
Biomechanics of Bone: Factors That Contribute to Osteoporosis and
Fractures and How to Combat This Risk
Boquan Jia
University of Sheffield (Sheffield, UK), Department of Animal and Plant Sciences, S10 2TN, U.K.
Keywords: Fracture, Osteoporosis, Risk, Bone Fragility.
Abstract: Reducing the risk of fracture and osteoporosis is particularly important as they have been a worldwide
health challenge, resulting in increased mortality and economic loss. According to relevant data, there are
about 23,700,000 people getting disease because of fracture and osteoporosis, which proves the
harmlessness of it (Al Anouti, F.et al, 2019). Bone delicacy is closely related with the chance of break and
osteoporosis, bone delicacy or bone quality is influenced by a assortment of variables, including bone
density, vitamin D, external forces and bone aging (Fonseca, et al, 2014). This paper addresses the factors
that influence bone fragility from a biomechanical perspective and suggests appropriate solutions to reduce
the risk of fracture and osteoporosis due to bone fragility. It was concluded that the natural aging of bones is
the main factor among many others, while the lack of vitamin D and external forces affect bone density to
accelerate this process.The risk of fracture and osteoporosis is reduced by appropriate exercise and moderate
intake of vitamin D.
1 INTRODUCTION
World widely, osteoporosis causes nearly 10 million
fractures each year, with an osteoporotic fracture
occurring every 3 seconds (Chao, et al, 2004). And
there is the academic result showing that the goal of
treating bone fragility is to increase strength and
reduce fragility (Turner, 2002). In the real
implementations, the following questions are shown
for this research topic. First and foremost, there are
few normal people except professional doctors
knowing the real principle of how fracture and
osteoporosis form and cause relevant bone diseases.
Besides, people who are getting these kinds of
diseases do not know much about Vitamin. As a
result, the lack of this information results in a more
serious degree of fracture and osteoporosis. Last but
not least, there is little attention and focus on this
kind of problem in the whole society, which requires
more relevant researches to change it. Based on
these conditions, this research focuses on the factors
that influence bone fragility from a biomechanical
perspective and suggests appropriate solutions to
reduce the risk of fracture and osteoporosis due to
bone fragility. To make the research aims in detail,
the following questions can be achieved.
RQ1. What are factors that influence bone
fragility from a biomechanical perspective?
RQ2. What are key factors that reduce the risk of
fracture and osteoporosis due to bone fragility?
Based on the information above, the significance
of this research can be summarized in the following
two aspects. On the one hand, there will be more
information for normal people to protect their bones
in daily life based on the solutions and other
recommendations mentioned in this research.
Therefore, this social problem can get solved more.
On the other hand, this research can provide
evidence for more professional and longer-term
academic research in the future.
2 DECLINE IN BONE MASS DUE
TO AGEING
Osteoporosis as a bone malady is clinically
characterized by decreased bone quality and a
propensity to break (Fonseca, et al, 2014). Several
studies have shown that skeletal changes are
age-related which maturing can bring almost a huge
number of skeletal changes within the tissue and
basic levels (Abraham, et al, 2016). Ageing causes a
926
Jia, B.
Biomechanics of Bone: Factors That Contribute to Osteoporosis and Fractures and How to Combat This Risk.
DOI: 10.5220/0011313200003443
In Proceedings of the 4th International Conference on Biomedical Engineering and Bioinformatics (ICBEB 2022), pages 926-931
ISBN: 978-989-758-595-1
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All r ights reserved
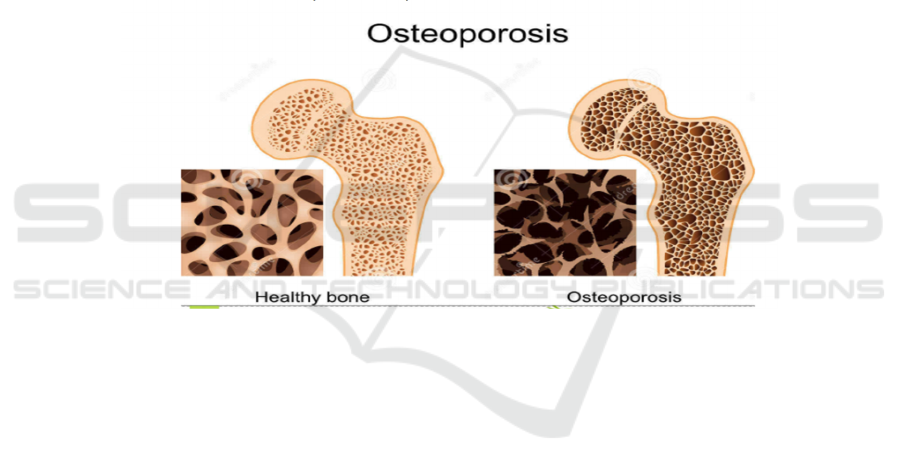
slowing of metabolism andisfortune of calcium from
the skeleton, leading to a general decline in bone
mechanics and a consequent expanded hazard of
break.
In expansion to this, age-related skeletal changes
are not simply a metabolic issue; the capacity of
tissues to stand up to break is decided by their
particular composition and structure. 1 Bone could
be a composite fabric comprising primarily of sort I
collagen, with a little sum of other non-collagenous
proteins and proteoglycans, and hydroxyapatite
gems develop amid biomineralisation.
This property of the bone strands permits them to
retain stretch through versatile misshapening and to
resist tall loads some time recently break. The
mineral stage is basically capable for the capacity to
stand up to distortion (hardness), whereas the
collagen strands can assimilate vitality (durability).
Changes in either composition may therefore affect
the mechanical properties of the bone and thus the
risk of fracture. 4The two-phase composition of
minerals and proteins in bone moreover gives it a
special combination of tall quality and durability.
While quality decides the most extreme stack that
the bone can withstand, sturdiness decides the bone's
capacity to scatter vitality, stand up to basic harm,
and subsequently trigger bone reconstruction to
repair microdamage to damaged bone tissue. Bone
toughness decreases with age and disease, thereby
increasing the risk of fracture (Abraham, et al, 2016);
(Hernandez, Keaveny, 2006). When strain reaches a
critical limit that cannot be tolerated, damage
gradually develops within the material as
microcracks develop, which can be shown in the
following figure
.
Figure 1: The comparison between different conditions of bones.
The quality and durability of bone are hence
profoundly subordinate on the capacity of the bone
to disseminate the stresses that cause expanded
strain, and on the microstructural properties that
prevent crack extension. Aging and disease lead to
an increase in intracortical porosity. Changes in
collagen and minerals in bone. As we age, bone cell
traps become smaller and more spherical.
Experimental studies have shown that as porosity
increases, the fracture toughness of bone decreases
significantly and the mineral content of bone
increases (Hemmatian, et al, 2017); (Ural, Vashishth,
2007).
Estrogen shows up to be a major controller of
skeletal digestion system not as it were in ladies but
also in men. 8 Bone deficiency is partly caused by a
deficiency of sex hormones. In particular,
post-menopausal women have reduced levels of
oestrogen, resulting in an overall negative balance
between bone resorption and formation rates. The
impaired bone structure may be due to a reduced
ability of osteoblasts to control local osteoblast
and/or osteoclast recruitment. Indeed, there is strong
evidence that reduced osteoclast sensitivity is
associated with age-related bone loss. For instance,
people who are aging are prone to getting
rheumatism (Al Anouti, et al, 2019). Thus,
oestrogen deficiency and impaired osteocyte
mechanosensitivity may be major risk factors for
osteoporotic fractures (Fonseca.et al, 2014).
The shape of osteoblasts and their traps changes
considerably with age. As osteocytes can directly
sense matrix strain through the cell body, changes in
osteocyte morphology may lead to alterations in
osteocyte mechanical sensitivity. Thus, the
load-adaptive response of osteocytes may change
with age, even when mechanical load remains
constant. Although substantial quantitative data are
lacking, there is evidence that osteocyte traps
Biomechanics of Bone: Factors That Contribute to Osteoporosis and Fractures and How to Combat This Risk
927
become smaller and more rounded with age
(Fonseca.et al, 2014).
3 VITAMIN D AND BONE
FRAGILITY
In studies of skeletal biomechanics in small animals,
a low calcium diet (LCD), reduced calcium
absorption and increased loss have been found to be
some of the important mechanisms that may
contribute to bone loss (Jiang.et al, 1997);
(Vashishth, 2008). Bone as a living tissue is more
accurately described as a mineralized tissue, and its
complexity is reflected in the fact that it undergoes
morphological changes in order to constantly adapt
to metabolic and structural demands. All changes in
the morphology of the external bone occur on the
surface of the periosteum, where complex anabolic
and catabolic processes take place (Roberts, et al,
2004). Thus, metabolic changes caused by food
intake and exercise can affect the chemistry of the
periosteal surface
.
Figure 2: How Vitamin D influences bones (Al Anouti, F.et al, 2019).
Vitamin D insufficiency may be a broad clutter
that plays an imperative part in human bone
wellbeing. Vitamin D lack causes maturing of the
human skeleton and increments the hazard of break.
In the presence of vitamin D deficiency, fracture
susceptibility is mainly associated with defects in
the mineralisation of the collagen matrix (bone-like
material). Vitamin D is broad in nature and one of
its parts in vertebrates and people is to advance the
retention of calcium and phosphorus so that bones
can mineralise appropriately. Vitamin D deficiency
in childhood predisposes to rickets, and defects in
growth plate cartilage and bone mineralisation lead
to altered long bone morphology, resulting in
curvature and deformity.In grown-ups, vitamin D
lack leads to osteochondrosis, a condition of flawed
mineralisation where the recently shaped bone
lattice (osteoid) falls flat to mineralise, driving to
bone torment, muscle shortcoming and an expanded
hazard of bone deformation and break (Busse, et al,
2013).
Vitamin D deficiency is a low serum 25(OH)D3
concentration associated with reduced serum
1,25-(OH)2D3 and calcium absorption. In turn, low
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
928

blood calcium leads to increased secretion of
parathyroid hormone (PTH), which promotes the
production of 1,25-(OH)2D3(5). As a result, serum
1, 25-(OH) 2D3 concentrations return to normal but
are accompanied by higher serum parathyroid
hormone concentrations, suggesting secondary
hyperparathyroidism. Increased serum parathyroid
hormone concentrations have been reported to
stimulate the rate of bone conversion, leading to a
reorganisation of bone structure. Secondary
hyperparathyroidism has therefore been suggested to
be a major factor in the increased susceptibility to
fracture due to vitamin D deficiency. Treatment with
vitamin D3 and calcium does significantly reduce
the incidence of non-vertebral fractures. However,
this was only achieved with little change in bone
mineral density (BMD) and serum parathyroid
hormone (PTH) concentrations, suggesting that
other factors play a role in reducing fracture risk.
Vitamin D deficiency increases bone turnover to
maintain normal calcium levels in the body,
producing bone-like bone that never mineralises
because of an overall calcium imbalance. The
deterioration in mechanical properties due to
vitamin D deficiency is associated with the
accumulation of large amounts of osteoid on the
bone surface and impaired resorption below the
bone surface, as evidenced by the accumulation of
unreconstructed traps of highly mineralised bone
cells. Localised tissue ageing is a key cause of the
increased risk of fracture in vitamin D deficiency
osteochondrosis, and tissue ageing affects the
resorption energy of bone by limiting bone plasticity
(Busse, et al, 2013).
4 METHODS OF REDUCING THE
RISK OF FRACTURES
Mechanical and biophysical stimulation can be
effective in promoting fracture healing in elderly
patients under less than ideal circumstances.
Different stimuli may limit their association with
specific healing mechanisms. However, accurate
repositioning is necessary for fracture healing,
regardless of the method of fixation used.
Misalignment of the fracture site will result in
delayed healing, deformed healing, or no healing.
When elderly patients with long bone fractures are
unable to perform the necessary rehabilitation
program, including partial weight-bearing exercises,
after fracture fixation, adjunctive physical or
biophysical stimulation can be applied to promote
bone healing and improve the quality of life of these
bedridden or wheelchair-bound patients.
Different types of stimulation have been shown
to be effective for fresh fractures or delayed healing.
As a result, more basic science research and clinical
trials are needed to make these potentially powerful
alternative medicine modalities more reliable.
Through signaling transducer design, tissue response
monitoring, dose and signal optimization, and
individualized and knowledge-based treatment
protocols for each patient and the fracture involved.
Considering all these special factors, the outcome of
fracture treatment in elderly patients and patients
with osteoporosis should not be different from other
fracture patients. Coordinated research and
development in relevant biomechanical areas is
needed to prepare us for the exponential growth of
the global aging population in the coming decades
(Chao, et al, 2004).
Bones benefit from regular physical activity.
Athletes typically have higher bone mass than
sedentary individuals, and prospective studies have
shown that exercise increases bone mass in humans
and experimental animals and experimental animals
(Turner, 2002). Although the increase in bone
density due to exercise is evident at younger ages,
the increase is small in adults. Despite this
apparently small effect, sedentary behavior is a
known risk factor for hip fracture, with men and
women who exercise regularly having up to half the
risk of hip fracture than sedentary men and women.
This reduction in fracture risk in physically active
adults must then be achieved by altering other
meaningful attributes that have an effect on bone
strength independent of BMD, as well as other
non-skeletal variables that significantly affect
fracture occurrence (e.g., fall risk), if only a slight
increase in BMD is obtained through exercise. Most
exercise intervention studies have shown that
exercise programs are either ineffective or have only
a small benefit in improving bone mineral density
(BMD) in patients with osteoporosis. Physical
activity has the potential to improve bone quality
and reduce fracture risk by influencing each of these
determinants. These findings have meaningful
clinical implications because they highlight the fact
that exercise interventions may benefit patients with
osteoporosis by improving other determinants of
bone strength, even if they do not lead to
improvements in BMD (Hemmatian, et al, 2017).
Biomechanics of Bone: Factors That Contribute to Osteoporosis and Fractures and How to Combat This Risk
929

5 CONCLUSION
This paper focuses on on the factors that influence
bone fragility from a biomechanical perspective and
suggests appropriate solutions to reduce the risk of
fracture and osteoporosis due to bone fragility. It can
be concluded that the natural aging of bones is a
major factor among many others, and lack of
vitamin D and external forces affecting bone density
accelerate this process. Aging slows down the
body's metabolism and thus affects the absorption of
vitamin D, which is an important component
involved in bone metabolism. Excessive physical
work depletes the durability of the bones and the
rate of bone metabolism does not keep up with the
rate of depletion, leading to fractures. There are two
ways to reduce the brittleness of bones and make
them stronger. Firstly, effective distribution of bone
mass can minimize the overuse of the bones. It is
important not to overuse a particular bone, but to
distribute the load on the bone appropriately.
Secondly, improving the material properties of bone
tissue can also make bone stronger at the tissue level,
controlling diet and exercising sensibly to improve
bone mass.
Here we have only discussed the general
framework of factors influencing osteoporosis and
fracture but we lack a quantitative analysis. As bone
fragility is ultimately a biomechanical event, further
research directions for this project should be based
on quantitative biomechanical tests to achieve more
intuitive data-based conclusions. The biomechanical
effects of bone mass can be quantified by analysing
the relationship between bone biomechanical
properties and bone density, with biomechanical
tests on bone at different physical scales (<1mm,
1mm, 1cm, etc.). Furthermore, data from analysis of
the relevant literature suggest that changes in bone
biomechanical properties with ageing, osteoporosis
or drug treatment remain unclear. We propose to use
the framework presented here, which represents the
basic bone biomechanical principles and will
provide new insights.
ACKNOWLEDGMENTS
To begin with and preeminent, I would like to thank
my proposal advisor, Professor Emad. During the
process of writing my dissertation, Prof. Emad gave
me careful guidance on the difficulties and doubts I
encountered. He gave me professional guidance and
recommendations on the direction of my thesis, and
helped me to improve this thesis. Then, I would like
to thank my partner for his support, we learned from
each other, helped each other, and had an
unforgettable time together. At long last, I would
like to thank my thesis analysts for their patient
work. My heartfelt thanks to my family for their
support.
REFERENCES
Abraham, A.C.et al, (2016). Microstructural and
compositional contributions towards the mechanical
behavior of aging human bone measured by cyclic and
impact reference point indentation. Bone.87, p37-43.
Al Anouti, F.et al, (2019). An insight into the paradigms
of osteoporosis: From genetics to biomechanics. Bone
reports, 11, p100-116.
Busse, B.et al, (2013). Vitamin D deficiency induces early
signs of aging in human bone, increasing the risk of
fracture. Science Translational Medicine.5 (193).
Chao, E.Y.S.et al, (2004). Biomechanical considerations
of fracture treatment and bone quality maintenance in
elderly patients and patients with osteoporosis.
Clinical Orthopaedics and Related Research.425,
p12-25.
Fonseca, H.et al, (2014). Bone quality: The determinants
of bone strength and fragility. Sports Medicine.44 (1),
p37-53.
Hemmatian, H.et al, (2017). Aging, Osteocytes, and
Mechanotransduction. Current Osteoporosis Reports.
15 (5), p401-411.
Hernandez, C. J., Keaveny, T. M., (2006). A
biomechanical perspective on bone quality. Bone.39
(6), p1173-1181.
Jiang, Y.et al, (1997). Long-term changes in bone mineral
and biomechanical properties of vertebrae and femur
in aging, dietary calcium restricted, and/or estrogen-
deprived/-replaced rats. Journal of Bone and Mineral
Research.12 (5), p820-831.
Khosla, S., (2013). Pathogenesis of age-related bone loss
in humans. Journals of Gerontology - Series A
Biological Sciences and Medical Sciences.68 (10),
p1226-1235.
Offord, E. A.et al, (2013). Nutrition and the biology of
human ageing: Bone health & osteoporosis /
sarcopenia / immune deficiency. Journal of Nutrition,
Health and Aging.17 (8), p712-716.
Roberts, W. E.et al, (2004). Bone modeling:
Biomechanics, molecular mechanisms, and clinical
perspectives. Seminars in Orthodontics.10 (2),
p123-161.
Tommasini, S.M.et al, (2005). Relationship between bone
morphology and bone quality in male tibias:
Implications for stress fracture risk. Journal of Bone
and Mineral Research.20 (8), p1372-1380.
Turner, C. H., (2002). Biomechanics of bone:
Determinants of skeletal fragility and bone quality.
Osteoporosis International.13 (2), p97-104.
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
930

Turner, C. H., (2002). Determinants of skeletal fragility
and bone quality. Journal of Musculoskeletal Neuronal
Interactions.2 (6), p527-528.
Ural, A., Vashishth, D., (2007). Effects of intracortical
porosity on fracture toughness in aging human bone:
A μCT-based cohesive finite element study. Journal of
Biomechanical Engineering.129 (5), p625-631.
Vashishth, D., (2008). Small animal bone biomechanics.
Bone.43 (5), p794-797.
Biomechanics of Bone: Factors That Contribute to Osteoporosis and Fractures and How to Combat This Risk
931
