
Identification of Bakuchiol Targeting Proteins in Human Skin Cells
Yucheng Li
Department of Biochemistry & Molecular Biology, University of Miami, Coral Gables, FL 33146, U.S.A.
Keywords: Bakuchiol, Retinol, Antiaging, Natural Product, Target Identification.
Abstract: Purpose: Bakuchiol is a natural product that is widely used for skin antiaging. Previous studies show that
bakuchiol and retinol have similar biological activities despite having no structural resemblance, but the
mechanism of action of bakuchiol remains largely unknown. We proposed a regulatory pathway of bakuchiol
that is based on the downstream activities of retinol. In this work, we will test a small portion of the proposed
pathway—that is, bakuchiol can bind to target proteins different than that of retinol (RARs). Methods: We
will design experiments to identify the molecular targets of bakuchiol. We will perform affinity
chromatography to isolate bakuchiol targeting proteins, followed by mass spectroscopy to sequence the target
proteins. The sequences will be identified using a database. Possible Results: At the end, there are three
possible results: (1) Bakuchiol does not bind the target proteins of retinol (RARs) and instead binds other
proteins; (2) Bakuchiol only binds RARs. (3) Bakuchiol binds RARs and other proteins. Conclusion: The
results of our study will pave the way for understanding the mechanism of action of bakuchiol.
1 INTRODUCTION
Bakuchiol is a natural product mainly obtained from
the seeds of the plant Psoralea corylifolia. Previous
studies have shown that bakuchiol can serve as a
functional analog to retinol even though bakuchiol
does not look like a retinoid (Figure 1). For example,
retinol has long been used as a therapeutic agent to
treat photo-aged human skin. Similarly, Chaudhuri
and Bojanowski showed through a pilot clinical study
that the clinical appearance of photo-aged human skin
is improved by bakuchiol, similar to retinol
(Chaudhuri, Bojanowski 2014). The functional
similarity of bakuchiol and retinol is also reflected in,
for example, their antioxidant and antiinflammation
effects (Chaudhuri, Sivamani, Jagdeo, Elsner,
Maibach 2015). To investigate the similarity of
bakuchiol and retinol on a molecular biology level,
the authors applied a comparative gene expression
profiling with both bakuchiol and retinol by using the
technique of DNA microarray (Chaudhuri,
Bojanowski 2014). They revealed that the volcano
plots of the two substances are similar in shape
(Figure 2), indicating their similar regulation on gene
expression. This leaves a question on why bakuchiol
and retinol, having no structural resemblance with
each other, regulates gene expression in a similar
pattern. One possibility is that bakuchiol binds to the
same targets as that of retinol (i.e., retinoic acid
receptors, or RARs). Another possibility is that
bakuchiol binds to different targets than retinol,
which somehow improves the availability of
endogenous retinol. The latter possibility is supported
by Chaudhuri and Bojanowski’s observation that
bakuchiol upregulates proteins (CRBP II, CRBP IV,
CRABP I, LRAT) that can help increase the cellular
storage of retinol to an extent greatly higher than that
of retinol (Chaudhuri, Bojanowski 2014). This
indicates a possible regulation pathway that
bakuchiol achieves its functional similarity to retinol
by binding to protein targets different than that of
retinol; the targets directly or indirectly activate
transcription factors that mediate the expression of
the retinol-storage-related proteins, which increases
the availability of endogenous retinol. In other words,
bakuchiol increases available retinol in cells, and it is
the increased retinol that ultimately acts to exhibit the
anti-aging activity of bakuchiol in human skin cells.
Figure 1: Structure of bakuchiol (left) and retinol (right).
Li, Y.
Identification of Bakuchiol Targeting Proteins in Human Skin Cells.
DOI: 10.5220/0011296600003443
In Proceedings of the 4th International Conference on Biomedical Engineering and Bioinformatics (ICBEB 2022), pages 803-809
ISBN: 978-989-758-595-1
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
803
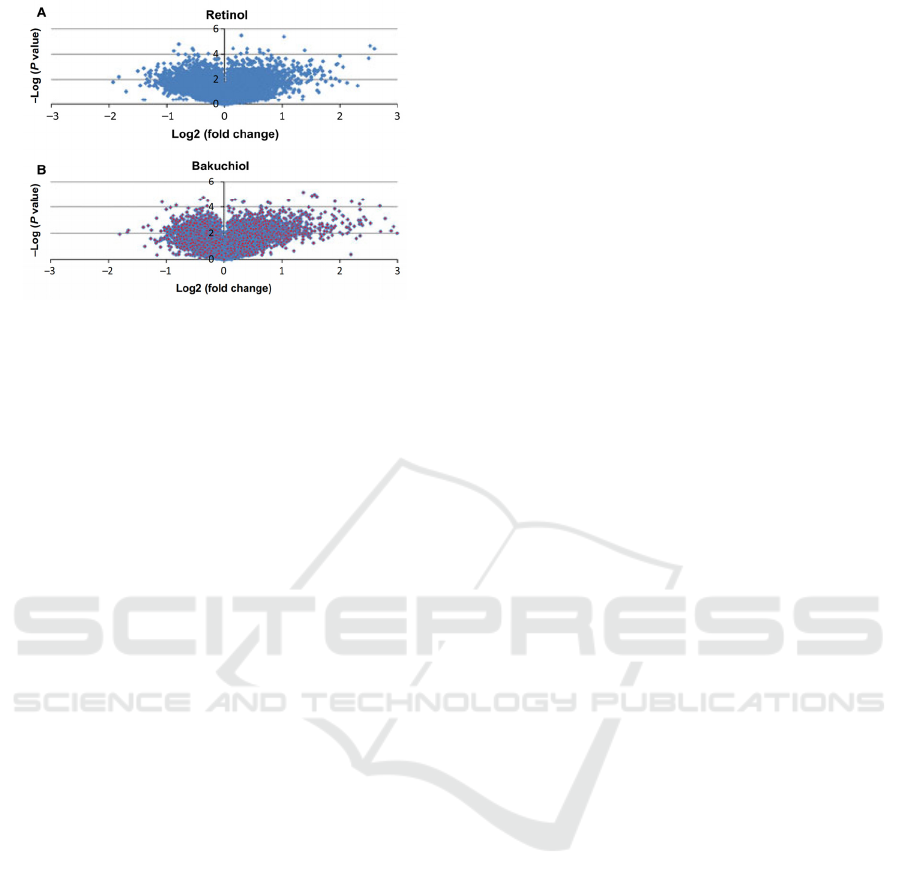
Figure 2. Volcano plots of DNA microarray data obtained
from retinol- (A) and bakuchiol- (B) treated skin
substitutes. The overall shapes of the two volcano plots are
similar, indicating that retinol and bakuchiol have similar
regulation on gene expression.
However, more evidence is still needed to support
this hypothesized pathway. It is also not clear what
are the targets of bakuchiol and how exactly they
regulate the expression of the retinol-storage-related
proteins. In this study, I will test a small portion of
the hypothesized pathway—bakuchiol can bind to
different targets than retinol. To test this hypothesis,
I will design an experiment to identify the bakuchiol
targeting proteins (BTPs) in cells.
I will perform bakuchiol affinity chromatography
on human dermal fibroblast lysates, followed by mass
spectrometry to sequence the BTPs. The use of
affinity chromatography assumes that modifications
at the 4-hydroxyl group of bakuchiol do not
significantly alter its anti-aging biological activity in
human skin cells. To test the assumption, I will
perform a structure-function study by substituting the
4-hydroxyl group of bakuchiol with methoxy group
and then comparing the biological activity of
bakuchiol and 4-methoxy-bakuchiol in stimulating
the expression of collagens by ELISA.
The result will provide hints for future researchers
to investigate the mechanism of action of bakuchiol.
2 MATERIALS AND METHODS
2.1 Structure-Function Study
2.1.1 Materials
Bakuchiol (INCI name), also known as Sytenol A
(trade name), will be purchased from Sytheon.
Sytheon derives bakuchiol from the plant Psoralea
corylifolia, which contains edible seeds that serve as
the source of bakuchiol. The plant itself is psoralene-
depleted bakuchiol with a purity of about 95%. The
Williamson-Ether method will be used to generate
methoxy-bakuchiol.
2.1.2 Synthesis of Methoxy-Bakuchiol
The Williamson-Ether method used to generate
methoxy-bakuchiol is as follows: 0.9 mmol/L
bakuchiol in acetone, treated with 4.8 mmol/L methyl
iodide, and 3.6 mmol/L potassium carbonate. The
solution will be stirred and refluxed for 24 hr;
afterward, there will be further room temperature
stirring for an additional 72 hr. The reaction will be
followed with thin-layer chromatography (25 ml of
methyl chloride and 3 ml of methanol). The acetone
and excess methyl iodide will be removed with a
stream of N2 gas. The remaining solid will be
resuspended in approximately 50 ml of ether and
vacuum filtered to remove the potassium carbonate.
The ether will be removed with N2 gas, leaving an
oily, yellow residue behind. This residue will be
resuspended in 50 ml of methanol and triturated with
water, then refrigerated (48C) overnight to produce
pale beige crystals of methoxy-bakuchiol. The
structural confirmation of methoxy-bakuchiol will be
determined through infrared spectrum (Nicolet IR
spectrometer, in KBr pellet) and a proton nuclear
magnetic resonance spectrum (Varian UnitccccPlus,
400 MHz, in DMSO). A sample of methoxy-
bakuchiol will be submitted for elemental analysis
(Desert Analytical, Tucson, AZ). The purity of the
sample will be assessed in two ways: 1) by measuring
its melting point, Rf on thin layer chromatography; 2)
by using high performance liquid chromatography
(Beckman Gold) that incorporates an isocratic 0.1%
trifluoro acetic acid:acetonitrile (50/50) solvent
system and a C18 reverse phase column at 0.8
ml/min.
2.1.3 Collagen ELISA
Bakuchiol and methoxy-bakuchiol will be assayed at
10 ug/mL on normal human fibroblasts grown in
DMEM with 5% calf serum (Hyclone, Salt Lake City,
UT, U.S.A.). Neonatal human dermal fibroblasts will
be used in analysis of Type I and IV collagen
quantification (low passage; American Type Culture
Collection, Manassas, VA, U.S.A. cat. no. PCS-201-
010, lot no. 58243223). Human epidermal fibroblasts
from a 68-year-old female donor will be used in
analysis of Type III collagen quantification (p. 5,
Zen-bio, cat. no. KR-F). Type I collagen
quantification requires cells to be subjected to test
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
804
materials for 3 days, whereas Type III and IV
collagen quantification requires cells to be subjected
to test materials for 7 days. Afterward, per standard
ELISA protocol, the sandwich ELISA, which uses
affinity-purified antibodies, will be used to assay the
collected cell culture conditioned media for type I,
type III or type IV collagen, which is then followed
by streptavidin-avidin-HRP conjugate and ABTS
(Dobak, Grzybowski, Liu et al 1994, Zhao, Alexeev,
Chang, et al. 2005). The collagen content is
proportional to a colorimetric signal. A BioRad
microplate spectrophotometer 3550-UV at 405 nm
with background subtraction at 660 nm will be used
to measure this colorimetric signal. Further analysis
of this colorimetric signal will be performed with
Microplate Manager v.2 software for Macintosh
(BioRad, Hercules, CA, U.S.A.).
2.2 Affinity Chromatography and
Mass Spectroscopy
2.2.1 Materials
Epoxy-activated agarose resin (12 atom linker, 33
µmol of epoxy group/ml of packed gel) will be
purchased from Sigma Chemical (St. Louis, MO).
Bakuchiol stock at a concentration of 12.5 mM will
be made in DMSO and stored at −20°C. Other
biochemical reagents will be procured from a variety
of chemical suppliers. The National Cell Culture
Center in Minneapolis, MN will source the large
amounts of cultured fibroblasts.
2.2.2 Preparation of Immobilized Bakuchiol
Affinity Column (BAC)
1 g of epoxy-activated agarose will be held in ice-cold
water for 5 min and thoroughly washed to get rid of
any additives or impurities. 23 mg of Bakuchiol
dissolved in 2.5 ml of 0.1 M NaOH will be added and
incubated with 1 ml of resuspended epoxy-activated
agarose for a night at room temperature to ensure that
the resin binds to the bakuchiol. 6 ml of 1 M sodium
acetate buffer (pH 5.0) containing 1 mM
dithiothreitol (DTT) will be added to the mixture to
neutralize unreacted epoxy groups and eliminate
further bakuchiol oxidation, thus halting the reaction.
Immobilized bakuchiol resin will be washed
successively with 0.1 M sodium acetate, pH 5.0,
containing 1 mM DTT and 70%, 30%, 10%, and 0%
ethanol, respectively. Mock-treated beads (which
utilize identical procedure except that there is no
added bakuchiol) or beads immobilized with a
tyrosine ligand will make up the controls.
2.2.3 Fractionation of Cytoplasmic Extracts
on BAC
Cultured mammalian cells will be lysed by 3 freeze–
thaw cycles with buffer containing 10 mM Hepes, pH
7.5, 90 mM KCl, 1.5 mM Mg (OAc)2, 1 mM DTT,
0.5% NP-40, 5% glycerol, 0.5 mM
phenylmethylsulfonyl fluoride (PMSF), and 10 µl/ml
of the protease inhibitor cocktail sourced from Sigma
Chemical. 10-min centrifugation using a refrigerated
microcentrifuge will yield the cell-free extracts.
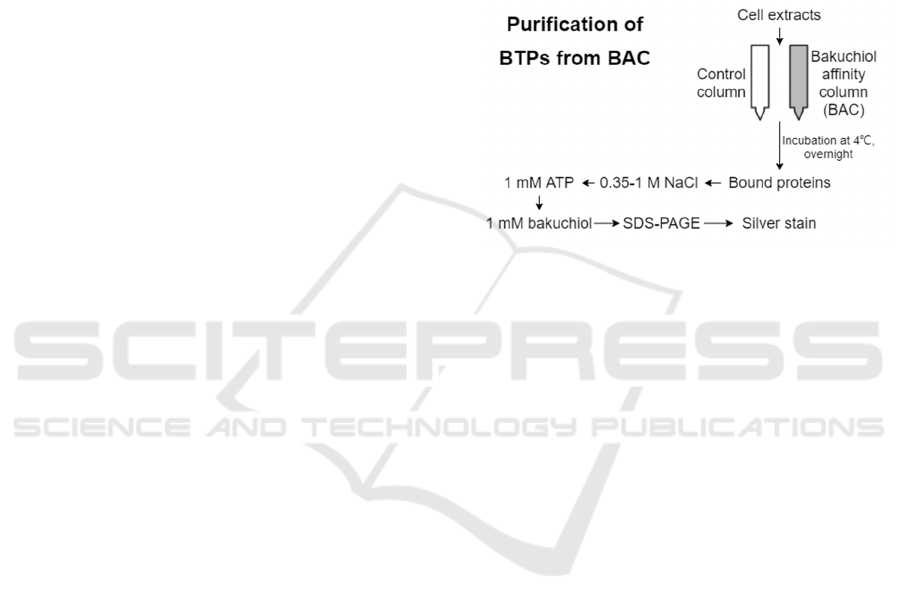
Figure 3. Purification strategy for the isolation of BTPs
from BAC. In step 1, a cell lysate is passed over BAC or a
mock-coupled or tyrosine-linked control column in
parallel, and washed exhaustively with lysis buffer to
remove nonspecific proteins. In step 2, the column is eluted
stepwise eluted with 0.35 and 1 M NaCl. In step 3, column-
bound proteins are eluted using 1 mM ATP. The last step
involves elution with 1–2 mM bakuchiol dissolved in 2%
DMSO.
To characterize BAC (Figure 3), 200 µl fibroblast
extract containing 0.6–1.0 mg protein will be
combined with 50 µl control (mock-treated or
tyrosine-linked) or bakuchiol-immobilized agarose
beads in a 1.5-ml Eppendorf tube. The tube will be
stored overnight at 4 °C using a modest tumbling
process. The protein extract, which resembles a gel
slurry, will be loaded onto a minicolumn (from Pierce
Chemical) and rinsed with 10–20 ml lysis buffer to
eliminate any proteins that did not successfully bind.
Elution of the column will be next occur 5–7 times,
each time with 0.5 ml lysis buffer containing 0.35 M
NaCl, and will be followed by the same number of
rinsing using 1 M NaCl supplemented buffer. Next,
the column will be equilibrated with the lysis buffer
and eluted with 1 mM ATP. For the final step, the
column will once more be re-equilibrated with the
lysis buffer and eluted with 1–2 mM bakuchiol
dissolved in 2% DMSO.
Identification of Bakuchiol Targeting Proteins in Human Skin Cells
805

2.2.4 Using MALDI-TOF MS to Identify
BTPs from Fibroblasts
We will use fibroblasts to prepare cytoplasmic
extracts which will be fractionated as described
(Figure 3). The middle silver-stained protein bands
will be excised, reduced, carbamidomethylated, and
cleaved with trypsin. The subsequent peptide mixture
will be desalted and concentrated using Zip-Tip C18
micro-columns (Millipore), and applied to the
MALDI target using solution phase nitrocellulose
method, as defined by Landry et at. (Landry,
Lombardo, Smith 2000). We will use a Voyager-DE
PRO MALDI-TOF mass spectrometer (Applied
Biosystems, Foster City, CA) set in reflector mode
with standard settings to calculate peptide masses.
Trypsin autodigestion products will be used for
internal mass calibration. MALDI-TOF MS
generates peptide mass fingerprinting which will be
compared to the theoretical tryptic digests of proteins
stored in NCBI nonredundant protein database using
the ProFound software
(http://prowl.rockefeller.edu/cgi-bin/ProFound).
This technique is used to identify proteins. The
identity of the BTPs will be obtained from the
database search result.
2.2.5 Statistical Analysis
The statistical significance of all numerical data will
be analyzed using the student’s T-Test on GraphPad
Prism® at (p <0.05).
3 POSSIBLE RESULTS
3.1 Confirmation of the Applicability
of Affinity Chromatography
Table 1 lists all the possible stimulatory effects of
bakuchiol and 4-methoxy bakuchiol on collagen I, II,
and IV.
Table 1. Possible stimulatory effects of bakuchiol and 4-
methoxy bakuchiol on collagen I, II, and IV. Bakuchiol and
4-methoy bakuchiol may have the same or different
stimulatory effects on each of collagen I, II, and IV. The
same stimulatory effect is defined as the two substances
decrease or increase the concentration of a collagen by the
same amount (p<0.05). In this table, the same stimulatory
effect is represented by “+”, and different stimulatory effect
is represented by “-“.
Collagen I Collagen III Collagen IV
Possible result 1 + + +
Possible result 2 + + -
Possible result 3 + - +
Possible result 4 + - -
Possible result 5 - + +
Possible result 6 - + -
Possible result 7 - - +
Possible result 8 - - -
3.2 Identification of Molecular Target
by Affinity Chromatography
Possible result 1: There is no silver-stained protein
shown in the gel.
Possible result 2: There is at least one silver-
stained proteins shown in the gel.
3.3 Identification of Molecular Target
by Mass Spectroscopy
Possible result 1: Bakuchiol does not bind RARs.
Possible result 2: Bakuchiol only binds RARs.
Possible result 3: Bakuchiol binds RARs and
other proteins.
4 DISCUSSION
4.1 Confirmation of the Applicability
of Affinity Chromatography
To secure and detect BTPs, we will immobilize
bakuchiol onto epoxy-activated agarose beads.
Residual bakuchiol found on column will be
eliminated with thorough rinsing. Chemical coupling
of bakuchiol occurs at the 4-hydroxyl group, which
yields a bakuchiol-immobilized affinity column
(BAC). However, it is not guaranteed that the
coupling between bakuchiol and epoxy-activated
agarose beads does not significantly affect the
antiaging biological activity of bakuchiol. We
therefore will perform a structure-function study to
examine if modifications at the 4-hydroxyl group of
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
806

bakuchiol alters the antiaging biological activity of
bakuchiol.
We synthesized 4-methoyl bakuchiol and will
compare its stimulatory effect with bakuchiol on the
expression of collagens in skin model.
The main components of the skin extracellular
matrix (type I and type III collagens) and basement
membrane (type IV collagen) include collagens
produced by dermal fibroblasts. Dermal fibroblasts
are reduced in amount and quality as found in aged
and photodamaged skin in addition to having less
new collagen pool. Consequently, we choose to
measure select collagens by ELISA method to
confirm the assumption that modifications at the 4-
hydroxyl group of bakuchiol does not alter the
antiaging biological activity of bakuchiol.
It is known from Chaudhuri and Bojanowski’s
work that bakuchiol can stimulate the amount of
collagen I, III, and IV in human dermal fibroblasts in
vitro (Chaudhuri, Bojanowski 2014). We therefore
choose collagen I, III, and IV to test our assumption.
Possible results are summarized in Table 1.
Possible result 1 the most strictly indicates that
modifications on the 4-hydroxyl group of bakuchiol
does not significantly alter its biological effect. As a
result, bakuchiol and 4-methoxyl bakuchiol has the
same stimulatory effect.
Possible results 2, 3, and 5 indicate that
modifications on the 4-hydroxly group of bakuchiol
can significantly alter its biological effect. As a result,
in the case of a substitution of 4-hydroxyl group with
4-methoxy group, the stimulation of one of the three
collagens has changed compared to bakuchiol.
Possible results 4, 6, and 7 indicate that
modifications on the 4-hydroxly group of bakuchiol
can alter its biological effect to a greater extent than
that of possible results 2, 3, and 5. As a result, in the
case of a substitution of 4-hydroxyl group with 4-
methoxy group, the stimulation of two of the three
collagens has changed compared to bakuchiol.
Possible result 8 indicates that the 4-hydroxyl
group of bakuchiol is necessary for it to exhibit its
biological effect, and that modifications on the 4-
hydroxyl group of bakuchiol can the most greatly
alter its biological effect. As a result, in the case of a
substitution of 4-hydroxyl group with 4-methoxy
group, the stimulation of all of the three collagens has
changed compared to bakuchiol.
The alteration of the biological effect of bakuchiol
as a result of modifications on the 4-hydryoxyl group
is most likely because the bakuchiol derivatives have
different binding affinities with biomolecules in the
cells. This can result in an alteration of the molecular
regulation, leading to an alteration of biological
effects.
If possible results 2-8 occur, the subsequent
affinity chromatography will not be applicable,
because the immobilization of bakuchiol at the 4-
hydroxyl group will affect the binding of bakuchiol
to putative BTPs. Other experimental method should
be designed to identify the molecular target of
bakuchiol.
If possible result 1 occurs, we are confident that
the subsequent affinity chromatography will be
applicable. The reason is that, as possible result 1
indicates, the immobilization of bakuchiol at the 4-
hydryoxyl group will not affect the binding of
bakuchiol to putative BTPs, and that the only position
of bakuchiol for epoxy-activated agarose beads to
bind is the 4-hydroxyl group. We can therefore claim
that column-bound bakuchiol should maintain similar
binding characteristics as bakuchiol in solution, and
thus affinity chromatography should be an applicable
method for the identification of the molecular target
of bakuchiol in photo-aged human skin cells.
4.2 Identification of Molecular Target
by Affinity Chromatography
Fibroblast lysates will be incubated with BAC and
eluted, as indicated in Materials and methods. We
will use SDS–PAGE to analyze eluted samples.
Furthermore, we will use silver staining to visualize
eluted samples. There may or may not be proteins that
show specific retention on BAC.
Possible result 1: There is no silver-stained
proteins.
The absence of silver-stained proteins suggests
that bakuchiol does not bind to any molecular target
in Fibroblasts. This is a very unlikely result given that
it have been proved that bakuchiol does have
biological affinity in photoaged human skin cells. If
this result occurs, there might be human errors during
the experiment, and further action should be done to
examine, e.g., if the cell lysates have active proteins.
Possible result 2: There is at least one silver-
stained proteins.
The presence of at least one silver-stained protein
suggests that bakuchiol has molecular target(s) in
fibroblasts, which bind to BAC and retain in the gel.
It is likely that some of the targets are involved in the
mechanism of action of the anti-aging activity of
bakuchiol. Next, we will perform mass spectroscopy
to reveal the identity of them.
Identification of Bakuchiol Targeting Proteins in Human Skin Cells
807

4.3 Identification of Molecular Target
by Mass Spectroscopy
Silver-stained proteins will be excised from the gel
and subjected to in-gel trypsin digestion to generate
peptide fragments that will be further determined by
MALDITOF MS. This method will produce
recognizable patterns that will be used in database
searches to match predicted tryptic peptide masses of
proteins with known identity, thus leading to
determining what the silver-stained proteins are.
We hypothesized that bakuchiol binds to different
molecular targets than that of retinol (RARs), but
there are other possibilities.
Possible result 1: Bakuchiol does not bind RARs
and instead binds other proteins.
This result is consistent with our hypothesis that
bakuchiol can bind to different target proteins other
than that of retinol. By binding to different target
proteins, it is plausible that bakuchiol exhibits its
biological activity by facilitating the intracellular
storage of retinol, and it is retinol that ultimately
function to achieve the biological activity of
bakuchiol. This explains why bakuchiol has similar
but not exactly the same gene regulation pattern as
retinol, and why bakuchiol has antiaging activity as
retinol does.
The hypothesized pathway proposed in the
Introduction part of this paper suggests that there are
transcription factors that mediate the enhanced retinol
storage. Further studies should examine whether or
not the identified targets are the above-mentioned
transcription factors, or the identified targets can
activate those transcription factors. It also needs to be
clarified whether or not the above-mentioned
transcription factors mediate the expression of
retinol-storage-related proteins (e.g. CRBP II, CRBP
IV, CRABP I, LRAT).
In addition, the hypothesized pathway may not be
sufficient to describe the biological activity of
bakuchiol. Maybe some bakuchiol targeting proteins
facilitates cellular uptake and/or retinol activation
and/or intracellular transportation of retinol. These
possibilities also need to be tested in future studies.
Possible result 2: Bakuchiol only binds RARs.
This result is contradictive with our hypothesis. If
bakuchiol only binds RARs, theoretically bakuchiol
should have the same biological activity as retinol,
which contradicts with the different gene expression
pattern (Figure 2). Therefore, this is not a likely
result.
Possible result 3: Bakuchiol binds RARs and
other proteins.
This result is consistent with our hypothesis that
bakuchiol can bind to different target proteins other
than that of retinol. Therefore, everything discussed
in possible result 1 is also applicable in possible result
3.
Furthermore, since bakuchiol can directly bind to
RARs, it is likely that bakuchiol can directly activate
the retinol-mediated gene regulation pathway. We
recommend conducting further studies to evaluate
this possibility.
5 CONCLUSION
Generally, this study tries to identify the molecular
target of bakuchiol in human skin cells. However, this
study assumes that the chemical coupling between
bakuchiol and epoxy-activated agarose beads does
not significantly affect the antiaging activity of
bakuchiol. If this assumption turns out to be false, the
subsequent affinity-chromatography is not
applicable, and other experimental approaches should
be designed to identify the molecular targets of
bakuchiol. If the assumption is true, most likely some
protein targets can be isolated and then identified by
mass spectroscopy. After the identification of target
proteins, we will be able to test the hypothesis that
bakuchiol can bind to proteins different than that of
retinol (i.e., RARs). After all, this hypothesis is not
likely to be false because Chaudhuri and Bojanowski
have revealed different gene expression patterns
regulated by bakuchiol and retinol (Chaudhuri,
Bojanowski 2014).
Further studies should be done to test the
hypothesized regulatory pathway of bakuchiol
proposed in the Introduction part of this paper.
Molecular details involved in this pathway needs to
be clarified. Further studies should also examine
whether or not the proposed pathway is sufficient to
explain the biological activity of bakuchiol. Overall,
after a series of studies, the mechanism of action of
bakuchiol can be elucidated.
REFERENCES
Chaudhuri, R. K., & Bojanowski, K. (2014). Bakuchiol: a
retinol‐like functional compound revealed by gene
expression profiling and clinically proven to have anti‐
aging effects. International journal of cosmetic science,
36(3), 221-230.
Chaudhuri, R. K., Sivamani, R., Jagdeo, J. R., Elsner, P., &
Maibach, H. I. (2015). Bakuchiol: a retinol-like
functional compound, modulating multiple retinol and
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
808

non-retinol targets. Cosmeceuticals and active
cosmetics, 1-18.
Dobak, J., Grzybowski, J., Liu, F.T. et al. 1,25-
Dihydroxyvitamin D3 increases collagen production in
dermal fibroblasts. J. Dermatol. Sci. 8, 18–24 (1994).
Landry F, Lombardo CR, Smith JW. A method for
application of samples to matrix-assisted laser
desorption ionization time-of-flight targets that
enhances peptide detection. Anal. Biochem. 2000;
279:1–8.
Zhao, H., Alexeev, A., Chang, E. et al. Lycium barbarum
glycoconjugates: effect on human skin and cultured
dermal fibroblasts. Phytomedicine 12, 131–137 (2005).
Identification of Bakuchiol Targeting Proteins in Human Skin Cells
809
