Study on NNRTIs Treatment for HIV-1 by Comparison of RPV and
EFV
Yichen Yan
Shanghai Weiyu High School, China
Keywords: AIDS, Treatment, RPV and EEV.
Abstract: AIDS (acquired immune deficiency)is a disease caused by progressive failure of immune system, allowing
cancer and opportunistic infection to survive. Without any therapy being taken, people who get HIV/AIDS
could only survive 9-11 years. AIDS are not only difficult to detect, Aids also spreads easily through several
ways. It could spread through the contact of blood, pre-ejaculate, semen, vaginal fluid and other body humor.
A simple contact with patient’s humor could cause the infection to happen. From the first-time researchers
discovered AIDS IN 1981, there are more than 32 million people died because of AIDS. The severity of the
disease could be seen in these large infection numbers. Until now, researchers had not found any useful
method to cure the disease. What researchers could do is only to try to make the patient live longer. This essay
is aimed on the comparison of two drugs (Rilpivirine and Efavirenz) which could help the Aids patient to live
longer and find out why the second generation rilpivirine (RPV) will be better than the first generation
Efavirenz (EFV). CCS concept: Professional topics, Life and medical sciences, Architectures.
1 INTRODUCTION
The first sample of AIDS was discovered by CDC in
1981 and published on the Morbidity and Mortality
weekly report. Then, after having some research, this
disease soon been named as AIDS. After then, AIDS
soon spread to every continent in the world and
become one of the most difficult disease to be cured.
The reason makes the disease spread so fast is
because Aids could spread easily by the contact of
humor of human body, including blood, semen and
vaginal fluid. It normally spread through sexually and
condomless contact with AIDS patient. On the other
way, a mother who have AIDS could also bring the
disease through pregnancy and the process during the
birth. These two particular ways to spread help us to
limit the ages people who are easily get AIDS, which
is 18-45. This is because normally people at this age
would have most of sex life in their whole life time
and most of mother will be pregnancy in this period.
The large year period which people could easily
get AIDS makes the disease spread quickly in the
world. By the spreading of disease, it causes huge
effects on society and makes people realize the
severity of the disease. In 2018, 37.9 million people
were living with HIV and it resulted in 770,000
deaths.
Between the time that AIDS was identified
(in the early 1980s) and 2018, the
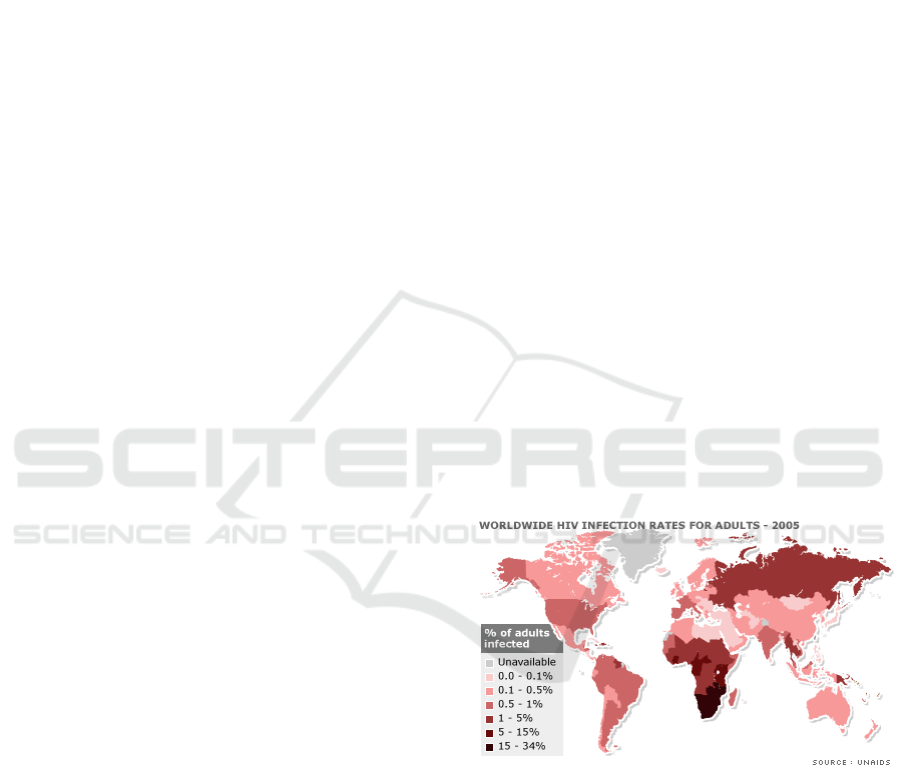
Figure 1. The global percentage of infecting HIV.
disease caused an estimated 32 million deaths
worldwide. As the graph shown here, most of the
countries have an infected rate more than 0.1%-0.5%,
even there is country has a rate of 15%-34%. The
graph could certainly prove that the speed it spread
and the large numbers of infections.
Large base numbers of patients show similar
symptoms after getting infected. Most of the patient
firstly get Flu in the early stage of AIDS and at the
later stage those patients will get fever, large lymph
nodes and weight loss.
264
Yan, Y.
Study on NNRTIs Treatment for HIV-1 by Comparison of RPV and EFV.
DOI: 10.5220/0011281100003438
In Proceedings of the 1st International Conference on Health Big Data and Intelligent Healthcare (ICHIH 2022), pages 264-271
ISBN: 978-989-758-596-8
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
AIDS has had a large impact on society, both as an
illness and as a source of discrimination. The disease
also has large economic impacts. This is because
particularly in China and some other country who may
still have a conservatism concepts to sexual life will
show extreme discrimination and stereotypes to the
people who get AIDS and considered them as
dissipated people. However, those people who get
AIDS could be infected by unconsciously contact with
the infected people. These stereotypes cause large
difficulties to the infected people in the society. On the
economically part, it also creates large burdens for the
patients to buy the particular drugs and take the
treatments. Because there are no drugs could
completely cure the AIDS, so the treatment will last a
long period, leading to a severe economic burden to
the family or individual.
2 TREATMENT
Currently, the most effective way to inhibit the AIDS
is to use antiviral treatment. These antiviral treatments
include NNRTIs (non-nucleoside reverse transcriptase
inhibitor). However, the first time we discover
NNRTIs and other drugs which could be used as a
treatment could be retrospect to decades before,
including the two drugs, RPV and EFV. In the
summer of 1981, the acquired immunodeficiency
syndrome was first discovered by people. Two years
later the etiological link to AIDS, the human
immunodeficiency virus (HIV) was identified. Since
the identification of HIV, the development of effective
antiretroviral drugs and the scientific achievements in
HIV research has been vital. The first
NNRTI(nevirapine) was discovered by researchers
at Boehringer Ingelheim . In 1996, it was approved by
FDA. In the next two years two other NNRTIs were
approved by the FDA, delavirdine and EFV in 1997
and 1998 respectively. These three drugs are the first
generation NNRTIs treatment. The yearning to get for
NNRTIs with better resistance profile led to the
development of the next generation of NNRTIs.
Janssens Foundation and Tibotec’s
researchers discovered the first drug in this
class, etravirine. It was approved in 2008 by FDA. The
second drug in this class, RPV, was also discovered by
Tibotec and was approved by the FDA in 2011.
2.1 Nomenclature
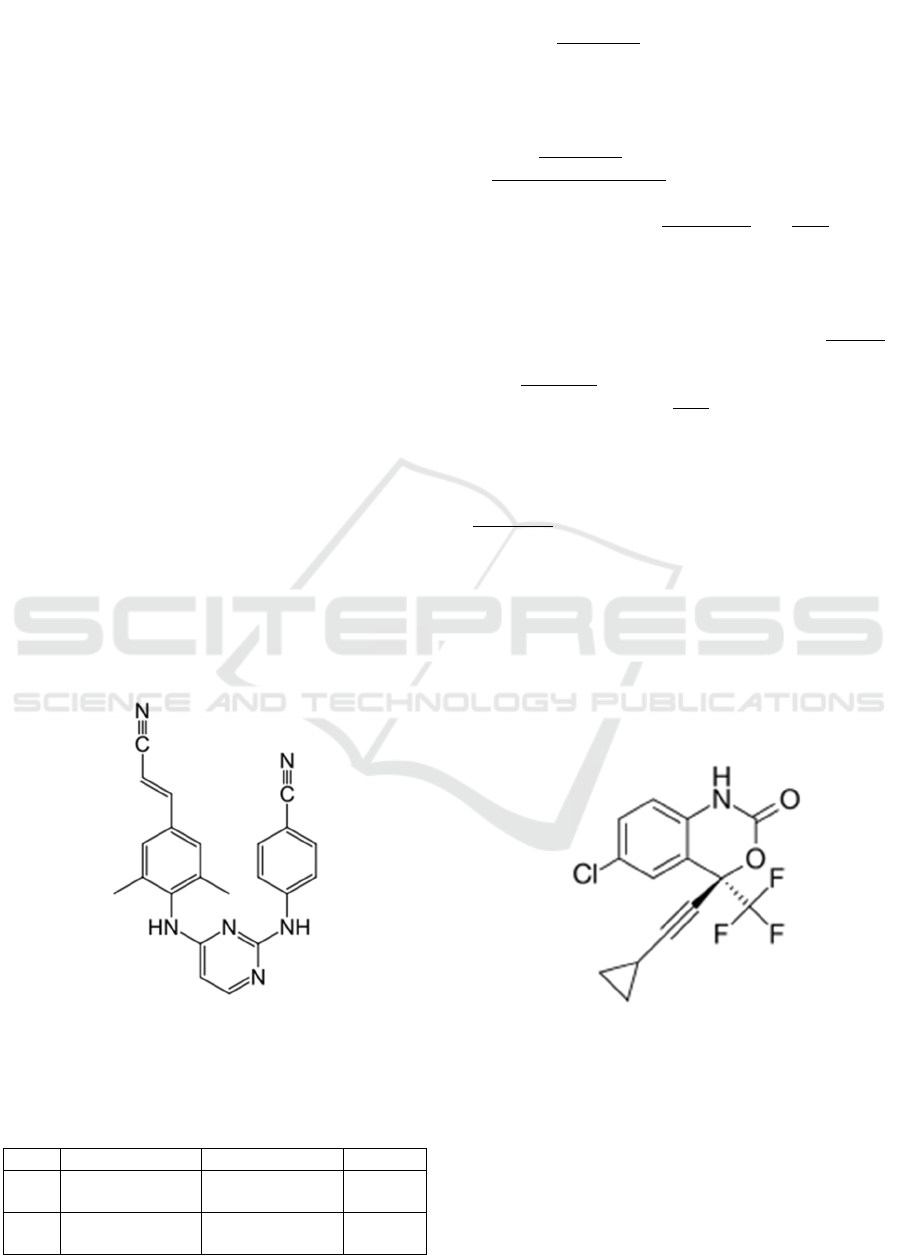
rilpivirine: 4-{[4-({4-[(E)-2-cyanovinyl]-2,6-
dimethylphenyl} amino) pyrimidin-2-yl] amino}
benzonitrile
Efavirenz: (4S)-6-Chloro-4-(2-
cyclopropylethynyl)-4-(trifluoromethyl)-2,4-
dihydro-1H-3,1-benzoxazin-2-one
Fi
g
ure 2. The structure of Ril
p
ivirine
Fi
g
ure.3 The structure of Efavirenz
2.2 Chemical Properties
Table 1 the basic properties of RPV and EFV.
Molar mass formula Half life
RPV 366.428 g·mol
−1
C
22
H
18
N
6
38
hours
EFV 315.68 g·mol
−1
C
14
H
9
ClF
3
NO
2
40–55
hours
Study on NNRTIs Treatment for HIV-1 by Comparison of RPV and EFV
265

3 THE PHARMACOLOGY
3.1 Introduction of RT: The Targets of
NNRTIs
RT is one of the most important enzymes for HIV to
spread in the host cell. During uncoating, the single-
stranded RNA genomes within the core or capsid of
the virus are released into the cytoplasm. HIV now
uses the enzyme
reverse transcriptase
to replicate the
RNA genome. Normal transcription in nature is when
the DNA genome is transcribed into mRNA which is
then translated into protein. In HIV reverse
transcription, RNA is reverse-transcribed into DNA.
3.2 The Targets of NNRTIs and the
Function Affected by NNRTIs
NNRTIs could inhibit the polymerization of HIV RT,
which is an essential viral enzyme in the process to
produce double-stranded viral DNA genomes from
the single strand viral DNA genome. RT is a
heterodimer of p66 and p51. P66 is formed like
people’s right hand. It contains the thumb, fingers,
palm and connections subdomains. Although the
exact mechanisms of NNRTI action is not clear yet, it
is commonly agreed that the using of NNRTIs to the
drug-binding pocket of HIV-1 RT would lead to a
reposition of the template-primer, therefore guarding
against the dNTP binding to form a competent RT-
DNA-dNTP complex. Moreover, non-competitive
NNRTIs allosterically target a hydrophobic pocket.
What needs to notice is that NNRTIs is only useful to
the HIV type 1 but not HIV type 2. This is because
HIV-2 RT possesses isoleucine at codon181 and
leucine at 188. Both of the amino acids there could
prevent the NNRTIs binding to the pocket. (Figure.
4).
Figure. 4: The binding process.
E.g. This is a graph showing the comparison
between the original hinge motion and the hinge
motion after using the NNRTIs (Ivetac, McCammon
2009). The thumb is colored in blue and the fingers is
colored in red. The hinge motion is the process that
thumb and fingers close and become a straight line.
However, as the graph has shown, the NNRTIs acts
like a wedge in the center of the thumb and fingers.
This “wedge effect” prevent the full closure of the
hinge motion. Then, the NNRTIs inhibits the RT to
replicate one single strand of DNA into double helix
structures.
3.3 Mode of Delivery
Depending on the different function of different
medicines and the different doses, each particular
medicine requires a different way to deliver them.
3.3.1 The Mode of Delivery of RPV
Traditionally, RPV is delivered orally by patients
with 25mg once-daily(Williams, Crauwels, Basstanie
2015, Sanford 2012). Rilpivirine should always be
taken with a meal to make sure that there is adequate
exposure. However, there are several recent studies
which are researching the effects of RPV-LA (Long
acting RPV). This type of RPV is focusing on the
different mode of delivery of RPV. Currently, the
RPV-LA pharmacokinetic data showed that
therapeutic concentrations of RPV can be maintained
for at least 28 days after intramuscular administration
of doses between 600 and 1200 mg (Ferretti, Boffito
2018), with high RPV levels achieved in rectal tissue
and in vulvovaginal secretions. With regards to HIV
prevention, a study in HIV negative women showed
that RPV-LA is generally safe and well tolerated and
accepted in this group (Cohen, Molina, Cahn, Clotet,
Fourie, Grinsztejn, Boven 2012). What makes the
new type of the mode of delivery attracting is that it
could overcome the adherence issues and relieve the
pill burden of patients. It is more patient-friendly than
orally available RPV tablets.
3.3.2 The Mode of Delivery of EFV
EFV is delivered orally by the patient once-daily. The
recommended dosage once-daily should be 600mg
(Ivetac, McCammon 2009), taken on an empty
stomach, and favorable at bedtime, to diminish
possible neuropsychiatric side effects. Efavirenz is
contraindicated in pregnancy (category D) because it
can cause fetal harm in the first three months.
However, a recent study conducted by Kamboj,
S., Sethi, S., & Rana, V. in 2018 gives a new idea in
ICHIH 2022 - International Conference on Health Big Data and Intelligent Healthcare
266
the mode of delivery in order to raise the
bioavailability of the EFV. The new deliver mode was
described as EFV1. It turns to a lipid base delivered
mode (Kamboj, Sethi, Rana 2018, Varshosaz,
Taymouri, Jahanian-Najafabadi, Alizadeh 2018)).
This particular mode focused on QbD-driven
systematic development of EFV loaded isotropic
mixture (IM). It contains long chain triglycerides that
have an ability to abolish unstable absorption and
obliterating the food associated variabilities which
could possibly enhances oral bioavailability.
3.3.3 Comparison of Different Modes of
Delivery
With all the existed data and clinical trials have
shown, the orally available RPV has a high
bioavailability than the orally available EFV for the
problems of different structures of the medicines.
However, researchers find that RPV-LA does not
significantly enhance the bioavailability of the RPV,
while the EFV1 (isotropic mixture) shows that it
could overcome the food associated effects and the
erratic absorption of the drug. EFV seems to have a
great potential on overcoming the existed problems
and becoming more bioavailable for patients.
3.4 Result
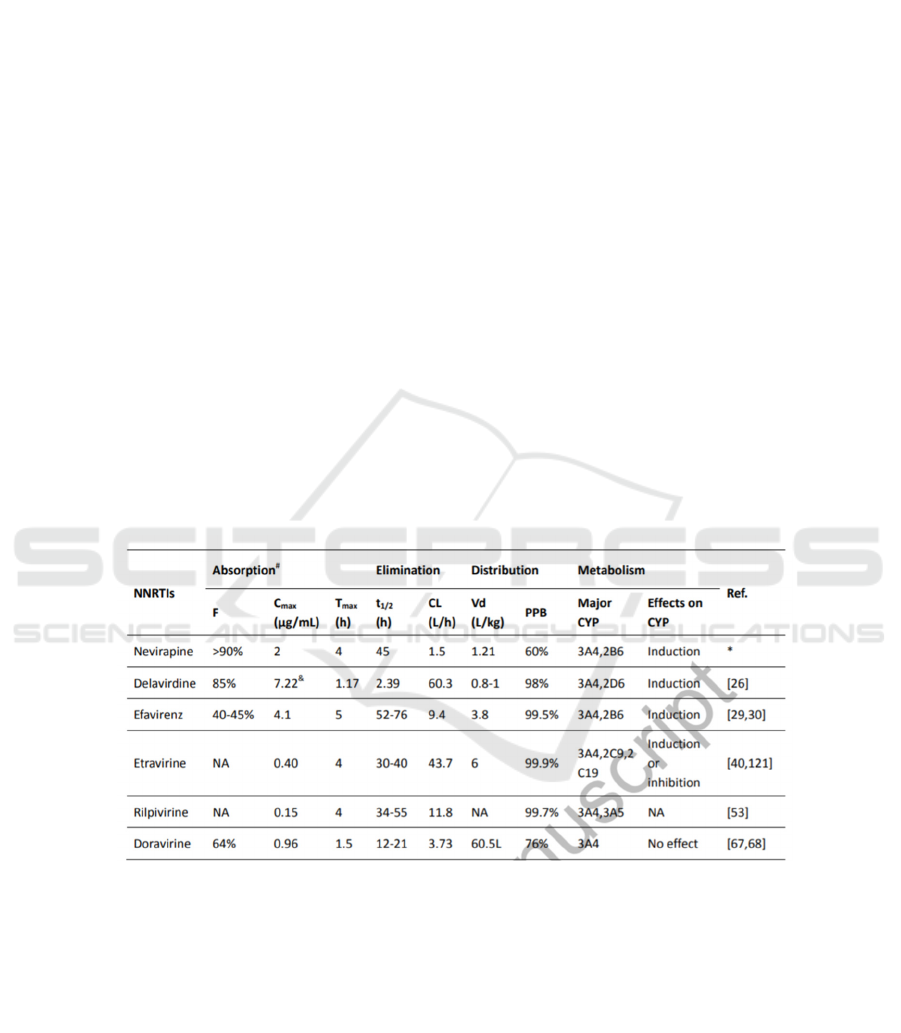
3.4.1 Pharmacokinetics of EFV
Efavirenz, a benzoxazinone compound, has 40%-
45% (Figure 2) oral bioavailability. After a single
dose of EFV orally administered to volunteers who
are not infected, its maximum plasma concentration
(Cmax) is 4.1 μg/mL by 5 hours. Efavirenz is suitable
for a once-daily regimen because the plasma half-life
(t1/2) of single-dose EFV (52 to 76 hours) is similar
to that of multiple-dose EFV (40 to 55 hours)
(Adkins, Noble, 1998) 90% of efavirenz is
metabolized in the liver by the cytochrome P450 3A4
and 2B6. About 14 to 34% of a radiolabeled dose of
efavirenz 400mg was excreted in the urine in the form
of metabolites and 16 to 61% was excreted in the
faeces as unchanged drug. Less than 1% of an
administered dose of efavirenz is excreted unchanged
in the urine.
Table 2: The properties and experimental data of different kinds of NNRTIS.
This is a snap shot taken from Current and
emerging non-nucleoside reverse transcriptase
inhibitors (NNRTIs) for HIV-1 treatment. (Wang, Y.,
De Clercq, E., & Li, G. (2019). Current and emerging
non-nucleoside reverse transcriptase inhibitors
(NNRTIs) for HIV-1 treatment. Expert Opinion on
Drug Metabolism & Toxicology.)
3.4.2 The Pharmacokinetics of RPV
RPV is a diarylpyrimidine compound with a high oral
bioavailability. After the oral administration, the
maximum plasma concentration (Cmax) of RPV is
generally achieved within 4 hours (Tmax). RPV has a
long half-life (t1/2=34-55h)(Wang, Clercq, Li, 2019;
Garvey, Winston, 2009), which is suitable for its
once-daily dosing. Since RPV is primarily
metabolized by CYP 3A4 and 3A5, administration
with other drugs that induce or inhibit CYP3A could
be influential to the concentration of RPV. RPV can
effectively inhibit HIV-1 wild-type strains (EC50:
0.51 nM). Moreover, the EC50(half maximal
effective concentration) values of RPV are generally
lower than that of NVP, EFV, and ETR in the
inhibition of HIV-1 group M isolates.
Study on NNRTIs Treatment for HIV-1 by Comparison of RPV and EFV
267

3.4.3 Comparison of RPV and EFV on the
Efficacy, Safety and Tolerability
Seemingly, EFV has a longer half life than RPV. It
means that the time of EFV to be metabolize half is
longer than RPV. However, the terminal half life of
RPV is 55h and it shows non-inferior efficacy than
EFV. For comparison of oral bioavailability of EFV
and RPV, it is only described as high oral availability
but does not give exact values. What the data presents
is only the bioavailability of EFV which is 40-45%. It
is not an effective number. Therefore, by researching
on a designed trial to test the efficacy, safety and
tolerability, it tells that 346 patients were randomly
assigned to take RPV and 344 to take EFV and take
at least one dose of study drug. The virological
failures of RPV was 13% versus 6% of EFV (11%vs
4% respectively by ITT-TLOVR). Rash, dizziness,
and nightmares, even thoughts to suicide were more
commonly appeared with EFV (Jackson, McGowan
2015). (This experiment is carried by Jean-Michel
Molina, Pedro Cahn, Beatriz Grinsztejn, Adriano
Lazzarin, Anthony Mills, Michael Saag, Khuanchai
Supparatpinyo, Sharon Walmsley, Herta Crauwels,
Laurence T Rimsky, Simon Vanveggel, Katia Boven,
ECHO study group). According to the results given in
the experiment, it shows that although RPV has a
higher rate of virological failure, it is safer and has a
higher tolerability than EFV. No doubt the virological
failure could affects the therapy taken by patients, but
the safety issues and the side effects should definitely
be more vital than the progression of the therapy.
3.5 Conclusion
For comparison, there are several differences could
help researchers to decide why the second generatioN
RPV is better than the first generation EFV. On the
side of the mode of delivery, RPV seems to be more
convenient. It does not need extra time to take the
pills and to notice when people have empty stomach.
To determine if people have an empty stomach will
be much difficult to determine whether people have
food in their stomach. Therefore, on the mode of
delivery, RPV is more convenient than EFV. On the
pharmacokinetics side, RPV has a similar
bioavailability as EFV does. However, considering
the safety issue, EFV has more side effects than RPV.
RPV is much safer than EFV. With the similar
efficacy, RPV will definitely becomes the more
popular one. Therefore, RPV could be the second
generation of HIV treatment of NNRTIs.
4 CHEMICAL SYNTHESIS
4.1 Chemical Synthesis of the RPV
Figure.5: The chemical synthesis method of RPV(Mordant, Schmitt, Pasquier, Demestre, Queguiner, Masungi, Guillemont
2007).
Compound 5(RPV) was synthesized in two
procedures from derivative 12. After heating (150 C)
the chloropyrimidine 12 and researchers use 3,5-
dimethyl-4-hydroxybenzaldehyde with sodium
hydride in a 1:1 ratio mixture of NMP dioxane to
displace the chlorine on the pyrimidine
(Namasivayam, Vanangamudi, Kramer, Kurup, Zhan,
Liu, Byrareddy 2018, Mordant, Schmitt, Pasquier,
Demestre, Queguiner, Masungi, Guillemont 2007). A
Wittig reaction on 5a then happened to form the RPV.
A Wittig reaction usually happened on the aldehyde.
There is an aldehyde on 5a and with same charge, it
could be replaced with a carbon connected to the
cyanide. Then, RPV is synthesized.
4.2 Chemical Synthesis of EFV
The synthesis of EFV starts with 4-chloroaniline (43).
In order to introduce the trifluoroacetyl group into the
chlorine, the amino group is protected as tert-butyl
carbamate, followed by reaction with n-
butyllithium/ethyl trifluoroacetate. The tert-butyl
carbamate was deprotected with HClCH3COOH in 45
to obtain 46, which was crystallized and purified at
5°C and separated with a yield of 87%. For further
ICHIH 2022 - International Conference on Health Big Data and Intelligent Healthcare
268

reactions, 46 forms need to be neutralized.
Therefore, it can be stirred with NaOAc in MTBE to
get 47. The p-methoxybenzylation reaction is carried
out by p-methoxybenzyl alcohol, which is a cheap and
less toxic alternative previously reported for 103,
forming 48 (90% yield). Enantioselective
alkynylation of compound 48 with 49 enantiomer 48
in the THF-toluene-hexane mixture at -50°C in the
presence of ligand 50, the yield was 51%, The body
excess (ee) is 98.5%. Cyclization of 51 by COCl 2
/TEA with a yield of 95%, and then deprotection of p-
methoxybenzyl by cerium ammonium nitrate to obtain
EFV (53) with a yield of 76%. However, p-
methoxybenzyl deprotection and cerium ammonium
nitrate are by-products such as p-
methoxybenzaldehyde and some cerium salts.
Therefore, another method was studied, in which 51
reacted with DDQ in toluene to obtain 54a/54b, and
then reacted with NaOH-MeOH to obtain amino
alcohol 55 (yield 94%). The by-product p-
methoxybenzaldehyde was It is converted into p-
methoxybenzyl alcohol by reduction with NaBH4.
Phosgene can achieve a ring closure of 55 and obtain
EFV (53) with a yield of 95% (purity> 99.5%,
enantiomeric excess or ee greater than 99.5% after ee-
heptane crystallisation), and try to convert it by methyl
carbamate After 55-ring closed recrystallization, only
EFV was obtained with a yield of 83%. Using p-
nitrophenyl carbamate, the yield reached 94%.
The synthesis of EFV starts with 4-chloroaniline
(43). In order to introduce the trifluoroacetyl group
into the chlorine, the amino group is protected as tert-
butyl carbamate, followed by reaction with n-
butyllithium/ethyl trifluoroacetate. The tert-butyl
carbamate was deprotected with HClCH3COOH in 45
to obtain 46, which was crystallized and purified at
5°C and separated with a yield of 87%. For further
reactions, 46 forms need to be neutralized. Therefore,
it can be stirred with NaOAc in MTBE to get 47. The
p-methoxybenzylation reaction is carried out by p-
methoxybenzyl alcohol, which is a cheap and less
toxic alternative previously reported for 103, forming
48 (90% yield). Enantioselective alkynylation of
compound 48 with 49 enantiomer 48 in the THF-
toluene-hexane mixture at -50°C in the presence of
ligand 50, the yield was 51%, The body excess (ee) is
98.5%. Cyclization of 51 by COCl 2 /TEA with a
yield of 95%, and then deprotection of p-
methoxybenzyl by cerium ammonium nitrate to obtain
EFV (53) with a yield of 76%. However, p-
methoxybenzyl deprotection and cerium ammonium
nitrate are by-products such as p-
methoxybenzaldehyde and some cerium salts.
Therefore, another method was studied, in which 51
reacted with DDQ in toluene to obtain 54a/54b, and
then reacted with NaOH-MeOH to obtain amino
alcohol 55 (yield 94%). The by-product p-
methoxybenzaldehyde was It is converted into p-
methoxybenzyl alcohol by reduction with NaBH4.
Phosgene can achieve a ring closure of 55 and obtain
EFV (53) with a yield of 95% (purity> 99.5%,
enantiomeric excess or ee greater than 99.5% after ee-
heptane crystallisation), and try to convert it by methyl
carbamate After 55-ring closed recrystallization, only
EFV was obtained with a yield of 83%. Using p-
nitrophenyl carbamate, the yield reached 94%.
Figure.6 The synthesis method of EFV (Namasivayam, Vanangamudi, Kramer, Kurup, Zhan, Liu, Byrareddy 2018)
Study on NNRTIs Treatment for HIV-1 by Comparison of RPV and EFV
269

Resource delivered from:
RPV
Invented RPV
The main building blocks 77 and 83 (Scheme 13)
are the main parts in the reaction.
117, 146-149 is the basic compound synthesis
requirements of RPV. 77 is prepared from thiouracil
(74), which can be purchased in bulk. Thiouracil (74)
used MeI/NaOH to form75% yield at 60°C with a
yield of 90%, then react with 68 at 150°C to obtain 76
with a yield of 70%. Then by using POCl3 to carry
out the halogenation reaction of 76 to obtain 80%
yield of 77
117,146−149 Compound 83 (another key
intermediate) is prepared from 3,5-4-bromo-2,6-
dimethylaniline in four steps
(78). Use N,N-dimethylformamide dimethylacetal to
protect the amino group in 78 to form dimethyl (79).
It forms 80, then react with n-butyl lithium to Give
81. Compound 81 carries the Wadsworth-Emmons
Reaction. Protection of dimethylformamide with
(diethoxyphosphino)acetonitrile followed by ZnCl2
gave the 83 compound. 77 and 83 are reacted together
at 150°C to obtain RPV
5 DRUG ECONOMICS
5.1 Cost of RPV and EFV
According to the research to different pharmacies, the
research shows that no matter how great the discount
the pharmacies are giving, all of the RPV is more than
1000 dollars for 30 tablets per month. Comparatively,
EFV has a price among $981–1,177, which is slightly
cheaper than RPV.
5.2 Potential
Edurant (Brand name of RPV), received a thumbs up
back in 2011 and has achieved annual sales growth
ever since. In 2017, the drug raked in $714 million,
an increase of 25% from the year prior. However,
according to the resources available, the Atripla
(efavirenz, emtricitabine, and tenofovir) has a sale
of $3.470 billion in 2014. Atripla has a higher sale
than RPV. However, RPV has a greater sale
increasing speed than atripla. Atripla even have lower
sales in 2014 than 2013. In the long term, RPV has
more potential than Atripla.
6 CONCLUSIONS
By collecting the data of RPV and EFV, we compared
and analyzed the two drugs from three dimensions:
pharmacology, chemical composition and price.
Based on the comparison of actual data, we finally
come to the conclusion that EFV is better in
application. I hope our work can play a reference role
in the medical application of life-prolonging drugs for
AIDS patients.
REFERENCES
Adkins, J. C., & Noble, S. (1998). Efavirenz. Drugs, 56(6),
1055–1064.
Cohen, C. J., Molina, J.-M., Cahn, P., Clotet, B., Fourie, J.,
Grinsztejn, B., … Boven, K. (2012). Efficacy and
Safety of Rilpivirine (TMC278) Versus Efavirenz at 48
Weeks in Treatment-Naive HIV-1–Infected Patients.
JAIDS Journal of Acquired Immune Deficiency
Syndromes, 60(1), 33–42.
Ferretti, F., & Boffito, M. (2018). Rilpivirine long-acting
for the prevention and treatment of HIV infection.
Current Opinion in HIV and AIDS, 13(4), 300–307.
Garvey, L., & Winston, A. (2009). Rilpivirine: a novel non-
nucleoside reverse transcriptase inhibitor. Expert
Opinion on Investigational Drugs, 18(7), 1035–1041.
Garvey, L., & Winston, A. (2009). Rilpivirine: a novel non-
nucleoside reverse transcriptase inhibitor. Expert
Opinion on Investigational Drugs, 18(7), 1035–1041.
Ivetac, A., & McCammon, J. A. (2009). Elucidating the
Inhibition Mechanism of HIV-1 Non-Nucleoside
Reverse Transcriptase Inhibitors through Multicopy
Molecular Dynamics Simulations. Journal of Molecular
Biology, 388(3), 644–658.
Jackson, A., & McGowan, I. (2015). Long-acting rilpivirine
for HIV prevention. Current Opinion in HIV and AIDS,
10(4), 253–257. doi:10.1097/coh.0000000000000160
Kamboj, S., Sethi, S., & Rana, V. (2018). Lipid based
delivery of Efavirenz: An answer to its erratic
absorption and food effect. European Journal of
Pharmaceutical Sciences, 123, 199–216
Molina JM, Cahn P, Grinsztejn B, et al. Rilpivirine versus
efavirenz with tenofovir and emtricitabine in
treatmentnaive adults infected with HIV-1 (ECHO): a
phase 3 randomised double-blind active-controlled
trial. Lancet 2011 Jul 16; 378 (9787): 238-46
Mordant, C., Schmitt, B., Pasquier, E., Demestre, C.,
Queguiner, L., Masungi, C., … Guillemont, J.
(2007). Synthesis of novel diarylpyrimidine analogues
of TMC278 and their antiviral activity against HIV-1
wild-type and mutant strains. European Journal of
Medicinal Chemistry, 42(5), 567–579.
Namasivayam, V., Vanangamudi, M., Kramer, V. G., Kurup,
S., Zhan, P., Liu, X., … Byrareddy, S. N. (2018). The
journey of HIV-1 non-nucleoside reverse transcriptase
inhibitors (NNRTIs) from lab to clinic. Journal of
ICHIH 2022 - International Conference on Health Big Data and Intelligent Healthcare
270

Medicinal
Chemistry. doi:10.1021/acs.jmedchem.8b00843.
Pozniak, A. L., Morales-Ramirez, J., Katabira, E., Steyn,
D., Lupo, S. H., Santoscoy, M., … Boven, K.
(2010). Efficacy and safety of TMC278 in
antiretroviral-naive HIV-1 patients: week 96 results of
a phase IIb randomized trial. AIDS, 24(1), 55–65.
Sanford, M. (2012). Rilpivirine. Drugs, 72(4), 525–541.
Varshosaz, J., Taymouri, S., Jahanian-Najafabadi, A., &
Alizadeh, A. (2018).Efavirenz oral delivery via lipid
nanocapsules: formulation, optimisation, and ex-vivo
gut permeation study . IET Nanobiotechnology, 12(6),
795–806.
Wang, Y., De Clercq, E., & Li, G. (2019). Current and
emerging non-nucleoside reverse transcriptase
inhibitors (NNRTIs) for HIV-1 treatment. Expert
Opinion on Drug Metabolism & Toxicology.
Williams, P. E., Crauwels, H. M., & Basstanie, E. D.
(2015). Formulation and pharmacology of long-acting
rilpivirine. Current Opinion in HIV and AIDS, 10(4),
233–238
Study on NNRTIs Treatment for HIV-1 by Comparison of RPV and EFV
271
