is
the first to figure out that the range of taurine levels
that can affect cardiomyopathy under the condition of
CDO knockdown. However, more studies can be
taken to understand the underlying molecular
mechanisms. For example, test the mitochondrial
apoptosis pathway by Western blotting.
If the results of this experiment are true as
expected to prove that the content of taurine in the
body can have a positive and positive effect on
myocarditis, then this technology can be achieved by
consuming more taurine-rich foods. It helps patients
with cardiomyopathy relieve their symptoms, and at
the same time help patients achieve a greater quality
of life.
REFERENCES
“Antibodies, Proteins, Kits and Reagents for Life Science.”
Abcam,
“Cardiomyopathy.” American family physician 96.10
(2017): 640.
Azuma, J et al. “Therapeutic effect of taurine in congestive
heart failure: a double-blind crossover trial.” Clinical
cardiology vol. 8,5 (1985): 276-82.
Azuma, J et al. “Therapy of congestive heart failure with
orally administered taurine.” Clinical therapeutics vol.
5,4 (1983): 398-408.
Bloemink, Marieke et al. “The Hypertrophic
Cardiomyopathy Myosin Mutation R453C Alters ATP
Binding and Hydrolysis of Human Cardiac β-Myosin.”
The Journal of biological chemistry 289.8 (2014):
5158–5167.
Chen, Yu-Han et al. “167-OR: Activation of Myocardial
Mitochondria Akt1 Improved Diabetic
Cardiomyopathy and Body Composition—The Heart
as a Metabolic Organ.” Diabetes (New York, N.Y.) 68.
Supplement 1 (2019): 167.
Debray, François-Guillaume et al. “Long-term outcome
and clinical spectrum of 73 pediatric patients with
mitochondrial diseases.” Pediatrics vol. 119,4 (2007):
722-33.
Guan H., Yang K. (2008) RNA Isolation and Real-Time
Quantitative RT-PCR. In: Yang K. (eds) Adipose
Tissue Protocols. Methods in Molecular Biology™, vol
456. Humana Press.
Han, Haiyong. “RNA Interference to Knock Down Gene
Expression.” Methods in molecular biology (Clifton,
N.J.) vol. 1706 (2018): 293-302.
Holmgren, D et al. “Cardiomyopathy in children with
mitochondrial disease; clinical course and cardiological
findings.” European heart journal vol. 24,3 (2003):
280-8.
https://www.abcam.com/.
Huang, Kun et al. “Intramyocardial Injection of siRNAs
Can Efficiently Establish Myocardial Tissue-Specific
Renalase Knockdown Mouse Model.” BioMed
research international 2016 (2016): 1267570–7.
Ichihara, Sahoko et al. “Involvement of Oxidative
Modification of Proteins Related to ATP Synthesis in
the Left Ventricles of Hamsters with Cardiomyopathy.”
Scientific reports 7.1 (2017): 9243–11.
Ladeira, M S et al. “Highly efficient siRNA delivery system
into human and murine cells using single-wall carbon
nanotubes.” Nanotechnology vol. 21,38 (2010):
385101.
Lambert, I. H et al. “Physiological Role of Taurine - from
Organism to Organelle.” Acta Physiologica 213.1
(2015): 191–212.
Ledderose, Carola et al. “Purinergic P2X4 Receptors and
Mitochondrial ATP Production Regulate T Cell
Migration.” The Journal of clinical investigation 128.8
(2018): 3583–3594.
Marcinkiewicz, Janusz, and Ewa Kontny. “Taurine and
Inflammatory Diseases.” Amino acids 46.1 (2014): 7–
20.
Meyers, Deborah E et al. “Mitochondrial cardiomyopathy:
pathophysiology, diagnosis, and management.” Texas
Heart Institute journal vol. 40,4 (2013): 385-94.
Moise, N S et al. “Dietary taurine deficiency and dilated
cardiomyopathy in the fox.” American heart journal
vol. 121,2 Pt 1 (1991): 541-7.
Knockdown of the Cysteine Dioxygenase Gene in Damaged Mitochondrial Cardiomyocytes May Be Protected by the Effective Range of
Taurine Level
949

Pion, P D et al. “Myocardial failure in cats associated with
low plasma taurine: a reversible cardiomyopathy.”
Science (New York, N.Y.) vol. 237,4816 (1987): 764-
8.
Polakof, Sergio et al. “Metabolic adaptations to HFHS
overfeeding how whole body and tissues postprandial
metabolic flexibility adapt in Yucatan mini-pigs.”
European journal of nutrition vol. 57,1 (2018): 119-
135.
Sampath W.W.H.A., et al. “Roles of dietary taurine in fish
nutrition.” Marine Life Science &
Technology2.4(2020)
Steiner, Jennifer L, and Charles H Lang. “Etiology of
Alcoholic Cardiomyopathy: Mitochondria, Oxidative
Stress and Apoptosis.” The international journal of
biochemistry & cell biology 89 (2017): 125–135.
Suzuki, Takeo et al. “Taurine as a constituent of
mitochondrial tRNAs: new insights into the functions
of taurine and human mitochondrial diseases.” The
EMBO journal vol. 21,23 (2002): 6581-9.
Tevatia, Rahul et al. “A Synthetic Cdo/csad Taurine
Pathway in the Green Unicellular Alga
Chlamydomonas Reinhardtii.” Algal research
(Amsterdam) 40 (2019): 101491.
Ueki, Iori, and Martha H Stipanuk. “Enzymes of the taurine
biosynthetic pathway are expressed in rat mammary
gland.” The Journal of nutrition vol. 137,8 (2007):
1887-94.
Wl, C, et al. "Molecular characterization and taurine
regulation of two novel CDOs (CDO1 and CDO2) from
Carassius auratus." Comparative Biochemistry and
Physiology Part B: Biochemistry and Molecular
Biology 235(2019):54-61.
Zhuge, Ruiqi et al. “Advances in Diagnosis and
Management of Mitochondrial Cardiomyopathy.”
Zhongguo yi xue ke xue yuan xue bao. Acta Academiae
Medicinae Sinicae vol. 39,2 (2017): 290-295.
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
950
