
Enemy Tolerance of Eupatorium Plants
Yingzhi Chen
1,2 a
, Shunbo Yang
1,3 b
, Ruiqiang Guo
1c
, Xingcheng Zhang
1d
, Gengyun Pan
1,* e
and Ruifang Wang
1,* f
1
College of Agriculture and Forestry, Puer University, Puer, Yunnan, 665000, China
2
College of Agriculture, Shanxi Agricultural University, Jinzhong, Shanxi, 030000, China
3
College of Agriculture, Yunnan Agricultural University, Kunming, Yunnan, 650000, China
Keywords:
Natural Enemy, Invasive Plant, Tolerance.
Abstract: Alien plants can tolerance the feeding of natural enemy by compensatory growth. In this study, the growth
responses of invasive Eupatorium adenophorum and native E. fortune plants to simulated insect feeding
were analyzed. The results showed that the total biomass of E. adenophorum had no significant change after
simulated insect feeding. The results indicated that E. adenophorum rapidly increasanges, but the leaf
biomass ratio increased significantly; the total biomass of E. fortune decreased signifed the biomass of
photozygous organs to cope with the simulated leaf loss. As a result, the invasive plant E. adenophorum is
more tolerant than the native plant E. fortune.
1 INTRODUCTION
1
E. adenophorum is native to Mexico in North
America and Costa Rica in Latin America (Qiang
1998). Due to its strong reproductive capacity,
adaptability and fast spread rate, it has caused
serious harm to human beings, livestock and even
the ecological environment (Xiao 2009). At present,
the control methods of E. adenophorum mainly
include three types: artificial mechanical control,
chemical control and biological control (Wang
2004).Among biological control, Procecidochares
utilis Stone, Orthezia quadrua, Dorylus orientalis
were reported to damage E. adenophorum.
Procecidochares utilis StoneProcecidochares
utilis Stone is the specialist enemy of E.
adenophorum , belonging to Diptera (Diptera:
Muscidae), and has certain inhibitory effect on the
growth and reproduction of E. adenophorum (Gao
2019, Lei 2014). In 1945, the United States
introduced Brasilia sinensis from Mexico to Maui
Island of Hawaii and established the population, and
a
https://orcid.org/0000-0001-8095-4848
b
https://orcid.org/0000-0002-8832-1604
c
https://orcid.org/0000-0002-8116-209X
d
https://orcid.org/0000-0003-2765-4090
e
https://orcid.org/0000-0002-9461-5158
f
https://orcid.org/0000-0003-4715-6240
succeeded in studying its biological characteristics
and the feasibility of using it to control the
population of E. adenophorum sinensis (Ming
2017). In July 1984, the Institute of Ecology of the
Chinese Academy of Sciences sent experts to
Yadong and Nyalam counties of Tibet, which are on
the border with Bhutan, Sikkim and Nepal, and
found the P. utili , which was introduced to some
areas of Yunnan, and then gradually spread to the
southwest of Sichuan, Guizhou and Guangxi (Wang
2013). It was found through experiments that the
oviposition only on the tender tips of E.
adenophorum plants, while the hatched larvae
crawled to the base of leaves, penetrated the
meristems, and entered the stems at the upper part of
the shoots, constantly feeding on the young parts at
the growth points of E. adenophorum, thus impeding
the circulation function of the three major vegetative
organs of E. adenophorum (Xin 1990). People make
full use of this habit to inhibit the normal growth and
development of E. adenophorum, and eventually
lead to the wilt of P. utili by E. adenophorum due to
malnutrition, so as to achieve the purpose of
biological control. In the course of its development
over the next 30 years, it was found everywhere, but
its control ability was very limited and it could not
achieve the desired effect.On the one hand, E.
adenophorum has the unique biological
characteristics of high seed yield, fast dispersal
Chen, Y., Yang, S., Guo, R., Zhang, X., Pan, G. and Wang, R.
Enemy Tolerance of Eupatorium Plants.
DOI: 10.5220/0011281200003443
In Proceedings of the 4th International Conference on Biomedical Engineering and Bioinformatics (ICBEB 2022), pages 739-743
ISBN: 978-989-758-595-1
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
739

speed, strong ability to regenerate tillers, and
vegetative reproduction of plant organs such as roots
and stems. On the other hand, it is only an insect, so
it takes some time to fully display its biological
characteristics. It is impossible to reach the degree
that E. adenophorum will wither and die
immediately once it is parasitic.For these two
reasons, the diffusion rate of E. adenophorum was
much faster than the parasitic rate of P. utili, so the
expected control effect could not be achieved .
In addition to specialist enemy, the growth of
some native generalist enemies is also a threat for
E. adenophorum. In April 2007, Chinese researchers
discovered another insect feeding on E.
adenophorum, O. quadrua, on the way from
Yingjiang to Tongbiguan Nature Reserve in Yunnan
Province. At the time, the researchers observed
damage in the field: the E. adenophorum had been
severely damaged, the whole plant turned brown and
withered. This insect mainly concentrates on the
stem of E. adenophorum, and mostly concentrates
on the node of the stem to suck the plant juice (Xu
2011).
It was found that the D. orientalis, which would
bite the stems and roots of E. adenophorum, form
holes or eat them completely, and damage the
epidermis, cortex, phloem, root cambium and xylem
tissues of E. adenophorum, thus resulting in the
death of E. adenophorum due to the nutrient
exchange between the broken roots and buds (Yao
2008). D. orientalis have a certain selectivity in
foraging activities. They usually prefer food with
foul or aromatic odor, and the strong and unique
odor of E. adenophorum is the chemical signal that
attracts D. orientalis to forage (Chen 2012).
However, plants tend to resist insect feeding
through their own defense mechanisms, such as
tolerance.Plant tolerance is the ability of a plant to
prevent, reduce, or repair damage by compensatory
growth. For example, Ellison (1960) believed that
the compensatory effect of plants was that the
feeding of herbivores was beneficial to the growth of
plants (Ellson 1960).Belsky believed that the
compensatory effect of plants was a positive
response to plant injury, and defined compensatory
growth as "the increase of plant biomass and seed
yield due to foraging" (Belsky 1986). Therefore,
after the research and induction of many scholars,
the increased biomass or seed yield of plants under
stress was defined as the compensatory effect of
plants.
Although several insect have been found and
damaged on E. adenophorum, we don't know much
about how E. adenophorum responds to native
generalists. In this study,we investigated the biomass
allocation of invasive E. adenophorum and native E.
fortune under natural enemies feeding. The findings
of this study are expected to improve our
understanding of the compensatory growth of E.
adenophorum and native plant, and to evaluate the
role of native generalist enemies in the process of
alienplant invasion.
2 MATERIALS AND METHODS
2.1 Materials
2.1.1 Seed Collection
Seed collection was carried out one year before the
trial. Since the seeds of E. adenophorum and E.
fortune belong to achenes with small and light size,
and difficult to collect manually, in order to prevent
the early shedding of seeds and the mixing of other
unknown seeds, so as to obtain a large number of
healthy, complete seeds with high germination rate,
the method of isolated seed collection was adopted
in this experiment. Isolation method of seed in
flowering plants (E. adenophorum flowering in
November - the following April, E. fortune
flowering for 7 - November), selected out from the
need for seed plants grow strong, no plant diseases
and insect pests, variety of pure plant, and then
according to the shape of the flowers of the plant to
choose appropriate to the size of the yarn pockets of
bagged processing.
After the seeds of bagged plants mature and fall
off, the collected seeds should be stored in a
ventilated and cool place to dry in the shade, and
then stored in the envelope bag prepared in advance
to prevent the seeds from being dampened and
mildewed.
2.1.2 Seedlings
The whole seedling breeding process of this
experiment was carried out under the condition of
50% light intensity in the greenhouse. To raise
seedlings, the prepared humus soil (0-10 mm Danish
Pinclop trophic soil) and sandy soil were evenly
mixed in a 3:2 ratio to form a seedling substrate.
Then put the mixed seedling substrate into the
seedling tray (size: 40 cm * 40 cm), until it is only
about 1 cm away from the hole plate, stop adding
soil. Then, continue to fill 19 seedling plates in the
same way.The 20 seedlings were divided into 2
groups (E. adenophorum group andE. fortune
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
740

group), with 10 seedlings in each group, and
corresponding labels were made. Next, lay a piece of
kraft paper on the ground, remove the previously
collected seeds from the envelope bag, and gently
rub them with your hands so that the seeds are
removed from the other non-seed structures and fall
onto the kraft paper. After the separation of all the
seeds is completed, the separated seeds are evenly
dispersed into the seedlings tray of the different
groups, and the number of seeds in each seedling
tray is controlled at about 100. Finally, the substrate
from the sieve is gently sifted evenly into the tray so
that the seeds in each tray are thinly coated with the
substrate and cannot be blown away by the wind or
washed away by water. When all this is done, water
the two groups of seeds with a water bottle. After
that, water it regularly every two days.
2.1.3 Thinning
When the palnt had three or four leaf, in order to
avoid crowded seedlings, mutual shading, save soil
moisture and nutrient, and cultivate strong seedling
and guarantee the seedling size is consistent.Thus
thinning for plant and dish were required, finally
every dish reserved 50-70 seedlings.
2.1.4 Transplant
When the height of seedlings were 5 cm,
transplanting was started. First, prepare a new
nutrient bowl (model: 30 cm * 35 cm) and an
adequate substrate (a mixture of river sand and soil
in a 1:2 ratio). Then, the transplanting substrate was
placed upto two-thirds of the bowl, and the
nutritional bowls were arranged in a pattern of 10
units per row and 4 units per column, with a
flowerpot tray under the arranged nutritional bowls.
After that, seedlings were raised.
The two plants that transplanted to nutritional
bowls should be watered thoroughly one day in
advance. When seedlings are raised, the root system
of the uprooted plants should be intact and not
damaged. One third of the soil at the base of the
roots should be removed, and the rotten roots and
rotten roots should be removed. In the process of
transplanting, the seedlings are held by one hand,
and the roots are placed in the dug soil nest. The
roots of the seedlings can not be twisted, but should
be smoothed, and the spaces around them should be
filled and compacted with soil. Transplanting should
not be too deep, the root of the plant can be planted.
After planting, seedlings should be watered again to
ensure the survival of seedlings after transplantation.
Since then, watering were taken once every two
days.If the growth of seedlings were slowed, and or
did not survive, these seedlings should be timely
supplemented. After two weeks of adaptation to 36
% light, the transplanted seedlings began to grow
under full light conditions.
2.1.5 Fertilization Management
The growth rate of plant seedlings is accelerated
after transplanting, then the vigorous growth period
begins. At this time, the seedling plant nutrients
gradually accumulated, rapid thickening of rhizome,
leaf number gradually increased, the demand for
fertilizer and water increased significantly.
Therefore, in order to promote the growth of the
stem and leaves, to meet the growth needs of the
plant seedlings, in this period, the corresponding
fertilization management should be carried out.
Firstly, according to the dosage of 0.5 g fertilizer
(N, P, K available nutrient content ≥ 35%) per kg of
soil, thus 2 g fertilizer per bowl was weighed. Then
dig a hole 5-10 cm deep in each of the four
directions about 5 cm from the seedlings. Finally,
0.5 g fertilizer was applied to each hole and then
covered with soil to prevent the volatile of fertilizer
and reduce waste.
2.2 Methods
Twenty sample plants of E. adenophorum and E.
fortune were divided into two groups. One group
was treated as simulated insect feeding (MN), in
which all leaves (except the top 2-4 young leaves) of
a single plant were removed about 50% of the area
of the single leaf. Another control treatment (CK) to
eliminate natural enemies was to apply a compound
insecticide to the leaves of the plants. After the
treatment was completed, the whole plant was
covered with a gauze net bag of suitable size.
At the end of the experiment, 5 samples out of 10
single plant replicates were randomly harvested in
both treatments (MN and CK) of E. adenophorum.
After the roots, stems and leaves of the plants were
thoroughly rinsed and dried, each plant was divided
into three parts: root, stem and leaves, and the
weight of root, stem and leaves was weighed by an
electronic balance (accurate to 0.001g). Then put the
weighed roots, stems and leaves into three different
envelope bags, and mark the corresponding numbers
under different treatment methods on the envelope
bags. After completion, all the envelope bags were
put into an oven, which was first dried at 105 ℃ for
1 h, and then baked at 65 ℃ for 48 h until the
Enemy Tolerance of Eupatorium Plants
741
sample was constant weight. Finally, after standing
for 10 h, the samples in each envelope bag were
weighed again. E. fortune did the same thing.
Total biomass (total biomass = root weight +
stem weight + leaf weight), root biomass ratio
(RMR, root weight/plant total weight), stem biomass
ratio (SBR, supporting structure biomass/plant total
weight), leaf biomass ratio (LMR, leaf weight/plant
total weight), root/shoot ratio (R/C, underground
biomass/aboveground biomass), tolerance
(BMN-BCK)/BCK).
3 RESULTS AND ANALYSIS
3.1 Dry Biomass Allocation
The root biomass ratio and root-shoot ratio of E.
adenophorum in the control group were significantly
lower than those of the same native plant, E. fortune.
While the leaf biomass ratio was significantly higher
than that of E. fortune. The root biomass ratio and
root-shoot ratio of E. adenophorum in the simulated
insect feeding group were significantly lower than
those of the native plant, while the total dry weight
and leaf biomass ratio of E. adenophorum were
significantly higher than those of the native plant.
The results showed that the total dry weight of the
native plant E. fortune decreased significantly, while
the leaf biomass ratio of the invasive plant E.
adenophorum increased significantly after the
simulated insect feeding treatment.
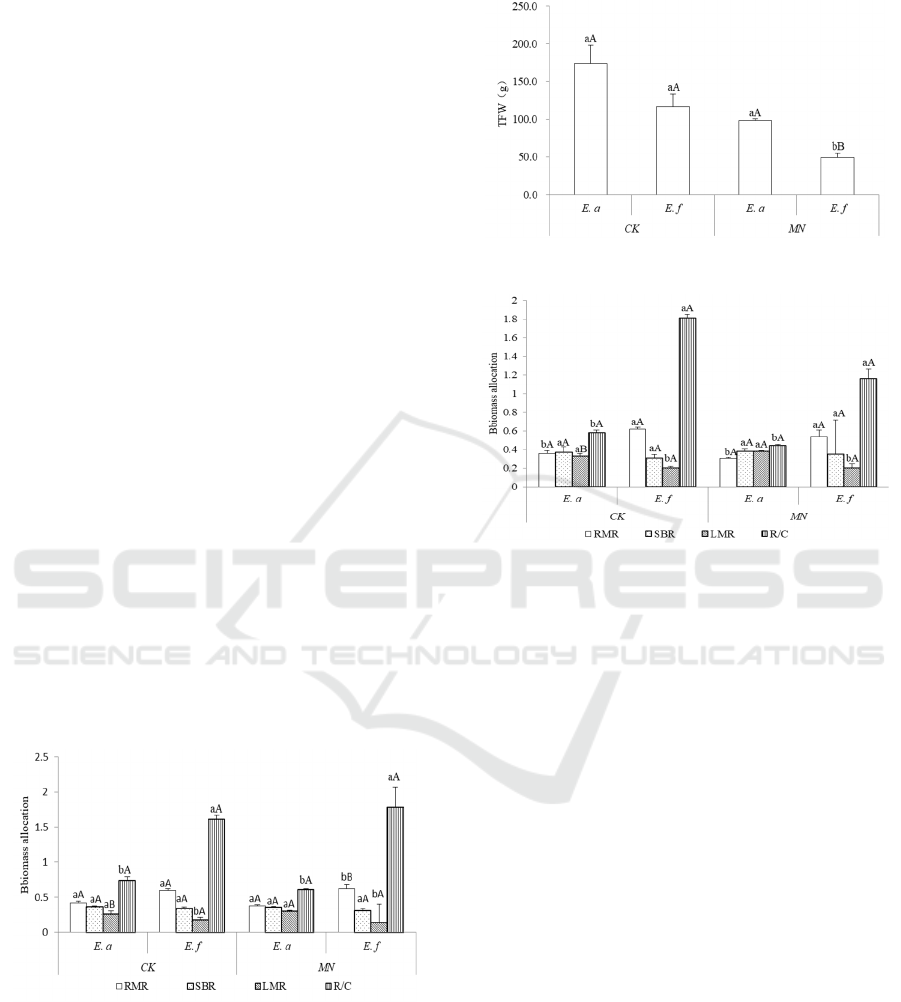
Figure. 1. Dry biomass allocation
3.2 Fresh Biomass Allocation
Figure 2: Toal fresh weight of biomass (TFW).
Figure 3: Fresh biomass allocation.
The root biomass ratio and root-shoot ratio of E.
adenophorum in the control group were significantly
lower than those of the same native plant, E. fortune.
While the leaf biomass ratio was significantly higher
than that of E. fortune. The root biomass ratio and
root-shoot ratio of E. adenophorum in the simulated
insect feeding group were significantly lower than
those of the native plant, while the total fresh weight
and leaf biomass ratio of E. adenophorum were
significantly higher than those of the native plant.
The results showed that the total fresh weight of the
plant decreased significantly, while the leaf biomass
ratio of E. adenophorum increased significantly after
simulated insect feeding.
3.3 Tolerance
There were no significant differences in the changes
of fresh stem water content and root-shoot ratio
water content of E. adenophorum and E. fortune.
While the changes of total water content, fresh root
water content and fresh leaf water content of E.
adenophorum were significantly lower than those of
E. fortune, the tolerance of E. adenophorum was
significantly higher than that of E. fortune.
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
742
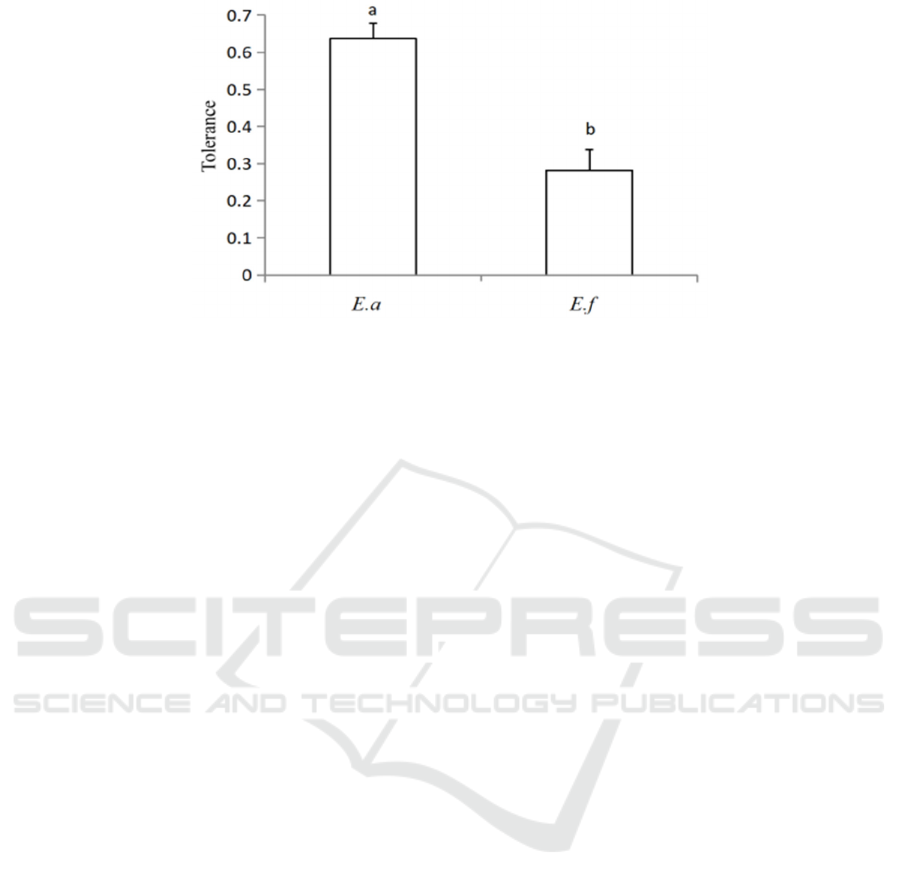
Figure 4: Tolerance in different plants.
4 DISCUSSION AND
CONCLUSION
The growth strategies in the biomass allocation
were different between E. adenophorum and E.
fortune under herbivory. More biomass of E.
adenophorum were allocated to leaf compared to E.
fortune, which can confer its higher carbon income
and tolerance for biomass accumulation than native
E. fortune. thus its total biomass was significantly
higher than E. fortune after herbivory. Therefore, the
biomass allocation pattern made a great contribution
to the tolerance and invasion ability of the invasive
plant E. adenophorum.
ACKNOWLEDGEMENTS
This work was supported by the National Natural
Science Foundation of China (31660170) and
Outstanding Young Teacher program
(2020GGJS006).
REFERENCES
Belsky Aj (1986). Does herbivore benefit plants? A
review of the evidence[J]. The American Naturalist.
127(6): 871-891.
Chen Yunyan, Qin Xunzheng, Chen Haihan, et al (2012).
Study on the damage characteristics and control
methods of the Dorylus orientalis to the young plants
of honeysuckle [J]. Hunan Agricultural Sciences.
(21):79-81.
Ellson L (1960). The influence of grazing on plant
succession[J]. Botanical Review. 26: 1-78.
Gao Xin (2019). Physiological and ecological mechanism
of adaptability of the Procecidochares utilis to the
invasive plant Eupatium adenophorum [D]. Yangzhou
University.
Lei Guisheng, Jiang Zhilin, Deng Dandan, et al (2014).
Effects of Procecidochares utilison the growth and
physiological response of Eupatium adenophorum [J].
Journal of Pu 'er College. 30(03):1-5.
Ming Xian Lan, Sha-Ma, Mou Zhang, et al (2017).
Advances in studies on Procecidochares utilis. Journal
of Southern Agriculture. 48 (03) : 459-464.
Qiang Sheng (1998). The history and status of studies on
the global malignant weed Euphorum adenophorum
[J]. Journal of Botany Research in Wuhan. (04) :
366-372.
Wang Jixiu, Gao Xi, Ma Sha, et al (2013). Study on the
biological enrichment of Cd, Pb and Zn in the
soil-Eupuphorum adenophorum and Procecidochares
utilis [J]. Chinese Journal of Eco-Agriculture. 21
(07) : 877-882.
Wang Lin, Qin Ruihao (2004). Journal of Southwest
Forestry University. (03) : 72-75+80.
Xiao Zhengqing, Zhou Guanhua, Quan Wenting (2009).
Distribution pattern of invasive invasive plant
Eupatorium adenophorum in Yunnan Province [J].
Journal of Natural Disasters. 18 (05) : 82-87.
Xin Liangdong (1990). Natural Enemies of Euphorum
adenophorum -- Procecidochares utilis[J]. Yunnan
Forestry. (03) : 19.
Xu Jin, Liu Ende, Xiang Chunlei, Chen Li, Peng Hua
(2011). Quadrua Orthezia (Homoptera: Ortheziidae), a
native nematode of Eupuphorum adenophorum and
Eupatorium lindleyanum[J]. Journal of Yunnan
Agricultural University (Natural Science Edition).
Yao Benyu, Zhang Yuanzi, Liu Xiaotie, et al (2008).
Research progress on biological characteristics and
control techniques of the Dorylus orientalis[J].
Modern Agricultural Science and Technology.
(04):64-67+69.
Enemy Tolerance of Eupatorium Plants
743
