
Azelastine Hydrochloride and Fluticasone Propionate in the
Alleviation of Allergic Rhinitis
Xiangkun Xu
Vision Academy, Shanghai, 200050, China
Keywords: Allergic Rhinitis, Azelastine Hydrochloride, Fluticasone Propionate.
Abstract: Allergic rhinitis is a global health problem. Although it does not endanger the life of patients with allergic
rhinitis, its symptoms, such as rhinorrhea, sneezing, nasal itching, and nasal obstruction, can seriously affect
the patients' quality of daily life and cause an economic burden to individual and country. In this paper, I
summarize the pathology of allergic rhinitis, as well as introduce three classes of drugs used to treat allergic
rhinitis, which includes corticosteroids, H1 receptor antagonists, leukotriene receptor antagonists. Among
them, azelastine hydrochloride and fluticasone propionate are taken as examples to introduce their respective
targets and specific pharmacological effects when contrasting H1 receptor antagonists and corticosteroids.
The reasons why corticosteroids became first-line drugs and their limitations are elaborated.
1 INTRODUCTION
Allergic rhinitis is a nasal symptomatic disease
caused by nasal mucositis after allergen exposure.
It belongs to type Ⅰ hypersensitivity
reactions(Vaillant et al 2020) and contains an early-
phase and late-phase allergic response (Sin, Togias
2011, Min 2010). The hallmark of allergic rhinitis is
nasal congestion, which often causes sleep
disturbances in patients. Approximately one-quarter
of 2500 adults in America reported that they would
wake up or be unable to sleep at night because of the
symptoms in a research in 2012(Meltzer et al 2012).
A survey of 35757 families in the United States
reported sleep disruption due to nasal allergic
symptoms in up to 45% of children in 2009(Meltzer
et al 2009). This phenomenon leads to mental fatigue
and low mood of people. It also causes cognitive
impairment, depression, and anxiety in patients by the
combination with other symptoms, which leads to a
decrease in work productivity of adults, and learning
disabilities of children, an inability of children to get
along better with their peers. As a result, there is a
negative impact on patients' quality of life (Meltzer,
2001, Muñoz-Cano R et al 2018).
2 THE DISEASE
2.1 Significance in China and Other
Countries in the World
Allergic rhinitis is a common disease that affects up
to 40% of the global population with about 23% to
30% of Europeans with a prevalence of 25% in
Sweden in 2012 (Bauchau, Durham 2004), and 12%
- 30% of the population in the United States (Nathan
et al 2008). People with allergic rhinitis are obliged
to ease their symptoms by using medicines. For
example, in 2017, fluticasone propionate that is a
drug to alleviate symptoms of allergic rhinitis was the
15th most commonly prescribed medication in the
United States, with more than 32 million
prescriptions (up from 16th place & 29 million in
2016). In 2018, azelastine hydrochloride that is also a
drug to alleviate symptoms of allergic rhinitis was the
240th most commonly prescribed medication in the
United States, with more than 2 million prescriptions.
The prevalence of allergic rhinitis is increasing
globally (Dykewicz, Hamilos 2010). It is usually a
long-standing disease and often goes unnoticed by
people due to its high universality of symptoms at its
initial stage. Although it is not life-threatening, its
symptoms are often bothersome and reduce the
quality of life and work of people, and it also causes
a significant burden on the individual and the country
916
Xu, X.
Azelastine Hydrochloride and Fluticasone Propionate in the Alleviation of Allergic Rhinitis.
DOI: 10.5220/0011313100003443
In Proceedings of the 4th International Conference on Biomedical Engineering and Bioinformatics (ICBEB 2022), pages 916-925
ISBN: 978-989-758-595-1
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
(Canonica et al 2007). The burden on society is the
costs associated with the treatment of allergic rhinitis
(Simoens, Laekeman 2009). Although the direct cost
of treating allergic rhinitis is not obvious, it incurs
substantial indirect costs (Bousquet et al 2008).
In 2016, the total cost of allergic rhinitis was
estimated at € 1.3 billion per year in Sweden, which
has a population of 9.5 million people. The total cost
per person per year due to allergic rhinitis was €
961.1, which includes mean direct and indirect costs
of € 210.3 and € 750.8, respectively in
Sweden(Cardell et al 2016)]. In Germany, the total
cost of allergic rhinitis was € 240 million in 2000,
which included both direct and indirect costs (Bachert
et al. 2006). It is difficult to search the prevalence of
allergic rhinitis in China in recent years because of
the paucity of relevant data on allergic rhinitis.
According to the data of The National Bureau of
Statistics of China, China had a population of 1.37
billion at the end of 2014. The prevalence of allergic
rhinitis reported in 18 major cities in China was
17.6%.
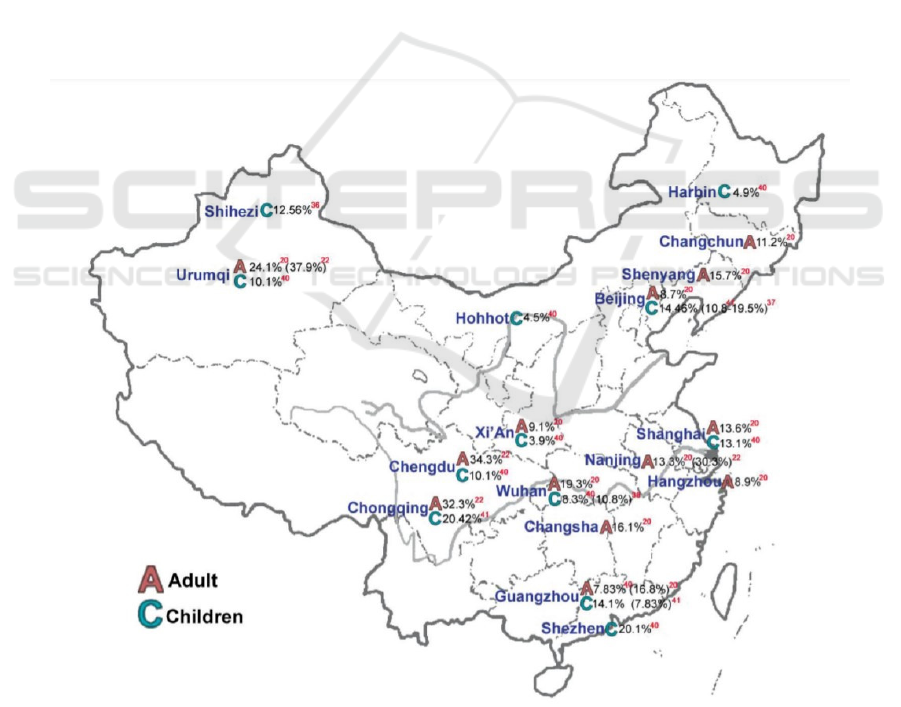
The overall prevalence in the four major cities of
Western China (Chengdu, Chongqing, Nanning, and
Urumqi) is 34.3%, 34.3% in Chengdu, 32.3% in
Chongqing, 30.3% in Nanning, and 37.9% in Urumqi
(Figure 1). The overall prevalence of allergic rhinitis
in several areas in northern China (rural areas of
Qingxian, Hebei; coastal fishing village of Bohai
Bay, Huanghua; area of Wuling Mountain, Chengde;
urban areas of Tianjin) are about 9.2% (Zhang, Zhang
2014). This preliminary suggests that different
geographical and climatic contribute to the
differences in prevalence. Meanwhile, occupational
factors are also considered as one of the reasons for
the different prevalence. For example, farmers have a
2.32-fold increased risk (Wang et al 2011, 2012).
Although there is variation in prevalence across
regions, the prevalence has generally increased in
both adults and children over the past 20 years. The
epidemic trends of allergic rhinitis in China and the
trends of other developing countries are the same.
Figure 1: Prevalence of allergic rhinitis in adults and children in different cities in China in 2008 (Zhang, Zhang 2014)].
Azelastine Hydrochloride and Fluticasone Propionate in the Alleviation of Allergic Rhinitis
917
2.2 The Pathology of Allergic Rhinitis
and Its Symptoms
Antigens that cause hypersensitivity reactions are
called allergens, which contain certain drugs, plant
pollen, dust mites, fungal spores, animal dander or
feathers, insects or their venoms as well as foods,
such as fish, shrimp, eggs, milk, and certain enzymes
classes, such as subtilisin.
When the allergens enter the human body, they
stimulate B-lymphocytes to convert into plasma cells
which can produce immunoglobulin E (IgE)
antibodies. IgE is found at very low levels in normal
human serum and is significantly increased in the
serum of hypersensitive patients, so it is often
considered by doctors as an important indicator for
the diagnosis of allergic diseases (Ansotegui et al.
2020).
IgE has a high affinity for basophils and mast
cells, so they can bind to high-affinity IgE receptors
(FcεRI) on the surface of tissue mast cells and blood
basophils (Turner, Kinet 1999). When there is a
reappearance of the same allergen and cross-links to
IgE on the cell surface, FcεRI activates mast cells or
basophils by signal transduction and releases
intragranular active mediators, such as histamine,
kinins, proteases, chemokines, and heparin
(Siraganian 2003), and several types of type 2
cytokines like interleukin (IL)-3. This process is
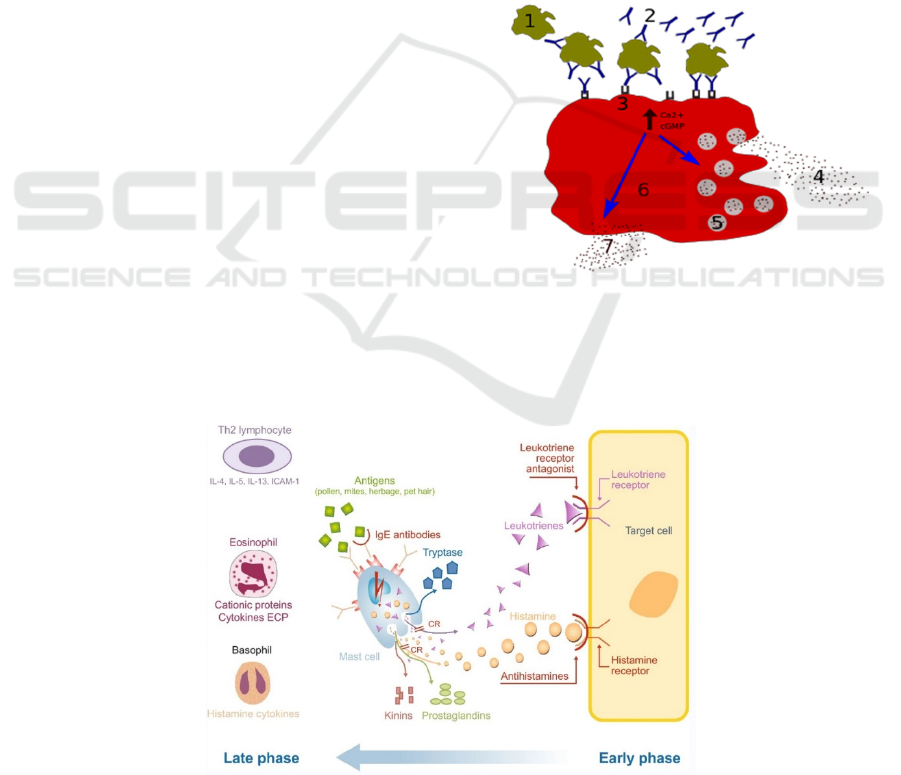
called degranulation (Figure 2), which is the feature
of the early-phase reaction (Figure 3). At the same
time, arachidonic acid is released from cell
membrane phospholipids of activated mast cells and
basophils, then it is catalyzed by lipoxygenases or
cyclooxygenases to form the inflammatory lipid
mediators that include leukotrienes (composed of
LTC4, LTD4, and LTE4) and prostaglandins,
respectively (Moon, Befus, Kulka 2014). Symptoms
triggered by histamine-mediated anaphylaxis and
inflammatory mediators in the early-phase response
include bronchoconstriction, vasodilation, smooth
muscle contraction, etc.
Late-phase allergic responses (Figure 3) appear
several hours after exposure to the allergen. The
responses are characterized by the cellular
recruitment of basophils, neutrophils, T-
lymphocytes, monocytes, and eosinophils. They can
release several mediators, including cytokines,
prostaglandins, and leukotrienes, which increase the
duration of the inflammatory response (Sin, Togias
2011, Min 2010). This means that the late-phase
response is related to the development and
persistence of tissue edema and nasal congestion.
Figure 2: Degranulation processes 1 - antigen; 2 - IgE
antibody; 3 - FcεRI receptor; 4 - preformed mediators
(histamine, proteases, chemokines, heparin); 5 - granules; 6
- mast cell; 7 - newly formed mediators (prostaglandins,
leukotrienes).
Figure 3: Early-phase and late-phase allergic response of allergic rhinitis (Bjermer, Westman, Holmström, Wickman 2019).
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
918
The main symptoms of patients with allergic
rhinitis are runny nose, sneezing, nasal itching, and
nasal congestion, it is usually accompanied by ocular
pruritus, redness, and/or lacrimation in 60% - 70% of
patients (Canonica et al 2007, Bousquet et al 2008,
Schatz 2007). Subsequent disease development may
lead to related conjunctivitis, postnasal drip,
eustachian tube dysfunction, otitis media, etc. At the
same time, about 20% - 50% of patients with allergic
rhinitis suffer from clinical asthma (Strachan et al
1997), which shows allergic rhinitis often coexists
with asthma.
2.3 Drugs Used to Treat the Disease
and Their Origins
There are three categories of drugs that are used to
treat allergic rhinitis. They have different modes of
action and different interactions in allergic rhinitis
cases.
a) H1 receptor antagonists
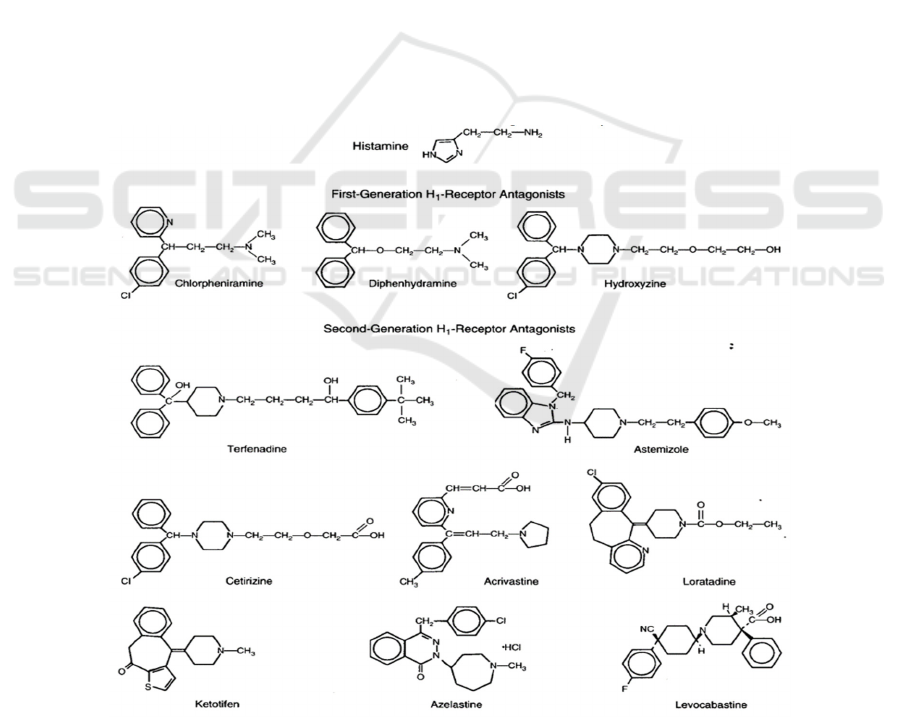
The first-generation of H1 receptor antagonists
(Figure 4), diphenhydramine (Benadryl),
carbinoxamine (Clistin), clemastine (Tavist),
chlorpheniramine (Chlor-Trimeton), and
brompheniramine (Dimetane) that were marketed
before the 1980s (Berdy et al 1991, Welch et al 2002)
can cross the blood-brain barrier and enter the central
nervous system because of their high lipid solubility,
which causes the side effects of central depression
and sedation(Kay 2000). At the same time, the
selectivity to the H1 receptor of the first generation of
H1 receptor antagonists is not strong enough
(Kalpaklioglu, Baccioglu 2012), so there are the side
effects, such as anti-cholinergic, anti-adrenergic,
analgesic effects (Ali Habibi ETR 1991).
Second-generation H1 receptor antagonists
(Figure 4), such as cetirizine, terfenadine, astemizole,
loratadine, azelastine, and acrivastine (Aaronson
1991), were invented to overcome these side effects
as much as possible. They are more hydrophilic and
less lipophilic, and they also have a higher selectivity
for the H1 receptor, which means that the possibility
of the drugs crossing the blood-brain barrier is greatly
reduced and the central side effects are attenuated
compared with the first generation of H1 receptor
antagonists (Kay 2000).
Figure 4: Chemical structures of H1 receptor antagonists (Simons, Simons 1994) The treatment of allergic rhinitis generally
requires intranasal and oral H1 receptor antagonists. After oral administration, the concentration of the drug in the nasal cavity
often cannot reach the effective value (Molimard, Diquet, Benedetti 2004, Urien et al 1999), so it is necessary to increase the
concentration through intranasal injection (Horak, Zieglmayer 2009).
Azelastine Hydrochloride and Fluticasone Propionate in the Alleviation of Allergic Rhinitis
919
b) Corticosteroids
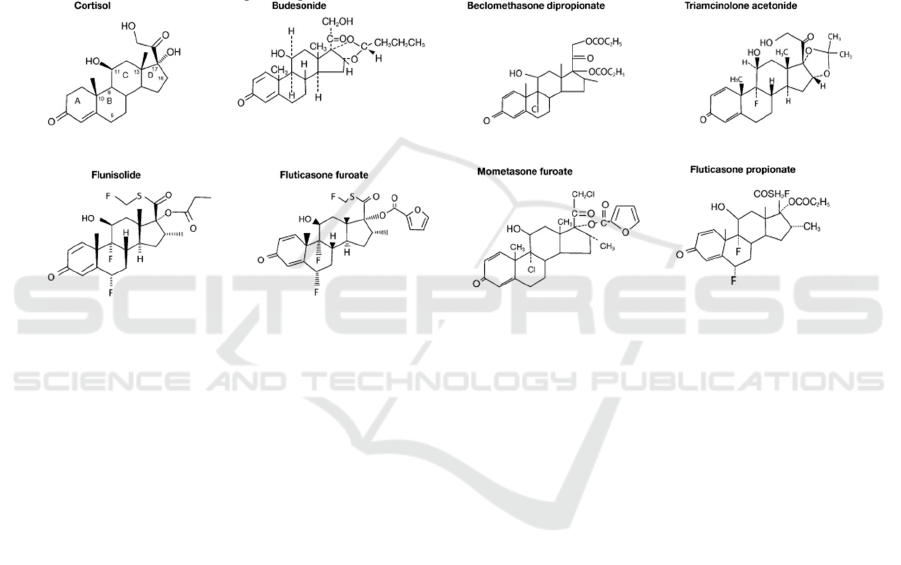
Corticosteroids are considered a safe and effective
first-line treatment for allergic rhinitis, although it
was initially reserved as a second-line agent (Group
IRMW 1994). Several intranasal corticosteroids are
available for allergic rhinitis (Figure 5), such as
beclomethasone dipropionate, budesonide,
flunisolide, fluticasone propionate, mometasone
furoate, and triamcinolone acetonide (Trangsrud,
Whitaker, Small 2002). The following contents
include the relationships of structure-activity of
corticosteroids.
The carbon skeleton of each corticosteroid
consists of three 6-carbon rings (rings A, B, and C)
and one 5-carbon ring (ring D). Common features
among each other are the ketone oxygen group at
position 3, unsaturated double bond between carbons
4 and 5, hydroxyl group at position 11, and a ketone
oxygen group on carbon 20. The changes at positions
16, 17, and 21, outside the D-loop, are the largest
differences between the individual molecules (Szefler
2001).
For example, the furoate group of mometasone
furoate can enhance the molecular affinity to the
glucocorticoid receptor binding site. Other groups
improve the activity of corticosteroid compounds.
Figure 5: Chemical structures of corticosteroids.
Modes of administration include intranasal
administration and systemic administration. The
common way is intranasal administration because
anti-inflammatory effects of the drugs can be
problematic if systemic concentrations of these drugs
are excessive, and intranasal administration can
achieve the efficacy of systemic administration while
minimizing side effects by oral administration for
patients with allergic rhinitis (Pichler, Klint, Blaser,
Graf, Sauter, Weiss et al.1988).
c) Leukotriene receptor antagonists
Leukotriene receptor antagonists, such as
montelukast, zafirlukast, and pranlukast, can block
the activity or secretion of cysteinyl leukotriene
(CysLT) that is an inflammatory mediator and
include leukotriene C4(LTC4), leukotriene
D4(LTD4), and leukotriene E4(LTE4) (Peters-
Golden, Henderson 2005). There are two ways to
block the action of leukotrienes. The first way is to
inhibit the synthetic pathway of leukotriene
metabolism by inhibiting 5-lipoxygenase. The second
way is to rely on the antagonistic effect of drugs on
cysteinyl-leukotriene type 1 (CysLT1) receptors,
such as montelukast and zafirlukast, which can block
the effect of CysLT on CysLT1 receptor of target
cells, such as bronchial smooth muscle. Therefore, it
can alleviate the symptoms of allergic rhinitis (Singh
2013).
3 AZELASTINE
HYDROCHLORIDE AND
FLUTICASONE PROPIONATE
3.1 Basic Information on Azelastine
Hydrochloride and Fluticasone
Propionate
a) Azelastine hydrochloride
The IUPAC name of azelastine hydrochloride is
4-[(4-chlorophenyl)methyl]-2-(1-methylazepan-4-
yl)phthalazin-1-one;hydrochloride. ‘Hydrochloride’
means that it is the hydrochloride salt of azelastine.
The molecular formula of azelastine
hydrochloride is C22H24ClN3O.HCl. Figure 6 and
figure 7 show the chemical structure of azelastine
hydrochloride.
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
920
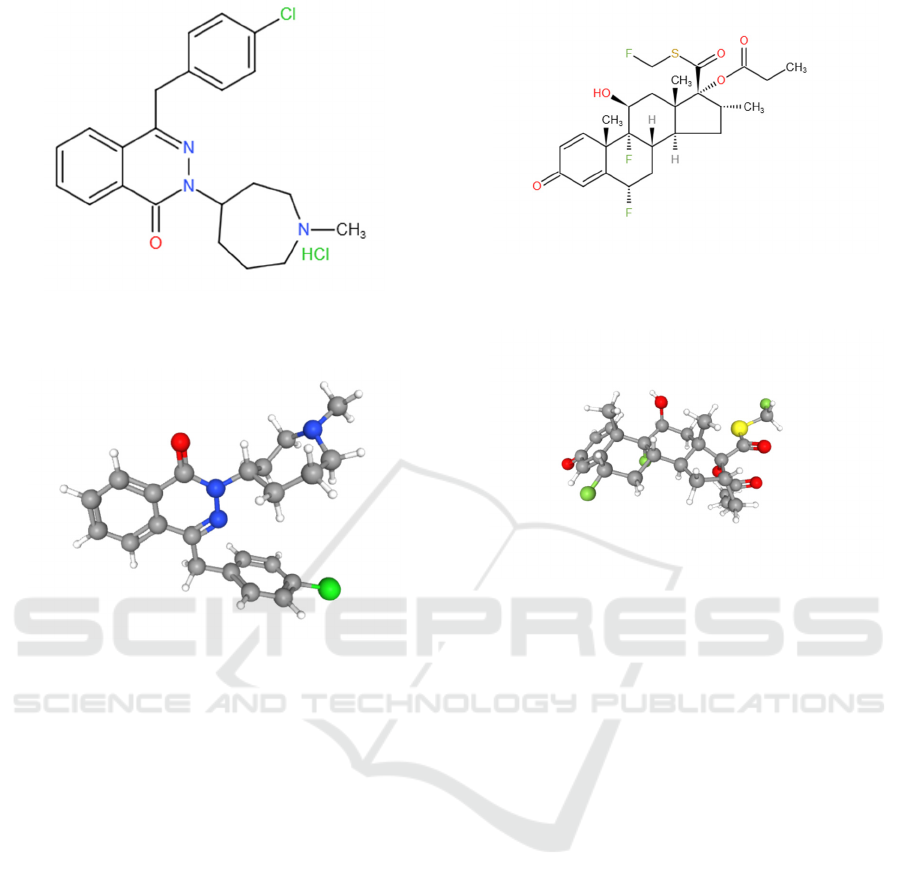
Figure 6: Chemical structure of azelastine hydrochloride
(2D).
Figure 7: Chemical structure of azelastine (3D).
Azelastine hydrochloride is a racemic mixture
with a melting point of 225°C, so it is a white
crystalline powder at room temperature with a
molecular weight of 418.37. It is sparingly soluble in
water, methanol, and propylene glycol, and slightly
soluble in ethanol, octanol, and glycerine. It is sold as
a form of the solution under the brand name Optivar.
b) Fluticasone propionate
The IUPAC name of fluticasone propionate is
[(6S,8S,9R,10S,11S,13S,14S,16R,17R)-6,9-
difluoro-17-(fluoromethylsulfanylcarbonyl)-11-
hydroxy-10,13,16-trimethyl-3-oxo-
6,7,8,11,12,14,15,16-
octahydrocyclopenta[a]phenanthren-17-yl]
propanoate. Fluticasone Propionate is the propionate
salt form of fluticasone.
The molecular formula of fluticasone propionate
is C25H31F3O5S. Figure 8 and figure 9 show the
chemical structure of fluticasone propionate.
Figure 8: Chemical structure of fluticasone propionate
(2D).
Figure 9: Chemical structure of fluticasone propionate
(3D).
The melting point of fluticasone Propionate is
261-273 °C, so it is solid at room temperature with a
molecular weight of 500.6. It is insoluble in water and
sold under the brands of Flovent and Florase. So, it is
not easy to dissolve in human bronchial fluid, the
deposition of drugs in the airways increases. The
release of drugs is slower, and the local action time is
longer.
3.2 Pharmacology of Azelastine
Hydrochloride and Fluticasone
Propionate
a) Azelastine hydrochloride
H1 histamine receptors belong to the family of
rhodopsin-like G-protein-coupled receptors. They are
activated by histamine and are expressed in smooth
muscles, vascular endothelial cells, the heart, and the
central nervous system. It can trigger anaphylaxis
mediated by histamine.
In the type Ⅰ hypersensitivity allergic reactions
process, once histamine is released from mast cells or
basophils by cellular degranulation, it can bind to H1
histamine receptors to initiate allergic reactions
(Lytina et al 2002, Tamaoki et al 1999). Azelastine
Azelastine Hydrochloride and Fluticasone Propionate in the Alleviation of Allergic Rhinitis
921
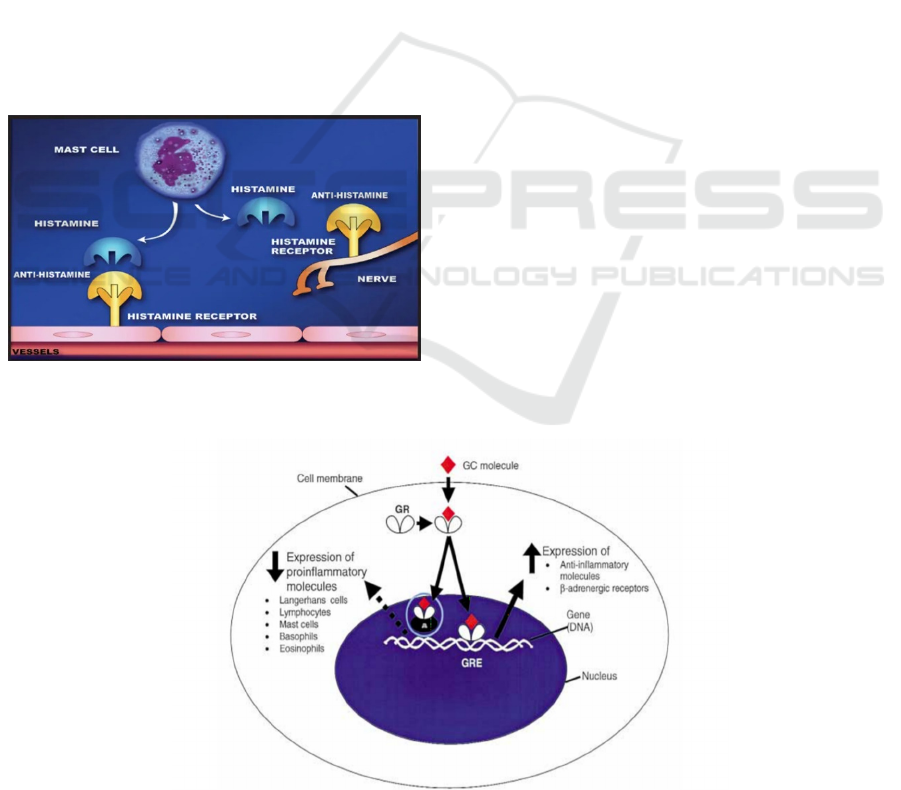
hydrochloride is useful in the treatment of allergic
rhinitis by competing with histamine for H1
histamine receptors (Figure 10).
Therefore, histamine-mediated symptoms of
anaphylaxis, such as rhinorrhea, itching, and sneezing
are reduced. This drug also has the effects of
stabilizing mast cells, anti-leukotrienes, and anti-
inflammatory (Horak, Zieglmayer 2009).
Azelastine hydrochloride can effectively block
the calcium channel regulated by IgE in the
degranulation process of mast cells and stabilize mast
cells, so it can prevent the release of histamine and
other mediators, such as prostaglandins, kinin, and
interleukin (Van Hoecke, Vandenbulcke, Van
Cauwenberge 2007, Kempuraj et al 2003).
Azelastine hydrochloride can inhibit
phospholipase A2 and LTC4 synthase and prevents
the release of leukotriene (LTB4 and LTC4) by
stabilizing mast cells (Hamasaki et al. 1996).
Anti-inflammatory properties of azelastine
hydrochloride are resulted from inhibiting the release
of inflammatory cells and mediators. These include
eosinophils and neutrophils as well as mediators.
Figure 10: The process of preventing mast cell
degranulation.
b) Fluticasone propionate
The glucocorticoid receptor (GR) is the receptor
to which cortisol that is the endogenous
glucocorticoid hormone and is produced in many
animals, mainly by the zona fasciculata of the adrenal
cortex in the adrenal gland (Thau, Gandhi, Sharma.
Physiology, cortisol. 2019.), and other glucocorticoid
receptor agonists (GC) bind. It is expressed in almost
every cell in the body. When cortisol or GC bind to
GR, it achieves anti-inflammatory effects by
regulating gene transcription (Lu et al 2006, Rhen
2005).
Glucocorticoid receptor agonists that have often
been used to treat allergic rhinitis belong to
corticosteroids. Fluticasone propionate, one of the
glucocorticoid receptor agonists (GC molecules),
binds to and activates glucocorticoid receptors (GR),
thereby activating lipocortins. Lipocortins can inhibit
cytosolic phospholipase A2 that can trigger a cascade
of responses involved in the synthesis of
inflammatory mediators, such as prostaglandins and
leukotrienes. The transcriptional activity of nuclear
factor kappa-B(NF-κB) is blocked, thereby inhibiting
the transcription of cyclooxygenase 2, which is
essential for the production of prostaglandin.
The complex formed from binding the molecules
of fluticasone propionate to GR can alter
transcriptional activity (Figure 11), which leads to
decreased expression of proinflammatory molecules
and cells, including Langerhans cells, lymphocytes,
mast cells, basophils, and eosinophils, and inhibits
the arrival of Langerhans cells, macrophages, mast
cells, T-lymphocytes, and eosinophils in the nasal
mucosa (Holm et al 2001). At the same time, it
increases anti-inflammatory molecules and β-
expression of adrenergic receptors (Mygind et al.
2001).
Figure 11: Actions of glucocorticoid (GC) molecule in the inflammatory process (Mygind et al. 2001).
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
922

c) Compare azelastine hydrochloride and
fluticasone propionate
Table 1: The differences between azelastine hydrochloride and fluticasone propionate.
azelastine hydrochloride fluticasone propionate
Targets of
drug
H1 histamine receptors on the
target cell.
The glucocorticoid receptors on the target cell.
Functions Compete with histamine for H1
histamine receptors.
Effects of stabilizing mast cells,
anti-leukotrienes, and anti-
inflammatory.
Bind to and activates glucocorticoid receptors, thereby activating lipocortins
and reducing the production of inflammatory mediators.
Alter transcriptional activity, that leads to decreased expression of
proinflammatory molecules and cells, an increase in expression of anti-
inflammatory molecules, and β-expression of adrenergic receptors.
The different pharmacology of the two drugs has
led to different therapeutic effects (see Table 1). Most
patients with allergic rhinitis who present to a
primary care physician have moderate to severe
symptoms, the use of fluticasone propionate is a
better option (Bousquet et al. 2003) because
fluticasone propionate is a potent inhibitor of
anaphylaxis in the late phase of allergic rhinitis. They
are more effective to control the symptoms of allergic
rhinitis, including nasal congestion, and rhinorrhea
than leukotriene receptor antagonists than H1
receptor antagonists (Weiner, Abramson, Puy 1998).
However, it is important to note that intranasal
corticosteroids are best initiated before exposure to
relevant allergens, as their peak effects may take
several days to develop, necessitating their regular
use to achieve better outcomes (Lee, Mace 2009).
4 CONCLUSION
This article has summarized the pathology of allergic
rhinitis and its symptoms, its significance in China
and other countries, and the current three classes of
drugs to treat it. At the same time, azelastine
hydrochloride and fluticasone propionate were used
as an example to explain the targets and
pharmacological effects of these two classes of drugs.
Typically, the effects of intranasal corticosteroids are
more obvious than those of second-generation
antihistamines. It is worth noting that current
medications only alleviate symptoms of allergic
rhinitis. Finding any approach to the thorough
treatment of allergic rhinitis is the direction of
research in the future.
REFERENCES
Aaronson D. Comparative efficacy of H1 antihistamines.
Annals of allergy. 1991;67(5):541-7.
Ali Habibi ETR. Antihistamines: H1- and H2-Blockers. in
Complications in Anesthesia (Second Edition). 2007.
Ansotegui IJ, Melioli G, Canonica GW, Caraballo L, Villa
E, Ebisawa M, et al. IgE allergy diagnostics and other
relevant tests in allergy, a World Allergy Organization
position paper. World allergy organization journal.
2020;13(2):100080.
Bachert C, Borchard U, Wedi B, Klimek L, Rasp G,
Riechelmann H, et al. Allergic rhinoconjunctivitis.
Guidelines of the DGAI in association with the DDG.
Journal der Deutschen Dermatologischen
Gesellschaft= Journal of the German Society of
Dermatology: JDDG. 2006;4(3):264-75.
Bauchau V, Durham S. Prevalence and rate of diagnosis of
allergic rhinitis in Europe. European respiratory
journal. 2004; 24(5):758-64.
Berdy GJ, ABELSON MB, GEORGE MA, SMITH LM,
GIOVANONI RL. Allergic conjunctivitis: a survey of
new antihistamines. Journal of Ocular Pharmacology
and Therapeutics. 1991;7(4):313-24.
Bjermer L, Westman M, Holmström M, Wickman MC. The
complex pathophysiology of allergic rhinitis: scientific
rationale for the development of an alternative
treatment option. Allergy, Asthma & Clinical
Immunology. 2019;15(1):1-15.
Bousquet J, Khaltaev N, Cruz AA, Denburg J, Fokkens W,
Togias A, et al. Allergic rhinitis and its impact on
asthma (ARIA) 2008. Allergy. 2008;63:8-160.
Bousquet J, Lund V, Van Cauwenberge P, Bremard‐Oury
C, Mounedji N, Stevens M, et al. Implementation of
guidelines for seasonal allergic rhinitis: a randomized
controlled trial. Allergy. 2003;58(8):733-41.
Canonica G, Bousquet J, Mullol J, Scadding G, Virchow J.
A survey of the burden of allergic rhinitis in Europe.
Allergy. 2007;62:17-25.
Cardell L-O, Olsson P, Andersson M, Welin K-O,
Svensson J, Tennvall GR, et al. TOTALL: high cost of
Azelastine Hydrochloride and Fluticasone Propionate in the Alleviation of Allergic Rhinitis
923

allergic rhinitis—a national Swedish population-based
questionnaire study. NPJ primary care respiratory
medicine. 2016;26(1):1-5.
Dykewicz MS, Hamilos DL. Rhinitis and sinusitis. Journal
of Allergy and Clinical Immunology. 2010;
125(2):S103-S15.
Group IRMW. International consensus report on the
diagnosis and management of allergic rhinitis. Allergy.
1994;49:5-34.
Hamasaki Y, Shafigeh M, Yamamoto S, Sato R, Zaitu M,
Muro E, et al. Inhibition of leukotriene synthesis by
azelastine. Annals of Allergy, Asthma & Immunology.
1996;76(5):469-75.
Holm A, Dijkstra M, Kleinjan A, Severijnena L-a, Boksa S,
Mulder P, et al. Fluticasone propionate aqueous nasal
spray reduces inflammatory cells in unchallenged
allergic nasal mucosa: effects of single allergen
challenge. Journal of allergy and clinical immunology.
2001;107(4):627-33.
Horak F, Zieglmayer UP. Azelastine nasal spray for the
treatment of allergic and nonallergic rhinitis. Expert
Review of Clinical Immunology. 2009;5(6):659-69.
Kalpaklioglu F, Baccioglu A. Efficacy and safety of H1-
antihistamines: an update. Anti-Inflammatory & Anti-
Allergy Agents in Medicinal Chemistry (Formerly
Current Medicinal Chemistry-Anti-Inflammatory and
Anti-Allergy Agents). 2012;11(3):230-7.
Kay GG. The effects of antihistamines on cognition and
performance. Journal of Allergy and Clinical
Immunology. 2000;105(6):S622-S7.
Kempuraj D, Huang M, Kandere-Grzybowska K, Basu S,
Boucher W, Letourneau R, et al. Azelastine inhibits
secretion of IL-6, TNF-α and IL-8 as well as NF-κB
activation and intracellular calcium ion levels in normal
human mast cells. International archives of allergy and
immunology. 2003;132(3):231-9.
Lee P, Mace S. An approach to allergic rhinitis. Allergy
Rounds. 2009;1:1.
Lu NZ, Wardell SE, Burnstein KL, Defranco D, Fuller PJ,
Giguere V, et al. International Union of Pharmacology.
LXV. The pharmacology and classification of the
nuclear receptor superfamily: glucocorticoid,
mineralocorticoid, progesterone, and androgen
receptors. Pharmacological reviews. 2006;58(4):782-
97.
Lytinas M, Kempuraj D, Huang M, Kandere K, Boucher
W, Letourneau R, et al., editors. Azelastine's inhibition
of histamine and tryptase release from human umbilical
cord blood-derived cultured mast cells as well as rat
skin mast cell-induced vascular permeability:
comparison with olopatadine. Allergy and asthma
proceedings; 2002: OceanSide Publications.
Meltzer EO, Blaiss MS, Derebery MJ, Mahr TA, Gordon
BR, Sheth KK, et al. Burden of allergic rhinitis: results
from the Pediatric Allergies in America survey. Journal
of Allergy and Clinical Immunology. 2009;124(3):S43-
S70.
Meltzer EO, Blaiss MS, Naclerio RM, Stoloff SW,
Derebery MJ, Nelson HS, et al., editors. Burden of
allergic rhinitis: allergies in America, Latin America,
and Asia-Pacific adult surveys. Allergy and Asthma
Proceedings; 2012: OceanSide Publications, Inc.
Meltzer EO. Quality of life in adults and children with
allergic rhinitis. Journal of allergy and clinical
immunology. 2001;108(1):S45-S53.
Min Y-G. The pathophysiology, diagnosis and treatment of
allergic rhinitis. Allergy, asthma & immunology
research. 2010;2(2):65-76.
Molimard M, Diquet B, Benedetti MS. Comparison of
pharmacokinetics and metabolism of desloratadine,
fexofenadine, levocetirizine and mizolastine in
humans. Fundamental & clinical pharmacology. 2004;
18(4):399-411.
Moon TC, Befus AD, Kulka M. Mast cell mediators: their
differential release and the secretory pathways
involved. Frontiers in immunology. 2014;5:569.
Muñoz-Cano R, Ribó P, Araujo G, Giralt E, Sanchez-Lopez
J, Valero A. Severity of allergic rhinitis impacts sleep
and anxiety: results from a large Spanish cohort.
Clinical and translational allergy. 2018;8(1):1-9.
Mygind N, Nielsen LP, Hoffmann H-J, Shukla A,
Blumberga G, Dahl R, et al. Mode of action of
intranasal corticosteroids. Journal of allergy and
clinical immunology. 2001;108(1):S16-S25.
Nathan RA, Meltzer EO, Derebery J, Campbell UB, Stang
PE, Corrao MA, et al., editors. The prevalence of nasal
symptoms attributed to allergies in the United States:
findings from the burden of rhinitis in an America
survey. Allergy and asthma proceedings; 2008:
OceanSide Publications.
Peters-Golden M, Henderson Jr WR. The role of
leukotrienes in allergic rhinitis. Annals of Allergy,
Asthma & Immunology. 2005;94(6):609-18.
Pichler W, Klint T, Blaser M, Graf W, Sauter K, Weiss S,
et al. Clinical comparison of systemic
methylprednisolone acetate versus topical budesonide
in patients with seasonal allergic rhinitis. Allergy.
1988;43(2):87-92.
Rhen T, Cidlowski JA. Antiinflammatory action of
glucocorticoids—new mechanisms for old drugs. New
England Journal of Medicine. 2005;353(16):1711-23.
Schatz M. A survey of the burden of allergic rhinitis in the
USA. Allergy. 2007;62:9-16.
Simoens S, Laekeman G. Pharmacotherapy of allergic
rhinitis: a pharmaco‐economic approach. Allergy.
2009;64(1):85-95.
Simons FER, Simons KJ. The pharmacology and use of
H1-receptor-antagonist drugs. New England Journal of
Medicine. 1994;330(23):1663-70.
Sin B, Togias A. Pathophysiology of allergic and
nonallergic rhinitis. Proceedings of the American
Thoracic Society. 2011;8(1):106-14.
Singh RK, Tandon R, Dastidar SG, Ray A. A review on
leukotrienes and their receptors with reference to
asthma. Journal of Asthma. 2013;50(9):922-31.
Siraganian RP. Mast cell signal transduction from the high-
affinity IgE receptor. Current opinion in immunology.
2003;15(6):639-46.
Strachan D, Sibbald B, Weiland S, Ait‐Khaled N,
Anabwani G, Anderson HR, et al. Worldwide
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
924

variations in prevalence of symptoms of allergic
rhinoconjunctivitis in children: the International Study
of Asthma and Allergies in Childhood (ISAAC).
Pediatric allergy and immunology. 1997;8(4):161-8.
Szefler SJ. Pharmacokinetics of intranasal corticosteroids.
Journal of allergy and clinical immunology.
2001;108(1):S26-S31.
Tamaoki J, Yamawaki I, Tagaya E, Kondo M, Aoshiba K,
Nakata J, et al. Effect of azelastine on platelet-
activating factor-induced microvascular leakage in rat
airways. American Journal of Physiology-Lung
Cellular and Molecular Physiology. 1999;276(2):L351-
L7.
Thau L, Gandhi J, Sharma S. Physiology, cortisol. 2019.
Trangsrud AJ, Whitaker AL, Small RE. Intranasal
corticosteroids for allergic rhinitis. Pharmacotherapy:
The Journal of Human Pharmacology and Drug
Therapy. 2002;22(11):1458-67.
Turner H, Kinet J-P. Signalling through the high-affinity
IgE receptor FcεRI. Nature. 1999;402(6760):24-30.
Urien S, Tillement J, Ganem B, Kuch M. A
pharmacokinetic-pharmacodynamic modelling of the
antihistaminic (H1) effects of cetirizine. International
Journal of clinical pharmacology and therapeutics.
1999;37(10):499-502.
Vaillant AAJ, Vashisht R, Zito PM. Immediate
Hypersensitivity Reactions. StatPearls [Internet]. 2020.
Van Hoecke H, Vandenbulcke L, Van Cauwenberge P.
Histamine and Leukotriene Receptor Antagonism in
the Treatment of Allergic Rhinitis. Drugs.
2007;67(18):2717-26.
Wang Z, Lin W, Li S, Zhao S, Wang L, Yang Z, et al.
Analysis of the correlation of prevalence in allergic
rhinitis and other allergic diseases. Zhonghua er bi yan
hou tou jing wai ke za zhi= Chinese journal of
otorhinolaryngology head and neck surgery.
2012;47(5):379-82.
Wang Z, Lin W, Li S, Zhao S, Wang L, Yang Z, et al.
Research on prevalence and related factors in allergic
rhinitis. Zhonghua er bi yan hou tou jing wai ke za zhi=
Chinese journal of otorhinolaryngology head and neck
surgery. 2011;46(3):225-31.
Weiner JM, Abramson MJ, Puy RM. Intranasal
corticosteroids versus oral H1 receptor antagonists in
allergic rhinitis: systematic review of randomised
controlled trials. Bmj. 1998;317(7173):1624-9.
Welch MJ, Meltzer EO, Simons FER. H1-antihistamines
and the central nervous system. Histamine and H1-
antihistamines in allergic disease. 2002:353-404.
Zhang Y, Zhang L. Prevalence of allergic rhinitis in china.
Allergy, asthma & immunology research. 2014;
6(2):105-13.
Azelastine Hydrochloride and Fluticasone Propionate in the Alleviation of Allergic Rhinitis
925
