
Study on the Antioxidant Capability and Microencapsulation of
Opuntia Ficus-indica Anthocyanins
Yun Zhang
1a
, Shuai Shao
2b
, Xuehui Ji
2c
, Lin Zhao
1d
, Ruiying Zhang
1,* e
and Souwen Zhang
2,* f
1
Heilongjiang Academy of Agricultural Sciences Postdoctoral Programme, China
2
College of food engineering, Heilongjiang East University, Harbin, China
*
zhangruiying@163.com,
*
zhangshouwen1956@163.com
*
Corresponding author
Keywords: Opuntia Ficus-indica, Anthocyanin, Antioxidant, Microencapsulation.
Abstract: Opuntia ficus-indica contains anthocyanins, flavonoids and other substances rich in biological activity.
Among them, anthocyanins have antioxidant, antitumor, anticancer, blood sugar, and blood lipid-lowering
effects. Through a DPPH free radical scavenging test, a hydroxyl free radical scavenging test, a superoxide
anion scavenging test and a test of the total reducing power to determine the in vitro antioxidant capacity of
opuntia ficus-indica anthocyanins, along with the use of complex agglomeration embedding technology, the
Opuntia ficus-indica anthocyanins were microencapsulated to achieve protection and sustained release. The
results showed that the DPPH scavenging ability, hydroxyl radical scavenging ability, and superoxide anion
scavenging ability of Opuntia ficus-indica anthocyanins were significantly higher than those of ascorbic
acid, with IC50 values of 0.59 mg/mL, 0.72 mg/mL and 0.80 mg/mL, respectively. Through single-factor
combined with response surface test analysis, it was determined that the best conditions for embedding
anthocyanins were a core-to-wall ratio of 1.2:1, a wall material concentration of 1.02 g/mL, and a pH of
3.36. Under these conditions, the predicted value of the prickly pear anthocyanin embedding rate was
64.50%. Under the conditions of microencapsulation, the stability of anthocyanins is significantly increased.
1 INTRODUCTION
1
The color of Opuntia ficus-indica is green or purple.
There are thorns inside and outside the skin. The size
depends on the variety. The flesh is purple and
slightly sour. Opuntia ficus-indica is rich in essential
amino acids, a variety of minerals and trace elements,
vitamins, polysaccharides, flavonoids and other
nutrients (Yahia,2011). Vegetables and fruits are rich
in anthocyanins. Because of their special functions
and effects, anthocyanins are used in many fields and
are most widely used in medicine, food, cosmetics
and other industries. There are a large number of
studies and records of the functions of anthocyanins,
a
https://orcid.org/0000-0003-1175-5735
b
https://orcid.org/0000-0002-4267-3110
c
https://orcid.org/0000-0002-9599-1294
d
https://orcid.org/0000-0002-2929-1826
e
https://orcid.org/0000-0001-6678-087X
f
https://orcid.org/0000-0002-3836-9212
which include antioxidation (Su, 2016), anticancer
(Stoner, 2009), and antiaging (Leichtweis, 2019);
moreover, they are a rich source of natural
antioxidant sugar (Wang, 2019) and can lower blood
lipids (Li, 2019), among other effects. Because
anthocyanins contain different numbers and positions
of hydroxyl groups and different types of binding
sugar groups, they exhibit different antioxidant
capabilities. The phenolic hydroxyl structure of
anthocyanins is easier to oxidize into quinones,
which allows anthocyanins to capture free radicals
(Kim, 2016). Moreno et al. found that red wine
contains mallow pigment and cyanidin, which causes
red wine to have antioxidant capacity (Sanchez,
2003). Joseph et al. mainly studied the antioxidant
components in the extracts of four Opuntia ficus-
indica varieties. The combined flavonoids, ascorbic
acid and carotenoids were separated from the extract.
Opuntia ficus-indica with purple skin has stronger
antioxidant activity than other varieties of fruit
838
Zhang, Y., Shao, S., Ji, X., Zhao, L., Zhang, R. and Zhang, S.
Study on the Antioxidant Capability and Microencapsulation of Opuntia Ficus-indica Anthocyanins.
DOI: 10.5220/0011297700003443
In Proceedings of the 4th International Conference on Biomedical Engineering and Bioinformatics (ICBEB 2022), pages 838-848
ISBN: 978-989-758-595-1
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

extracts. The data also show that opuntia ficus-indica
is suitable as food (Kuti, 2004).
The principle of microencapsulation technology
is to use embedding technology to embed unstable
solid, liquid or gaseous substances in tiny closed
capsules to achieve protection and controlled release
effects. In this technology, the wall material is the
material performing the embedding, the core
material is the material to be embedded, and
microencapsulation is the process of embedding
(Zhang, 2015). Because anthocyanins are easily
affected by factors such as pH, temperature, and
light, their stability is reduced (Tarone, 2020);
therefore, embedding anthocyanins through
microcapsule technology will increase their stability.
There have been studies on the extraction and
purification of wild Opuntia ficus-indica
anthocyanins, but there have been few studies on
their antioxidation and microencapsulation
preservation technology. This article hopes to
investigate the antioxidant capacity and
microencapsulation of Opuntia ficus-indica
anthocyanins, to increase the development and
utilization of wild Opuntia ficus-indica and to
provide a theoretical basis for the follow-up in-depth
study of anthocyanins in Opuntia ficus-indica.
2 MATERIALSANDMETHODS
2.1 Chemicals and Solvents
Opuntia ficus-indica (purchased in Hainan),
anhydrous ethanol, sodium acetate, potassium
chloride, concentrated hydrochloric acid,
macroporous resin HPD-100, sodium acetate,
potassium chloride, trichloroacetic acid, glacial
acetic acid, ethyl acetate, 30% hydrogen peroxide,
ferrous sulfate, salicylic acid, potassium
ferricyanide, pyrogallol, hydroxymethyl
aminomethane, formic acid, ascorbic acid (Tianjin
Guangfu Technology Development Limited
company), DPPH (West Asia Chemical Limited
company), gelatin, an gum arabic (Shanghai
Xiangrui Biological Technology Limited company)
2.2 Study on the Antioxidant Ability of
Cactus Fruit Anthocyanins in Vitro
2.2.1 Extraction of Opuntia Ficus-indica
Anthocyanin
The frozen Opuntia ficus-indica was thawed in a
water bath, homogenized with a juicer, and frozen in
an ultralow-temperature freezer at -80℃ until the
sample became solid, after which it was placed in a
vacuum freeze dryer. Two grams of powder was
added to a 100-mL Erlenmeyer flask, and 50%
ethanol at pH=2 (concentrated hydrochloric acid for
pH adjustment) at a material-to-liquid ratio of 1:25
g/mL was added. A stir bar was added to the flask,
after which the flask was sealed with a sealing film
and placed in a magnetic stirrer at 60 ℃for 70
minutes of extraction. After that, it quickly entered
the cooling state and was centrifuged for 15 min (the
centrifuge speed was 4500 r/min). The supernatant
was concentrated under reduced pressure at 50 ℃
and freeze-dried for 48 h to obtain the Opuntia ficus-
indica anthocyanin extract.
To determine the Opuntia ficus-indica
anthocyanin content, 1 ml of the extracted
supernatant was placed in a 25-mL volumetric flask,
10 mL each of pH=1 and pH=4.5 buffer solutions
were added to constant volume, and the solution was
allowed to stand for 60 minutes. The absorbance was
measured at 530 nm with an ultraviolet
spectrophotometer. The content of Opuntia ficus-
indica anthocyanin was calculated by the pH
difference method using the following calculation
formula (Ryu, 2018):
=
100×
××
×××
mL
VDFMWA
β
(mg/100g) (1)
here: A=(A530-A700) pH1.0MW-The molecular
weight of Bluebonnet-3-glucoside is 449.2 g/mol; -
(A530-A700)pH4.5;
β-The molar extinction coefficient is 26900 L•mol-
1•cm-1;
DF-The dilution factor; L-The optical path (cm);
V-Extract volume (mL); m-Raw material mass (g)
2.2.2 Determination of the Scavenging
Capacity of DPPH Free Radicals
This article uses the Abdel (Abdel, 2018) method
with slight modification. Two milliliters of different
mass concentrations of Opuntia ficus-indica
anthocyanin solution was added to a test tube with a
stopper, and 0.2 mmol/L DPPH solution was added.
The solution was shaken well and put in a dark place
for 60 minutes. The blank group was treated without
anthocyanin solution to remove the influence of
sample color. The experimental blank control is the
sample without DPPH. The scavenging rate of
DPPH free radicals in the sample was calculated
using ascorbic acid. After a controlled experiment
was performed, the absorbance at a wavelength of
517 nm was measured:
Study on the Antioxidant Capability and Microencapsulation of Opuntia Ficus-indica Anthocyanins
839

Clearance rate/%=
%1001
0
21
×
−
−
A
AA
(2)
where A
0
(517 nm)-is the absorbance of the blank
tube solution without sample solution;
A
1
(517 nm)-is the absorbance of the reaction
solution;
A
2
(517 nm)-is the absorbance of the control tube
without DPPH solution
2.2.3 Determination of Hydroxyl Radical
Scavenging Ability
This study was performed using a slightly modified
protocol based on Cásedas(Sang, 2015, Cásedas,
2017): 1.0 mL of H
2
O
2
(8.8 mmol/L), 1.0 mL of
FeSO4 (10 mmol/L), and 1.0 mL of 10 mmol/L
salicylic acid-ethanol solution were added to test
tube, followed by 1.0 mL of sample solution and 1.0
mL of H2O2 to react in a 37℃ water bath for 0.5 h.
Distilled water was used as a blank test and ascorbic
acid was used as a control test. The absorbance was
measured at 510 nm, and the clearance rate was
calculated:
Clearance rate/%=
%1001
0
21
×
−
−
A
AA
(3)
where A
0
(510 nm)-is the absorbance of the blank
tube solution without sample solution;
A
1
(510 nm)-is the absorbance of the reaction
solution;
A
2
(510 nm)-is the absorbance of the control
group (containing 1.0 mL of H2O2 and 1.0 mL of
sample solution)
2.2.4 Superoxide Anion Free Radical
Scavenging Ability
In this paper, according to the method of
Chen(Damar, 2012, Chen, 2020), 50 mmol/L of
Tris-HCL buffer (4.5 mL, pH 8.2) and 4.5 mL of
distilled water were added to a dry test tube, mixed
well, and kept warm for 20 minutes (25 ℃), after
which 1.0 mL of sample solution, 3.5 mL of distilled
water, and 0.3 mL of the pyrogallol solution
(concentration 3 mmol/L) preheated at 25 ℃ were
added, and the solution was shaken quickly until it
was uniform. The solution was then transferred to a
cuvette, and the absorbance of the solution was
measured at 320 nm every 0.5 min, stopping after 5
min. The increase in absorbance A0 (320 nm) within
1 min was calculated in the linear range. Ascorbic
acid was used as a control experiment. The clearance
rate can be calculated as follows:
Clearance rate/%=
%100
0
0
×
−
A
AA
(4)
where A
0
(320 nm)-is the autooxidation rate of
pyrogallol.
A (320 nm)-represents the autooxidation rate of
pyrogallol after adding the sample solution.
2.2.5 Determination of Total Reducing
Power
Tsai (Tsai, 2002) and other methods were referenced
to determine the reducing ability. Using the Prussian
blue method, 1.0 mL aliquots of samples with
different concentrations were measured and placed
in 5 dry test tubes, and 3.0 mL of 0.2 mol/L
phosphate buffer (pH=6.6) and 2.5 mL of
hexacyanoferric acid were added in sequence.
Potassium solution (concentration of 1%) was kept
in a water bath (50 °C) for 20 minutes, removed and
quickly cooled. Then, 2.5 mL of 10% trichloroacetic
acid was added and centrifuged for 4500r,10
minutes, and the supernatant was extracted. Then,
3.0 mL of distilled water and 0.5 mL of 0.1% ferric
chloride solution were added in sequence. After
mixing them thoroughly, they were allowed to stand
at room temperature for 10 min. Ascorbic acid was
used as a control test, and then the absorbance was
measured at 700 nm. The reduction ability of
Opuntia ficus-indica anthocyanins was judged
according to the absorbance value. The stronger the
absorbance value, the greater the reduction
ability(Cerezo, 2010).
2.3 Study on Microcapsules of Prickly
Pear Anthocyanins
Weigh a certain amount of gum arabic and gelatin to
dissolve them, then weigh a certain amount of
anthocyanins and mix them with gelatin,
homogenize and emulsify at a high speed for 3 min,
while slowly adding the gum arabic solution, and stir
with a cantilever agitator for 40 min (300 r~450 r),
measure the absorbance value A, add 10% acetic
acid solution dropwise, adjust the pH value to 4.0,
stir at 50℃ for 1 h, cool to below 15℃, adjust pH 8
with 10% sodium hydroxide solution -9, add 4 mL
10% tannic acid, stir at low temperature for 3 h,
stand still for 10 h, pour it into a centrifuge tube and
centrifuge at 4500 r/min for 15 min, take the
supernatant and measure its absorbance B.
Preparation of anthocyanin microcapsules and
calculation of the embedding rate;
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
840
Certain amounts of gum arabic and gelatin were
weighed to dissolve them, and then certain amounts
of anthocyanins and gelatin were weighed and
mixed homogeneously. Then, gum arabic was
slowly added while stirring, the pH was adjusted
with acetic acid, and the mixture was stirred at high
speed at 50 ℃. After cooling, the pH value was
adjusted again with sodium hydroxide and solidified
with tannic acid. After standing for 10 hours, a solid
powder was obtained by freeze drying.
The embedding rate is an important indicator
used to evaluate the quality of microcapsules. The
higher the embedding rate, the better the embedding
effect, and the less exposed the core material, which
increases the stability of the product, which can then
be stored for a long time.
E=
%100
)(
×
−
A
BA
(5)
Where: A-The absorbance value of the solution
before embedding
B-The absorbance value of the solution after
embedding
E-The embedding rate (%)
The influence of the core-wall ratio on the
process of anthocyanin microcapsules
Accurately weigh 5 portions of gelatin and gum
arabic 0.5 g each, put them into a beaker containing
50 ml of distilled water and dissolve them in a water
bath at 50°C, accurately weigh out 0.3 g, 0.5 g, 1 g,
2 g, and 3 g of anthocyanins, and wait for the
gelatin. After dissolving the anthocyanin and gum
arabic, put the anthocyanins into the gelatin solution
for high-speed homogenization and emulsification,
prepare microcapsules according to the above
method, and study the core-to-wall ratio (3:1, 2:1,
1:1, 1:2, 1:3) Influence on the process of
anthocyanin microcapsules.
2.3.1 The Influence of Wall Material
Concentration on the Process of
Anthocyanin Microcapsules
Accurately weigh 0.25 g, 0.475 g, 0.5 g, 0.75 g, and
1 g of gelatin and gum arabic, respectively, and
dissolve them in a beaker containing 50 ml of
distilled water in a water bath at 50°C. Weigh
accurately 5 parts of anthocyanins, 1 g each,
According to the above method to microcapsule,
study the influence of different wall material
concentration (0.5%, 0.75%, 1.0%, 1.5%, 2.0%) on
the process of anthocyanin microcapsule.
2.3.2 The Influence of pH Value on the
Process of Anthocyanin
Microencapsulation
Accurately weigh 5 parts of gelatin and gum arabic
0.5 g each, put them into a beaker containing 50 ml
of distilled water and dissolve them in a water bath
at 50°C, accurately weigh 5 parts of anthocyanins 1
g each, prepare microcapsules according to the
above method, and add them dropwise The pH
value of 10% acetic acid solution was adjusted to
3.0, 3.5, 4.0, 4.5, 5.0, respectively, and the influence
of pH value on the process of anthocyanin
microcapsules was studied.
2.3.3 Box-Behnken Experimental Design
According to the principle of the Box-Behnken
experimental design and the single-factor results,
three factors that had a significant embedding rate of
anthocyanin microencapsulation were selected: core-
wall ratio X1, wall material concentration X2, pH
X3 is the influencing factor, and the response
surface test design with 3 factors and 3 levels is
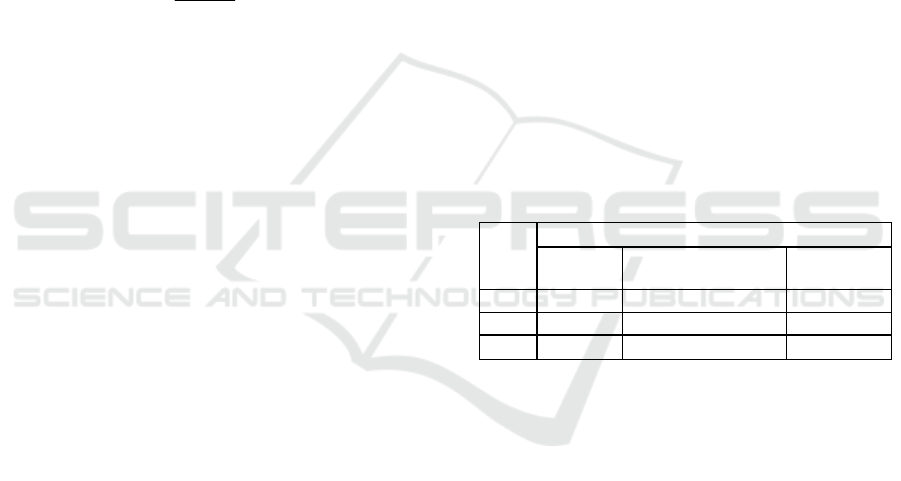
carried out. Table1shows the test factors and levels.
Table 1: Independent variables and levels for optimization
Level
Factor
X
1:
Core
wall ratio
X
2:
Wall material
concentration
X
3:
pH
-1 2:1 0.75 3.5
0 1:1 1 4.0
1 1:2 1.5 4.5
2.3.4 Stability of Microcapsules
The effect of light on the stability of microcapsules
Two grams of Opuntia ficus-indica anthocyanin
and Opuntia ficus-indica anthocyanin microcapsules
were accurately weighed, dissolved in 50 mL of pH
3.0 citric acid-sodium citrate buffer solution, and
placed under natural light at 0, 2, 4, and 6. Samples
were taken at 8 and 10 days, and the absorbance was
measured at 530 nm.
2.3.5 The Effect of Light on the Stability of
Microcapsules
Fifty-two grams of Opuntia ficus-indica anthocyanin
and Opuntia ficus-indica anthocyanin microcapsules
were accurately weighed, dissolved in 50 mL of pH
3.0 citric acid-sodium citrate buffer solution, and
placed in 20, 40, 60, 80, and 100 °C water baths.
The absorbance values were measured after 3 h.
Study on the Antioxidant Capability and Microencapsulation of Opuntia Ficus-indica Anthocyanins
841
2.3.6 Stability of Microcapsules
The single-factor application SPSS 19.0 software
was used to analyze the variance of the data,
OriginPro8.5 was used for graphing, and the
response surface was analyzed and graphed using
Design-Expert 8.0.6 software.
3 RESULTS AND DISCUSSION
3.1 Antioxidant Ability Measurement
Results
3.1.1 DPPH Free Radical Scavenging Ability
Measurement Results
The lone pair electrons of DPPH free radicals have
strong absorption at 517 nm. When free radical
scavengers are present in the reaction system, the
absorption will gradually disappear. This is because
the free radical scavengers pair with DPPH single
electrons. The relationship is proportional, so the
ability of free radical scavengers can be measured by
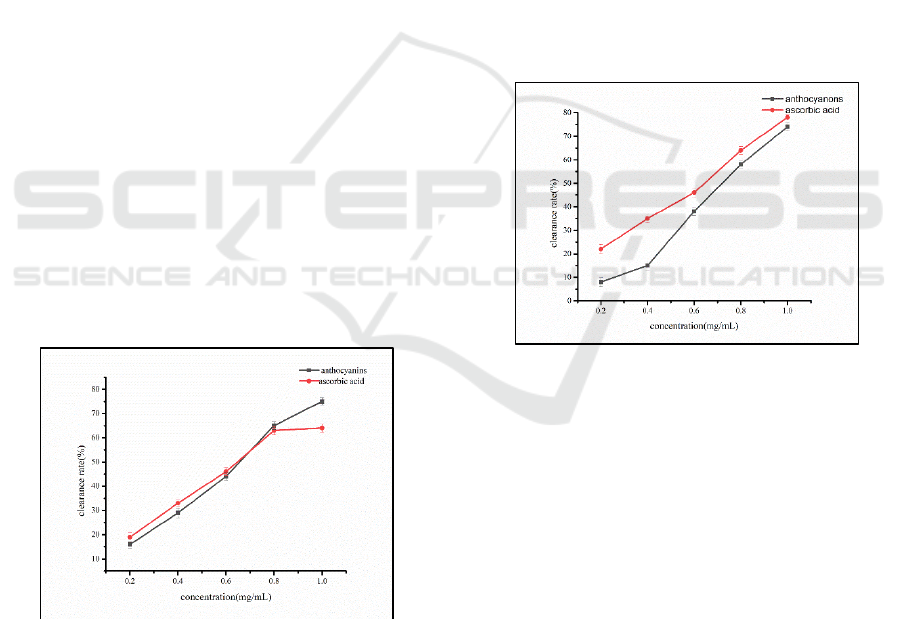
the reduced absorbance value(Corrales, 2019). Fig. 1
shows that the DPPH free radical scavenging ability
of Opuntia ficus-indica anthocyanins is significantly
higher than that of ascorbic acid at 0.2-0.6 mg/mL.
The clearance rate reaches its maximum at 0.8
mg/mL, and the scavenging ability of ascorbic acid
DPPH free radicals is higher than that of
anthocyanins at 1.0 mg/mL.
Figure 1: Determination of DPPH free radical scavenging
rates.
3.1.2 Measurement Results of the Hydroxyl
Radical Scavenging Ability
Fig. 2 shows that the scavenging ability of Opuntia
ficus-indica anthocyanins was positively correlated
with the scavenging ability at 0.2-0.4 mg/mL. The
scavenging ability was significantly higher than that
of ascorbic acid at the same concentration.
Thereafter, the scavenging ability of anthocyanins
was also stronger than that of ascorbic acid. This is
because anthocyanins have an aromatic ring
structure, so the provided hydrogen can react with
hydroxyl radicals to generate inert substances. The
hydrogen peroxide produced by ascorbic acid in its
self-oxidation process can promote the generation of
hydroxyl groups in the reaction, and the ability to
scavenge hydroxyl radicals is low(Szymanowska,
2018). According to statistical analysis, the
scavenging ability of anthocyanins on hydroxyl free
radicals was significantly higher than that of
ascorbic acid (P<0.05).
Figure 2: Determination of hydroxyl radical scavenging
rates.
3.1.3 Superoxide Anion Free Radical
Scavenging Capacity Results
Fig. 3 shows that Opuntia ficus-indica anthocyanins
scavenge superoxide anions. Within a certain
concentration range, the scavenging ability of
Opuntia ficus-indica anthocyanins on superoxide
anions increases with increasing concentration. The
same concentration of anthocyanins has the effect of
scavenging superoxide anions. The clearance of
anthocyanins is significantly higher, and the
clearance rate of anthocyanins is 1.23 times that of
ascorbic acid at 0.8 mg/mL. The clearance rate of
Opuntia ficus-indica anthocyanins reached 1.0
mg/mL.
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
842
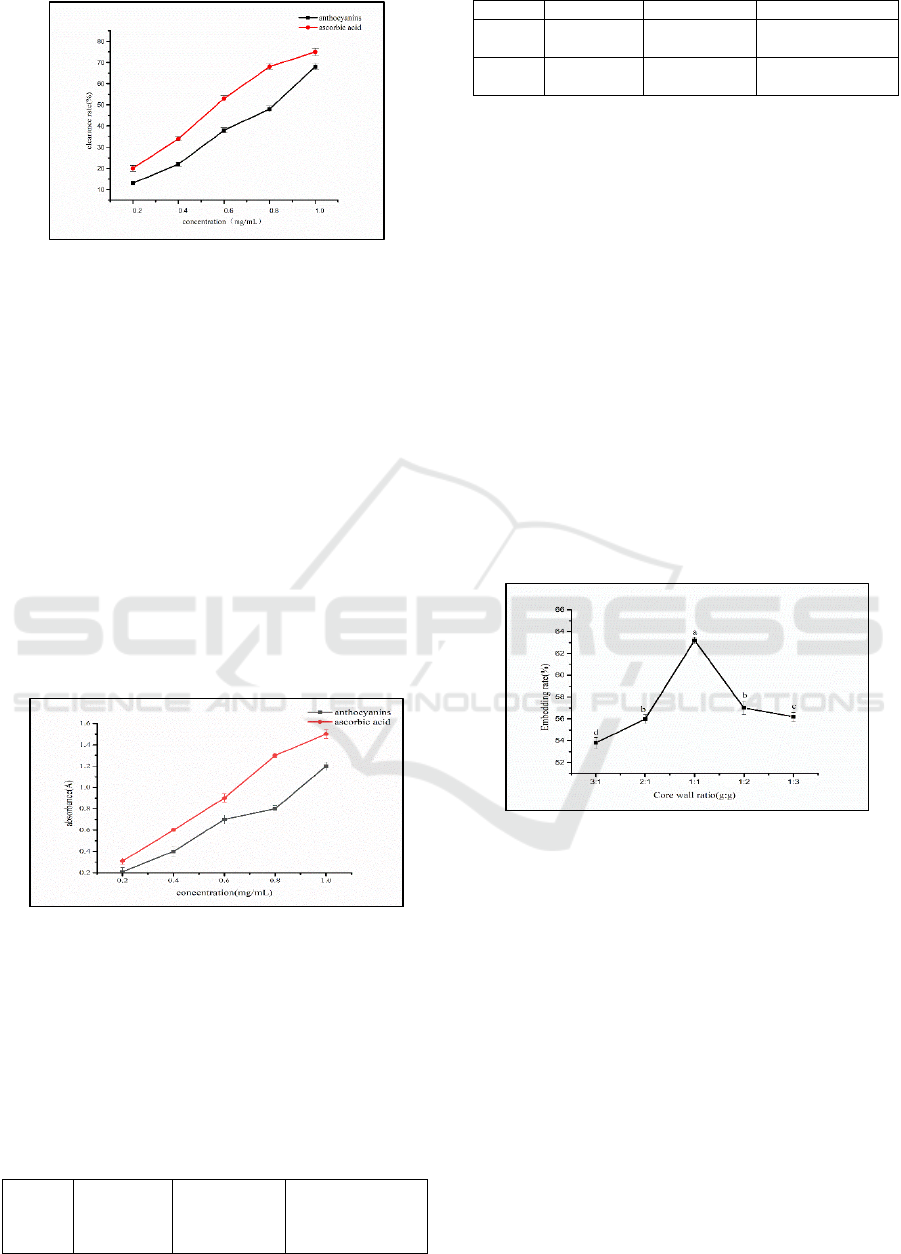
Figure 3: Determination of superoxide anion free radical
scavenging rates.
3.1.4 Superoxide Anion Free Radical
Scavenging Capacity Results
Fig. 4 shows that the total reducing power of
Opuntia ficus-indica anthocyanins and ascorbic acid
both show increasing trends, but the total reducing
power of the same concentration of anthocyanins is
lower than that of the same concentration of ascorbic
acid, and the total reducing power of anthocyanins is
lower than that of ascorbic acid. This may be due to
the reaction of Opuntia ficus-indica anthocyanins
with oxidants and their reducing power, but because
the number of hydroxyl groups of anthocyanins was
greater than ascorbic acid, the total reducing power
is still slightly lower than that of ascorbic
acid(Grobelna, 2019).
Figure 4: Determination of total reduction ability.
3.1.5 Comparison of the IC50 Values of
Opuntia Ficus-indica Anthocyanin and
Ascorbic
Table 2: The IC
50
value of anthocyanin and ascorbic
acid ascorbic acid antioxidant capacity
Table 2: The IC
50
value of anthocyanin and ascorbic acid
ascorbic acid antioxidant capacity.
name
DPPH
clearance
(mg/mL)
Hydroxyl
radical
scavenging
ability
Superoxide anion
scavenging
capacity
(mg/mL)
(mg/mL)
Anthoc
yanin
0.55 0.55 0.53
Ascorb
ic acid
0.59 0.72 0.80
3.2 Single-factor Test Results
3.2.1 Determination of the Optimal
Core-wall Ratio for Anthocyanin
Microencapsulation
While keeping other conditions unchanged, the
different core-wall ratios that affect the embedding
rate of the microcapsules were studied, and the
results are shown in Fig. 5. The embedding rate first
increased and then decreased with the wall-core
ratio. When the wall-core ratio was 1:1, The
embedding rate of microcapsules reached a
maximum of 63.7%, and there was a significant
difference between the sexes of each group
(P<0.05). The results are the same as Gao Yan's
optimal core-to-wall ratio in the preparation of
capsaicin microcapsules by the complex
coacervation method (Chen, 2018).
Figure 5: The relationship between the wall core ratio and
the embedding rate.
3.2.2 Determination of the Optimal Wall
Material Concentration for
Anthocyanin Microencapsulation
Under other conditions unchanged, by changing the
concentrations of the gelatin and gum arabic
solutions, the influence of different wall material
concentrations on the embedding rate of
microcapsules was investigated. When the wall
material concentration is large, a cohesion reaction
will occur between the wall materials. As a result,
empty sacs are generated, and the embedding rate is
reduced; when the wall material concentration is
small, the core material cannot be completely
embedded (Meng, 2019), as shown in Fig. 6. As
shown in the figure, the wall material concentration
Study on the Antioxidant Capability and Microencapsulation of Opuntia Ficus-indica Anthocyanins
843
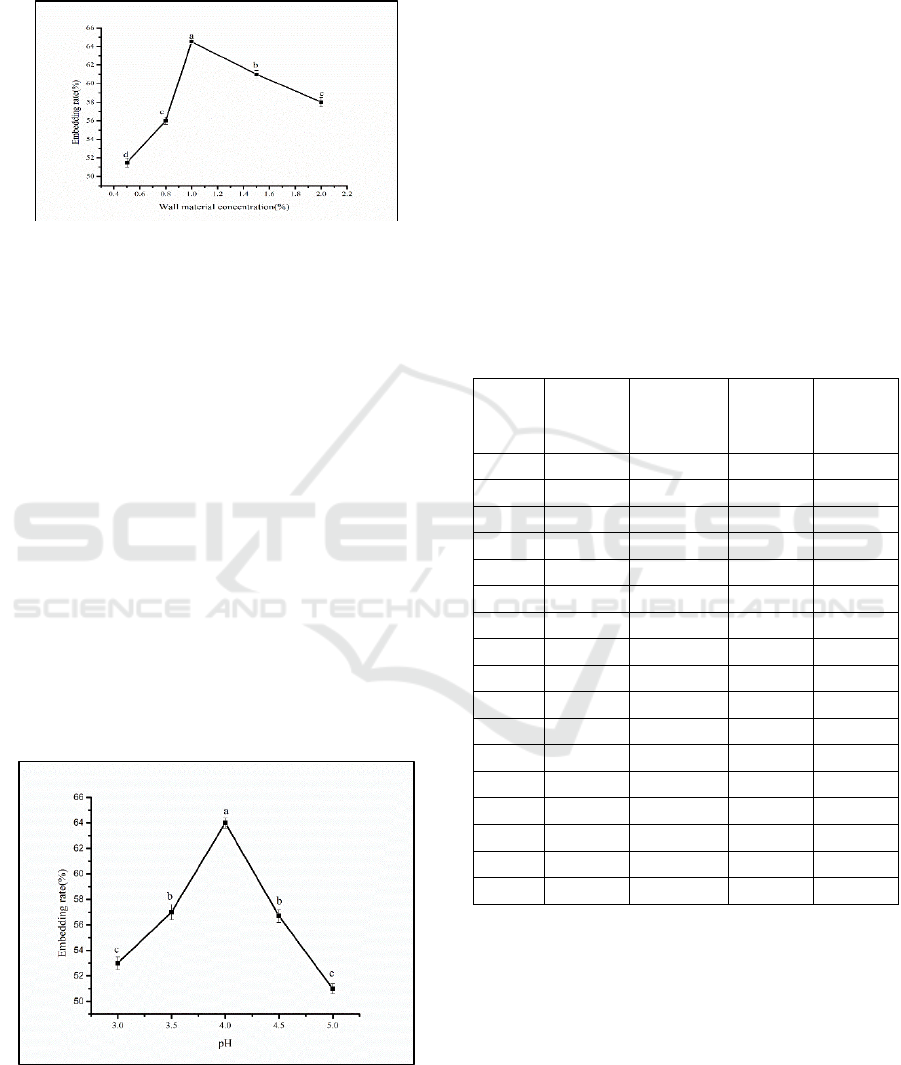
was 1.0%, the embedding rate reached a maximum
of 64.5%, and the difference between each
concentration was significant (P<0.05).
Figure 6: The relationship between wall material
concentration and embedding rate
3.2.3 Determination of the Optimal pH
Value for Anthocyanin
Microencapsulation
Keeping other conditions unchanged, by changing
the pH value, the effect of different pH values on the
embedding rate of microcapsules was investigated,
and the results are shown in Fig. 7. There were
significant differences between the groups (P<0.05).
When the pH was 4, the embedding rate reached its
maximum of 63.2%. This may be because at pH 4,
gelatin and gum arabic have a better charge–
chemical balance. At other pH values, the polymer
formed between the wall materials will change,
which will reduce the affinity with anthocyanins,
which will then reduce the embedding rate(Wang,
2016). This was the same as the optimal pH value in
the preparation process of VE microcapsules
described by Feng Yan et al.
Figure 7: The relationship between pH and embedding
rate.
3.3 Response Surface Test Design and
Results
3.3.1 Response Surface Test Model
Establishment and Results
According to the design principle of the Box-
Behnken test, based on the above single-factor test,
the wall-to-core ratio, the wall material
concentration and the pH value were used as the
three main influencing factors, and the anthocyanin
microcapsules were optimized through response
surface experiments (three factors and three levels)
to determine the embedding rate (Y). According to
statistical regulations, the regression fit various
regression coefficients. Table 3 shows the specific
experimental design and results.
Table 3: Box-Behnken design with the observed
responses.
Numb
ering
X
1
Core
wall
ratio
/(g:g)
X
2
Wall
material
concentrati
on/(%)
X
3
pH
Embeddi
ng
rate/(%)
1 1:2 1 4.5 55.03
2 2:1 0.75 4.0 61.9
3 1:1 0.75 4.5 51.86
4 2:1 1.5 4.0 53.57
5 1:2 1.5 4.0 60.21
6 1:1 1.5 4.5 51.01
7 2:1 1 3.5 57.02
8 1:1 1 4.0 64.11
9 1:1 1 4.0 64.34
10 1:1 1 4.0 64.33
11 1:1 1.5 3.5 56.00
12 1:2 1 3.5 60.55
13 1:1 1 4.0 64.01
14 1:1 0.75 3.5 54.81
15 1:1 1 4.0 64.45
16 1:2 0.75 4.0 55.87
17 2:1 1 4.5 54.86
Design Expert 8.0.6 statistical software was used
to perform regression fitting on the test data in Table
3 through stepwise regression and to obtain a
quadratic polynomial regression model of 3 factors
for the anthocyanin microcapsule embedding rate:
Y=64.25+0.53X
1
-0.47X
2
-1.95X
3
+3.19X
1
X
2
-
0.84X
1
X
3
-0.51X
2
X
3
-1.45X
12
-4.89X
22
+5.94X
32
Analysis of variance was performed on the
model, and the results are shown in Table 4.
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
844
Table 4: ANOVA for the regression model
Source of variance
Sum of
squares
Degree of
freedom
Mean square F value p value Significance
model 359.48 9 39.94 73.05 <0.0001 **
X
1
2.24 1 2.24 4.09 0.0828
X
2
1.74 1 1.74 3.18
<0.1177
X
3
30.50 1 30.50 55.78 0.0001 **
X
1
X
2
40.64 1 40.64 74.33
<0.0001
**
X
1
X
3
2.82 1 2.82 5.16 0.0573
X
2
X
3
1.04 1 1.04 1.90 0.2102
X
1
2
8.83 1 8.83 16.14
<0.0051
**
X
2
2
100.80 1 100.80 184.35
<0.0001
**
X
3
2
148.33 1 148.33 271.28
<0.0001
**
Residual 3.83 7 0.55
Lack of fit 3.70 3 1.23 37.42 0.0622
Errors 0.13 4 0.033
Total deviation 363.31 16
R
2
=0.9895
By comparing the absolute value of the primary
coefficient (from the multiple regression equation),
the order of factors affecting the embedding of
Opuntia ficus-indica anthocyanin microcapsules is
pH value > core wall ratio > wall material
concentration. From the results of the analysis of
variance shown in Table 3, we can conclude that the
significance level of the model was p=0.0022<0.05,
meaning that the regression variance was
significantly different; the coefficient of
determination was R2=0.9895, meaning that the
model was highly reliable; the proposed item was
0.0622, and the lack of fit item was not significant.
This shows that the fitting model composed of the
pH value, the wall-to-core ratio and the wall material
concentration can be used as a prediction and
analysis model for prickly pear anthocyanin
embedding.
3.3.2 Response Surface Experiment Results
Fig. 8 shows the interaction of the core-wall ratio
and pH on the embedding rate of Opuntia ficus-
indica anthocyanin microcapsules. As shown in the
contour map, the shape of the contour was close to
an ellipse, indicating that the core-wall ratio and the
pH value interacted strongly. Moreover, based on
the slope of the response surface, the interaction
between the core-wall ratio and the pH value was
obvious. Because the ellipse or circle in the figure
was in a closed state, it was the largest in this range.
Figure 8: Response of contour plots and surface plots of
the extraction yield under the interaction of the wall core
ratio and the pH.
Fig. 9 shows the interaction between the core-
wall ratio and the wall material concentration on the
embedding rate of Opuntia ficus-indica anthocyanin
microcapsules. As shown in the contour map, the
Study on the Antioxidant Capability and Microencapsulation of Opuntia Ficus-indica Anthocyanins
845
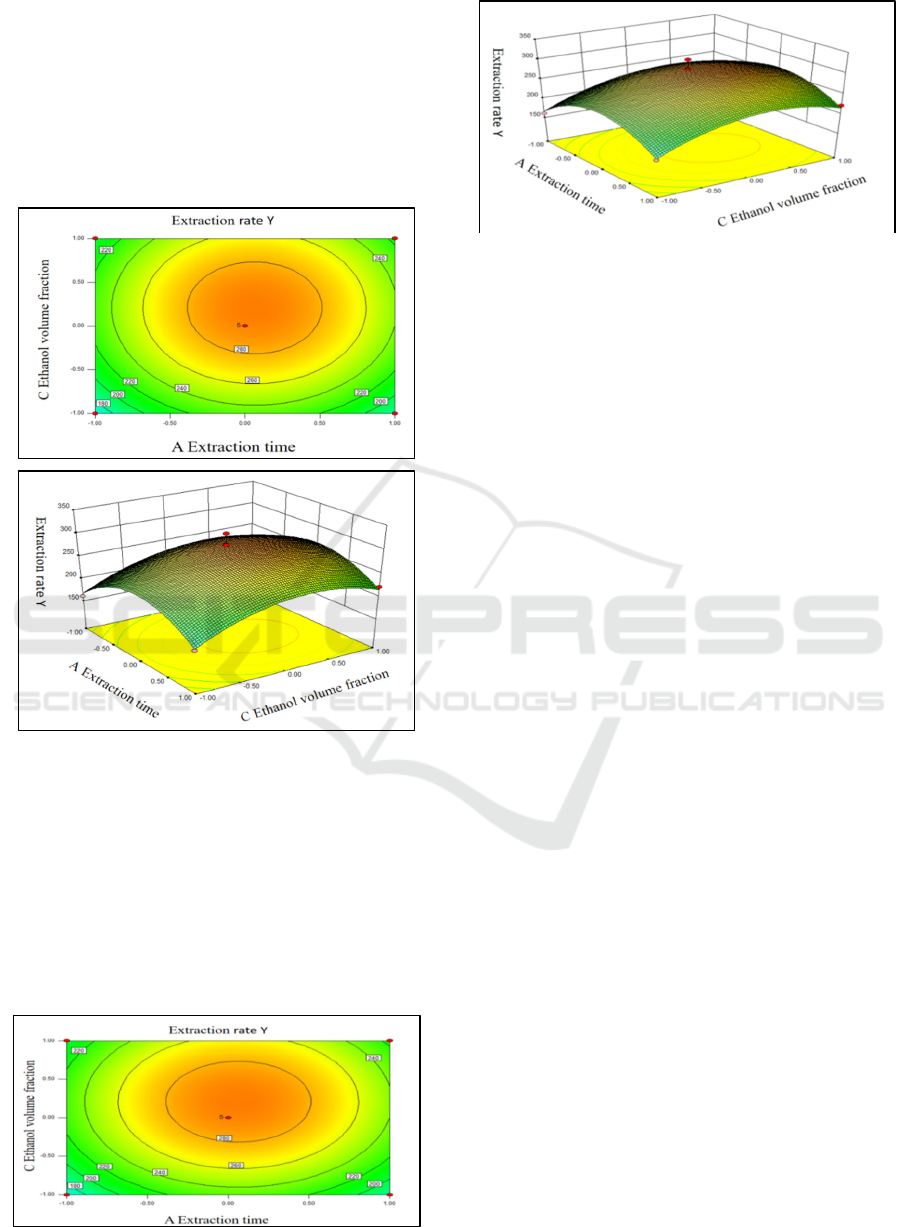
shape of the contour was elliptical, indicating that
the core-wall ratio and the wall material
concentration have a strong interaction. From the
steep slope of the response surface, the interaction
between the core wall ratio and the wall material
concentration was obvious. Because the ellipse or
circle in the figure was in a closed state, it was the
largest in this range.
Figure 9: Response of contour plots and surface plots of
the extraction yield under the interaction of the wall core
ratio and the wall material concentration amount.
Fig. 10 shows the interaction of the wall material
concentration and the pH on the embedding rate of
Opuntia ficus-indica anthocyanin microcapsules. As
shown in the contour map, the shape of the contour
was circular, indicating that the two factors of wall
material concentration and pH value did not have a
strong interaction.
Figure 10: Responses of contour plots and surface plots of
the extraction yield under the interaction of wall material
concentration and pH.
From the analysis results of the Box-Behnken
design model, the optimized process parameters of
Opuntia ficus-indica anthocyanin microcapsules are a
core-to-wall ratio of 1.2:1, a wall material
concentration of 1.02%, and a pH of 3.36. Under
these conditions, the predicted value of the prickly
pear anthocyanin embedding rate was 64.50%. To
verify the reliability of the Box-Behnken model,
three experiments performed according to the
optimal process of the model optimization revealed
that the embedding rate of the Opuntia ficus-indica
anthocyanin microencapsulation was 65.12%,
demonstrating that the model optimized the Opuntia
ficus-indica anthocyanin microcapsules. The process
has certain application value.
3.4 Stability Analysis of Microcapsules
3.4.1 Analysis of the Stability of
Microcapsules under Light
As shown in Fig. 11, as time increases, the
absorbance values of the anthocyanins before and
after the microcapsules show a downward trend, but
the anthocyanins after microencapsulation decreased
more slowly than the anthocyanins before
microencapsulation. This finding indicates that after
the microcapsule wall material embedded the
Opuntia ficus-indica anthocyanin, the influence of
light on the anthocyanin was reduced, thereby
improving the stability of the anthocyanin.
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
846
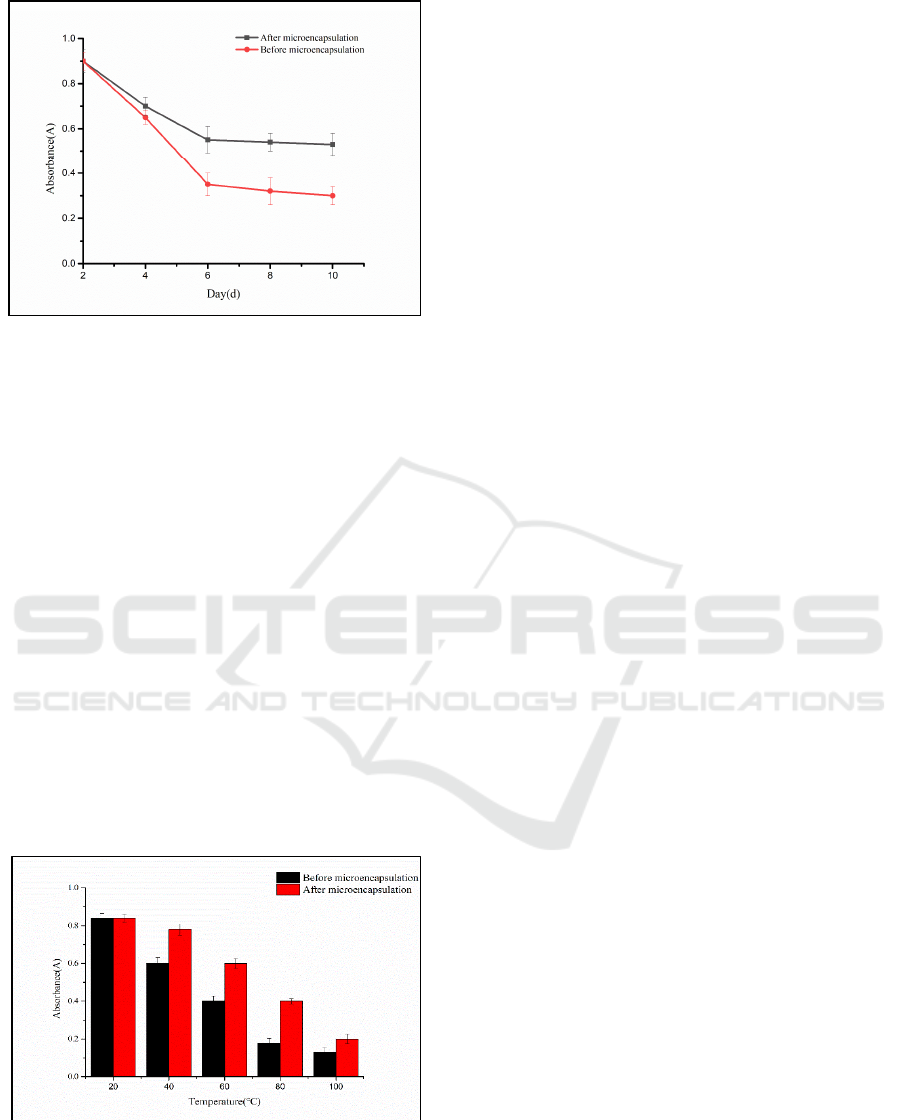
Figure 11: Relationship between light and microcapsule
stability.
3.4.2 Analysis of the Stability of
Microcapsules under Light
As shown in Fig. 12, within 3 h, as the temperature
increased, the absorbance of anthocyanins gradually
decreased, but it was clear that the decrease in
absorbance of anthocyanins after microcapsules were
formed was lower than that before microcapsules
were formed. At 20 ºC, the absorbance values of the
anthocyanins before and after microencapsulation
were the same, indicating that the anthocyanins were
stable at 20 °C, and then as the temperature
increased, the absorbance values of the anthocyanins
before and after microencapsulation had significantly
different trends. These results show that after the
Opuntia ficus-indica anthocyanins were embedded in
the microcapsule wall material, the influence of
temperature on the anthocyanins was reduced,
thereby improving the stability of the anthocyanins.
Figure 12: Relationship between temperature and
microcapsule stability.
4 CONCLUSIONS
The antioxidant capacity in vitro of Opuntia ficus-
indica anthocyanins was determined, and the results
showed that the IC50 values of Opuntia ficus-indica
anthocyanin and ascorbic acid were 0.55 mg/mL and
0.59 mg/mL for DPPH scavenging capacity, the
IC50 values of the hydroxyl radical scavenging
capacity were 0.55 mg/mL and 0.72 mg/mL,
respectively, and the IC50 values of the superoxide
anion scavenging capacity were 0.53 mg/mL and
0.80 mg/mL, respectively. Taken together, Opuntia
ficus-indica anthocyanins were determined to have
strong antioxidant capacity in vitro.
The process of optimizing the
microencapsulation of Opuntia ficus-indica
anthocyanins was determined by the compound
coacervation method. The best process was a core-
to-wall ratio of 1.2:1, a wall material concentration
of 1.02%, and a pH of 3.36. Under these conditions,
the predicted value of the prickly pear anthocyanin
embedding rate is 64.50%. By comparing the effects
of light and temperature on the stability of the
anthocyanins before and after the microcapsules, the
results show that the absorbance value of the
anthocyanins after 6 days of light is 1.6 times that
before and after embedding at 60℃. The
anthocyanin stability is 1.4 times that before
embedding. Taken together, the stability of
anthocyanins is significantly increased after
microencapsulation.
ACKNOWLEDGEMENTS
This project was strongly supported by Quality &
Safety institute of Agricultural Products,
Heilongjiang Academy of Agricuitural Sciences
Heilongjiang Academy of Agricuitural. The author
thanks Heilongjiang East University for provided
raw materials. This research was funded by Key
Projects of Heilongjiang East University, grant
number HDFKY200105.
REFERENCES
Abdel-Aal E, Hucl P, Rabalski I(2018). Compositional
and antioxidant properties of anthocyanin-rich
products prepared from purple wheat. Food
Chemistry, 254(15):13-19.
Cásedas G, Les F, Gómez-Serranillos MP, et al(2017).
Anthocyanin profile, antioxidant activity and enzyme
inhibiting properties of blueberry and cranberry juices:
Study on the Antioxidant Capability and Microencapsulation of Opuntia Ficus-indica Anthocyanins
847

a comparative study. Food & Function, 8(11): 4187-
4214.
Cerezo A B, Cuevas R, Winterhalter R, et al(2010).
Isolation, identification, and antioxidant activity of
anthocyanin compounds in Camarosa strawberry.
Food Chemistry, 123(3): 574-582.
Chen H, Xiao-Mei W U, Yue LI , et al(2018). Stability
and targeting release characteristics of anthocyanin
microcapsules of Lycium ruthenicum Murr. Science
and Technology of Food Industry, 39(01): 22-28.
Chen L, Xin X, Feng H, et al(2020). Isolation and
Identification of Anthocyanin Component in the Fruits
of Acanthopanax Sessiliflorus (Rupr. & Maxim.)
Seem. by Means of High Speed Counter Current
Chromatography and Evaluation of Its Antioxidant
Activity. Molecules, 25(8): 1781-1795.
Corrales-García JE, García-Mateos MDR, Martínez-López
E, et al(2019). Anthocyanin and Oil Contents, Fatty
Acids Profiles and Antioxidant Activity of Mexican
Landrace Avocado Fruits. Plant Foods for Human
Nutrition, 74(6): 389-397.
Damar R, Eki A(2012). Antioxidant capacity and
anthocyanin profile of sour cherry (Prunus cerasus L.)
juice. Food Chemistry, 135(4): 2910-2914.
Grobelna A, Kalisz S, Kieliszek M(2019). The Effect of
the Addition of Blue Honeysuckle Berry Juice to
Apple Juice on the Selected Quality Characteristics,
Anthocyanin Stability, and Antioxidant Properties.
Biomolecules, 9(11): 744-750.
Kim J, Keum Y S(2016). NRF2, a Key Regulator of
Antioxidants with Two Faces towards
CancerOxidative Medicine and Cellular Longevity,
22(2): 1-7.
Kuti J O(2004), Antioxidant compounds from four
Opuntia cactus pear fruit varieties. Food Chemistry,
Food Chemistry, 2004, 85(4): 527-533.
Leichtweis M G, Pereira C, PrIeto M A, et al(2019).
Ultrasound as a Rapid and Low-Cost Extraction
Procedure to Obtain Anthocyanin-Based Colorants
from Prunus spinosa L. Fruit Epicarp: Comparative
Study with Conventional Heat-Based Extraction.
Molecules, 24(3): 573-580.
Li A, Xiao R, He S, et al(2019). Research Advances of
Purple Sweet Potato Anthocyanins: Extraction,
Identification, Stability, Bioactivity, Application, and
Biotransformation. Molecules, 24(21): 3816-3832.
Meng X Y, Zhao Y Q, Yu-Kun L I, et al(2019).
Preparation of Anthocyanin from Hibiscus sabdariffa
Microcapsules and Evaluation of Stability and
Release. Science and Technology of Food Industry,
40(14): 174-181.
Ryu D, Koh E(2018). Application of Response Surface
Methodology to Acidified Water Extraction of Black
Soybeans for Improving Anthocyanin Content, Total
Phenols Content and Antioxidant Activity. Food
Chemistry, 261(30): 260-266.
Sanchez-Moreno C, Cao G, Ou B, et al(2003).
Anthocyanin and proanthocyanidin content in selected
white and red wines. Oxygen radical absorbance
capacity comparison with nontraditional wines
obtained from highbush blueberry. Journal of
Agricultural and Food Chemistry, 51(17): 4889-4896.
Sang G L, Vance T M, Nam T G, et al(2015).
Contribution of Anthocyanin Composition to Total
Antioxidant Capacity of Berries. Plant Foods for
Human Nutrition, 70(4): 427-432.
Stoner G D, Wang L S, et al(2007). Cancer prevention
with freeze-dried berries and berry components.
Seminars in Cancer Biology, 17(5): 403-410.
Su X, Xu J, Rhodes D, et al(2016). Identification and
quantification of anthocyanins in transgenic purple
tomato. Food Chemistry, 202(29): 184-188.
Szymanowska U, Baraniak B, Bogucka-Kocka A(2018).
Antioxidant, Anti-Inflammatory, and Postulated
Cytotoxic Activity of Phenolic and Anthocyanin-Rich
Fractions from Polana Raspberry (Rubus idaeus L.)
Fruit and Juice-In Vitro Study. Molecules, 23(7):1812.
Tarone A G, Cazarin C, Junior M(2020). Anthocyanins:
New techniques and challenges in microencapsulation.
Food Research International, 133: 109092.
Tsai P J, Mcintosh J, Pearce P, et al(2002). Anthocyanin
and antioxidant capacity in Roselle (Hibiscus
Sabdariffa L.) extract. Food Research International,
35(4): 351-356.
Wang J, Mazza G(2002). Inhibitory effects of
anthocyanins and other phenolic compounds on nitric
oxide production in LPS/IFN-gamma-activated RAW
264.7 macrophages. Journal of Agricultural and Food
Chemistry, 50(4): 850-857.
Wang W(2016). Investigation of Optimal Extraction
Conditions and Microencapsulation Technique for
Stabilizing Anthocyanins and Polyphenols from Fruit
Materials. America: Oregon State University.
Yahia E M, Saenz C, et al(2011). Cactus pear (Opuntia
species). Postharvest Biology and Technology of
Tropical and Subtropical Fruits, 19(1): 290-329.
Zhang C, Li X, Liu Y N, et al(2015). Utilization of
Microcapsule Technology in Foods. Journal of
Nanoscience and Nanotechnology, 15(12): 9330-9340.
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
848
