
Optimal Social Limitation Reduction under Vaccination and Booster
Doses
Paolo Di Giamberardino
a
and Daniela Iacoviello
b
Department of Computer, Control and Management Engineering, Sapienza University of Rome, Rome, Italy
Keywords:
Epidemic Modeling, COVID-19, Vaccination, Optimal Control, Social Behaviour.
Abstract:
In the paper an optimal control solution is provided for the containment of the number of infected individuals
in COVID-19 pandemic under vaccination campaign. The possibility to dynamically change the cost of the
controls according to the ongoing evolution within the design procedure allows to get great efforts in presence
of very serious disease conditions, saving resources otherwise. The different contribution of vaccinated and
unvaccinated individuals to the epidemic spread is investigated, optimising the controls which describe the
individual contact restrictions separately for the two classes and showing that it would have been possible
to reduce all the social limitations introduced by many governments for the vaccinated individuals since the
beginning of the vaccination campaign.
1 INTRODUCTION
COVID-19 is a sanitary emergency since January
2020 and the scientific community has started to
study the evolution and the spread of the infection
by means of mathematical models which had to fol-
low the changes and the novelties in the knowledge
of the illness, updated day by day, and in the pos-
sibility to fight the virus, as for examples (Di Gi-
amberardino et al., 2021),(Giordano et al., 2020),
(Tang et al., 2020), (Di Giamberardino and Iacoviello,
2021), (Radulescu et al., 2020). In particular, since
the end of 2020, the availability of an effective vac-
cine makes it possible to introduce in all the models a
new important control input, especially for the initial
problem of scheduling the vaccinations among popu-
lation categories. There is for example (Diagne et al.,
2021), with the study of the efficacy of the vaccination
with respect to the amount of population immunized.
There is a great relationship between the level of
immunization of the population and the relax of the
individual containment limitations. The necessity of
a booster dose after the two doses cycle of vaccina-
tions highly increased the people immunization, al-
lowing to strongly reduce or even eliminate social re-
strictions in many places. Different control actions
have been proposed in literature to reduce the num-
ber of infected patients; in particular, optimal control
a
https://orcid.org/0000-0002-9113-8608
b
https://orcid.org/0000-0003-3506-1455
allows to allocate efficiently the available limited re-
sources, as in (Silva et al., 2021) referred in particular
to the Portugal situation, where an optimal strategy is
proposed to maximize the number of people that can
return to normal life minimizing the number of in-
fected patients, taking into account the level of hospi-
talization. In (Olivares and Staffetti, 2021) the effects
of various vaccination strategies are studied consid-
ering realistic scenarios in which constraints on vac-
cine administration are introduced. Optimal solutions
for vaccination strategies are also proposed improv-
ing existing models by the addition of compartments
which take into account new knowledge on epidemic
behaviour, like in (Liu et al., 2021) with the introduc-
tion of class of people for which the vaccine is inef-
fective.
In this paper, following the approach described in
(Di Giamberardino and Iacoviello, 2017), it is pro-
posed an optimal control obtained minimizing a suit-
able cost index in which the controls are weighted
on the basis of the severity of the pandemic situa-
tion. In particular, this approach is used to act on the
most invasive containment measure represented by
the individual contact limitations with consequences
on the everyday life and economy. A new mathemat-
ical model, which takes into account the possibility
of a periodic reiteration with respect to the vaccina-
tion procedure, is here proposed as an improvement of
previously adopted ones to better fit the present epi-
demic conditions.
38
Di Giamberardino, P. and Iacoviello, D.
Optimal Social Limitation Reduction under Vaccination and Booster Doses.
DOI: 10.5220/0011277500003271
In Proceedings of the 19th International Conference on Informatics in Control, Automation and Robotics (ICINCO 2022), pages 38-48
ISBN: 978-989-758-585-2; ISSN: 2184-2809
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

The paper is organized as follows; in Section 2,
the new mathematical model is introduced and then
analysed in Section 3. In Section 4 the optimal control
strategy is presented. Numerical results are shown in
Section 5 and a discussion is provided in Section 6.
2 THE MATHEMATICAL MODEL
Since the beginning of 2021, the mathematical mod-
elling of COVID-19 in a population can assume vac-
cination as a real possibility to contain virus spread.
As long as information about vaccine efficacy, level
of immunization and time of protection duration have
been acquired, the possibility of a booster dose has to
be introduced in any model; the number of categories,
i.e. compartments, in the mathematical models must
take into account the different level of vaccination, in
addition to the different condition with respect to the
disease. The model here adopted is based on a prelim-
inary split into two main categories with respect to the
vaccination conditions: those who have not received
any vaccination dose and those who have started the
vaccination cycle. The two groups are further particu-
larized, depending to their specific condition with re-
spect to the disease. For the population not vaccinated
the well known SEIR model is chosen. A SEIR–like
model is adopted also for the vaccinated population,
but distinguishing the healthy subjects on the num-
ber of vaccination doses received: there is the class P,
including those that have received only the first dose,
the class V including the ones that have completed the
vaccination cycle (second dose for two doses vaccine
or unique dose for single dose ones), and the class B,
containing the subjects that, after a full vaccination
cycle or healed from infection, are losing immunity
and are suggested to get the booster dose; they are not
completely susceptible to the infection but the protec-
tion of the vaccine decreases with time and they can
be infected with a course of the illness that could de-
pend on the time elapsed since the completion of the
vaccination cycle. This aspect is still under investiga-
tion by the researchers; in this paper we will assume
that if a subject in B is infected he will have, proba-
bly, the same reaction to the virus as that of a subject
in the P or V condition.
More precisely, the population is partitioned into
10 compartments:
• S: susceptible subjects, composed by the healthy
part of the population which is not vaccinated yet;
• E: exposed individuals, i.e. the subjects in the
incubation period; they are infected but can not
infect;
• I: infected patients, that can infect the susceptible
individuals and the subjects in P and V compart-
ments;
• R: removed subjects, immunised by vaccination
or because healed from the virus;
• P: healthy individuals that received the first vac-
cination dose;
• V : healthy subjects that received both the vacci-
nation doses;
• E
V
: exposed vaccinated individuals, i.e. the vacci-
nated subjects in the incubation period; as E, they
are infected but cannot infect;
• I
V
: vaccinated infected patients, that can infect
both the susceptible non vaccinated individuals,
but also the subjects in P and V compartments;
• R
V
: removed vaccinated subjects;
• B: individuals which are losing immunization and
then require a booster vaccine dose.
The dynamics describing the epidemic spread and
its evolution can be written as
˙
S = N − β
SI
(1 − u
S
)SI − β
SI
V
(1 − u
S
)SI
V
− d
S
S
−u
1
S − u
4
S (1)
˙
E = β
SI
(1 − u
S
)SI + β
SI
V
(1 − u
S
)SI
V
− d
E
E
−kE −u
1
E (2)
˙
I = −d
I
I + kE − γ
I
I (3)
˙
R = −d
R
R + γ
I
I − r
2
R − u
3
R (4)
˙
P = −β
PI
(1 − u
P
)PI − β
PI
V
(1 − u
P
)PI
V
− d
P
P
+u
1
S − u
2
P (5)
˙
V = −β
V I
(1 − u
V
)V I − β
V I
V
(1 − u
V
)V I
V
− d
V
V
−r
3
V + u
2
P + u
3
R + u
4
S + u
5
B (6)
˙
E
V
= β
V I
(1 − u
V
)V I + β
V I
V
(1 − u
V
)V I
V
+β
PI
(1 − u
P
)PI + β
PI
V
(1 − u
P
)PI
V
+β
BI
(1 − u
B
)BI + β
BI
V
(1 − u
B
)BI
V
−d
E
V
E
V
− kE
V
+ u
1
E (7)
˙
I
V
= kE
V
− d
I
V
I
V
− γ
V
I
V
(8)
˙
R
V
= −d
R
V
R
V
+ γ
V
I
V
− r
1
R
V
(9)
˙
B = r
2
R + r
1
R
V
+ r
3
V − u
5
B − d
B
B
−β
BI
(1 − u
B
)BI − β
BI
V
(1 − u
B
)BI
V
(10)
The block diagram of the proposed model is shown
in Fig.1 The controls u
i
, i = 1,...,5, denote the vacci-
nation strategy. More precisely, a susceptible subject
in S is vaccinated a first time with rate u
1
(with u
1
S
representing the daily number of doses administered)
and then he receives the second dose after an average
time
1
u
2
. An exposed subject in E is still not aware
of being infected so, in a vaccination campaign he
Optimal Social Limitation Reduction under Vaccination and Booster Doses
39

Figure 1: Block diagram of the considered model.
could be vaccinated with the first dose; he will never
receive the second one because in the period
1
u
2
he
probably becomes a diagnosed infected vaccinated I
V
after having passed through E
V
. It is considered also
the vaccination u
3
for the healed subjects in R; in fact,
at present the scientific community is suggesting to
vaccinate this class of subjects with only one dose.
Finally, the possibility of using a one dose vaccine,
with a vaccination rate u
4
, is introduced; this implies
that a susceptible subject in S could enter directly the
V class with just one dose of this vaccine. For sim-
plicity, it is assumed a mean vaccination coverage,
without distinguishing among the different vaccines
efficacy. The control u
5
denotes the third dose, the
booster one.
The controls u
S
, u
P
, u
V
and u
B
, following the ap-
proach adopted for example in (Di Giamberardino
and Iacoviello, 2021) and (Di Giamberardino and Ia-
coviello, 2020), denote the reduction of social inter-
actions as well as the individual distancing; they can
be represented by different levels of lock–down con-
ditions or limits on public places occupancy or certi-
fications for free mobility and/or by personal protec-
tive equipment like masks, disinfectant products and
so on. They are referred to the four classes of popula-
tion which denote the different vaccination conditions
in view of a possible differentiation of restrictive de-
cisions according to the vaccination.
The parameters appearing as coefficients in (1)–
(10) have the following meanings: d
∗
denotes the
death rate in each compartment identified in the sub-
script;
1
k
is the incubation time; it is assumed equal
both for the vaccinated and non vaccinated subjects;
β
i j
, i = S,P,V,B, j = I, I
V
represents the transmission
rate between the healthy subjects i (S, P, or V , or B)
and the infectious one j (I or I
V
);
1
r
i
, i = 1,2,3 rep-
resents the time from which the antibodies decrease
and the subject turns to the B class from the R
V
, R and
V classes respectively;
1
γ
V
and
1
γ
I
are the incubation
times; they are assumed different for the vaccinated
and non vaccinated subjects, since for the vaccinated
ones it is frequent to have a faster healing; N repre-
sents the rate of new incomers.
Defining
X =
S E I R P V E
V
I
V
R
V
B
T
U =
u
1
u
2
u
3
u
4
u
5
u
S
u
P
u
V
u
B
T
dynamics (1)–(10) can be written in the compact form
˙
X = F(X,U) (11)
3 MODEL ANALYSIS
The basic analysis of the model requires the computa-
tion of the disease free equilibrium point; by equating
to zero the system (11), once U = 0 is set, the solu-
tion with zero infected patients can be found; some
calculations bring to:
X
e
= (
S
e
E
e
I
e
R
e
P
e
V
e
E
e
V
I
e
V
R
e
V
B
e
)
T
=
N
d
S
0 0 0 0 0 0 0 0 0 0
T
(12)
To study its local stability, it is possible to analyse the
sign of the eigenvalues of the Jacobian J
X
e
=
∂F
∂X
X
e
under null inputs. Thanks to a large number of null
entries, it is possible to get directly from the matrix
structure eight eigenvalues; they are
λ
1
= −(d
V
+ r
3
) λ
2
= −d
S
λ
3
= −d
P
λ
4
= −(d
R
V
+ r
1
) λ
5
= −(d
R
+ r
2
) λ
6
= −d
B
λ
7
= −(d
E
V
+ k) λ
8
= −(d
I
V
+ γ
V
)
(13)
The remaining two eigenvalues come from the block
of the Jacobian matrix
−d
E
− k β
SI
S
e
k −d
I
− γ
I
(14)
and can be computed as the roots of the equation λ
2
+
(d
E
+ k +d
I
+ γ
I
)λ + (d
E
+ k)(d
I
+ γ
I
) − kβ
SI
N
d
S
.
By applying the Descartes’ rule of signs, it can be
claimed that the disease free equilibrium point X
e
is
locally asymptotically stable if and only if
(d
E
+ k)(d
I
+ γ
I
) > kβ
SI
N
d
S
(15)
This implies a limit in the contact rate β
SI
to reduce
the spread of the virus. In fact, this result can be
compared with that characteristic parameter adopted
for studying the spread velocity under endemic con-
ditions or the reduction to zero, asymptotically: the
basic reproduction number R
0
. Its analytical expres-
sion with respect to the model parameters can be
ICINCO 2022 - 19th International Conference on Informatics in Control, Automation and Robotics
40

deduced by evaluating the next generations matrix
(Van Den Driessche, 2017); its calculus requires the
study of the part of the system (11) involving the evo-
lution of the subjects infected, in this case the individ-
uals belonging to the classes E, I. The control actions
(in our case the vaccination effort) are all set equal
to zero. The reduced system involving the variables
(E I) and the corresponding variations (2), (3), may
be written enhancing the contributions due to the in-
fection, F , and the ones due to changing the health
condition, V :
˙
E
˙
I
= F − V (16)
where
F =
β
SI
SI + β
V I
V
SI
V
0
(17)
V =
d
E
E + kE
−kE +d
I
I + γ
I
I
(18)
The variations of these matrices with respect to
the variables E, I, evaluated in the disease free equi-
librium point X
e
, yield the matrices F and V respec-
tively:
F =
∂F
∂(E, I)
X
e
=
0 β
SI
N
d
S
0 0
, (19)
and
V =
∂V
∂(E, I)
X
e
=
d
E
+ k 0
−k d
I
+ γ
I
(20)
Under these positions, the reproduction number
R
0
is given by the dominant eigenvalue of the next
generation matrix
FV
−1
=
kβ
SI
N
d
S
(d
E
+k)(d
I
+γ
I
)
β
SI
N
d
S
(d
I
+γ
I
)
0 0
!
(21)
Then, the basic reproduction number R
0
is obtained
from the non–null eigenvalue and is given by
R
0
=
kβ
SI
N
d
S
(d
E
+ k)(d
I
+ γ
I
)
(22)
By comparison of this expression (22) with the
stability condition (15), the known relationships be-
tween the epidemiological and stability characteris-
tics of the system is remarked: the epidemics tends
to extinguish if R
0
< 1, so that the condition can be
written as
kβ
SI
N
d
S
(d
E
+ k)(d
I
+ γ
I
)
< 1 (23)
that can be rearranged as (15).
4 DEFINITION OF THE
OPTIMAL CONTROL
PROBLEM FOR BEST
STRATEGY
Since the beginning of 2021, the availability of vacci-
nations added an important, and hopefully definitive,
action to the previous non pharmaceutical ones which
have always represented a freedom limitation of indi-
viduals.
The proposed study aims at evaluating, in an op-
timal control framework, the possibility and the opti-
mal time of relaxing the contact limitations between
individuals and the constraints on the use of protective
devices under a full vaccination campaign. The gen-
eral cost function should consider time, state and con-
trols. For sake of clarity in the following discussion,
simple but effective choices are performed. The state
considered in the minimization are the two meaning-
ful for evaluating the epidemic spread, the two in-
fected classes I(t) and I
V
(t); the controls representing
the vaccinations are set constant under the hypothe-
sis of a full rate vaccination while the ones denoting
the contact limitations are part of the control to be de-
fined. Due to their significant differences in terms of
infection spread contributions, the unvaccinated indi-
viduals and the vaccinated ones are maintained dif-
ferent, so that the two controls u
S
and u
V
are kept
distinguished; in view of a realistic correspondence
with social behaviour, people with one dose only is
considered as unvaccinated, that is u
P
= u
S
, while the
class of people requiring the booster dose is consid-
ered equivalent to the vaccinated ones: u
B
= u
V
.
The design approach here followed is the same
as the one introduced in (Di Giamberardino and Ia-
coviello, 2017): the control is weighted by a function
of the current state, so that different costs can be as-
sociated to different dangerousness levels of the epi-
demics effects. Under the choice of I(t) and I
V
(t) as
the most meaningful ones, the weights are defined as
functions of these two state variables only.
Under the present choices, the cost function is
J(U,T ) =
Z
T
0
(K
1
+ K
2
I(t) + K
3
I
V
(t)
1
2
(W
S
u
2
S
(t) +W
V
u
2
V
(t)))dt (24)
with W
S
=W
S
(I(t),I
V
(t)) and W
V
=W
V
(I(t),I
V
(t)) to
be defined according to the severity of the spread and
the population health situation. This can be reformu-
lated as the necessity of a stronger intervention under
higher number of infected patients, I(t) and I
V
(t)
The controls are chosen bounded to consider lo-
gistic, economic, technical and physical constraints,
Optimal Social Limitation Reduction under Vaccination and Booster Doses
41

so that U
Sm
≤ u
S
(t) ≤ U
SM
and U
V m
≤ u
V
(t) ≤ U
V M
;
according to the optimal control problem formulation,
such bounds yield to the constraints formulation
q
1
(t) = U
SM
− u
S
(t) < 0
q
2
(t) = u
S
(t) −U
Sm
< 0
q
3
(t) = U
V M
− u
V
(t) < 0
q
1
(t) = u
V
(t) −U
V m
< 0
(25)
For the definition of the weight function
W
i
(I(t),I
V
(t)), a Cartesian decomposition of the sub-
space ℜ
2
= I ×I
V
of the state space is performed. The
domain interval [0,+∞], in which the number of in-
fected non–vaccinated patients, I(t), can vary, is par-
titioned into n
1
+ 1 subintervals [I
i
,I
i+1
), i = 0,...,n
1
,
with I
0
= 0, I
1
= I
m
, and I
n
1
+1
= +∞; the same is
performed for I
V
(t): its domain definition [0, +∞] is
partitioned into n
2
+ 1 subintervals [I
V,i
,I
V,i+1
), i =
0,...,n
2
, with I
V,0
= 0, I
V,1
= I
V m
, and I
V,n
2
+1
= +∞.
The weight function chosen W
i
(I(t),I
V
(t)) for the
control in the cost index aims at improving the con-
trol effort in case of severe epidemiological situation,
while relaxing the measures or even cancelling any
containment action when the number of infected pa-
tients is low. The values I
m
and I
V m
represent the min-
imum number of infected non vaccinated and vacci-
nate patients, respectively, below which any control
action is no more motivated.
For the control u
S
it is assumed that, when I(t) ∈
[I
i
,I
i+1
) and I
V
(t) ∈ [I
V j
,I
V, j+1
), the weight is con-
stant and set equal to a certain value α
S
i, j
chosen
so that the higher is the severity of the disease, the
lower is the cost of the control action. The same
is performed for the control u
V
. So, defining I
i, j
=
[I
i
,I
i+1
) × [I
V j
,I
V, j+1
), one has
W
h
(I(t),I
V
(t)) = α
h
i j
for (I(t),I
V
(t)) ∈ I
i, j
(26)
with α
h
i j
∈ R
+
, i = 1,.. . ,n
1
, j = 1,... , n
2
, h ∈
{
S,V
}
.
For their meanings, one has
α
h
i, j
≤ α
h
i+1, j
(27)
α
h
i, j
≤ α
h
i, j+1
(28)
α
h
i, j
< α
h
i+1, j+1
(29)
The control is not applied, and hence not defined,
when (I(t),I
V
(t)) ∈ I
0,0
.
The piecewise definition of the weight functions
W can be formulated as a decomposition over each in-
terval I
i, j
of a constant weighted optimal control prob-
lem. With the choice of the cost index (24), it follows
that when the number of infected non–vaccinated pa-
tients I(t) falls in the interval [I
i
,I
i+1
) and the num-
ber of infected vaccinated patients falls in the interval
[I
V j
,I
V, j+1
), the Hamiltonian can be defined as
H(X ,U, λ)=K
1
+ K
2
I(t) + K
3
I
V
(t) +
1
2
α
S
i j
u
2
S
(t)
+
1
2
α
V
i j
u
2
V
(t) + λ
T
(t)F(X,U ) (30)
with λ the 10–dimension costate function. Note that,
obviously, the Hamiltonian and the costate functions
depend on the specific interval [I
i
,I
i+1
) × [I
V j
,I
V, j+1
);
to avoid weighing down the notation, the subscript i, j
are not written.
The solution follows an iterative computation.
Starting from the initial condition, the first inter-
val I
i, j
is defined with I(0) ∈ [I
i
,I
i+1
) and I
V
(0) ∈
[I
V, j
,I
V, j+1
). If i 6= 0 and j 6= 0, the optimal con-
trol problem can be posed, with W
S
(I(t),I
V
(t)) =
α
S
i, j
and W
V
(I(t),I
V
(t)) = α
V
i, j
used in the Hamil-
tonian (30), along with all the boundary condi-
tions and constraints, for getting the optimal solution
(X(t),λ(t),U(t)). This is the optimal solution until
(I(t),I
V
(t)) ∈ I
i, j
. Along the solution, at the first time
¯
t
1
in which the number of infected people I(
¯
t
1
) and
/or I
V
(
¯
t
1
) crosses upper or lower boundary values of
the domain I
i, j
a switch occurs since at least one of the
functions W
i
(I(t),I
V
(t)) changes its value; a new opti-
mal control problem must be defined and then solved
in the new region; the Hamiltonian is defined as in
(30) but with
W
S
(I(t),I
V
(t)) = α
S
i−1, j
if (I(
¯
t
+
1
),I
V
(
¯
t
+
1
)) ∈ I
i−1, j
W
S
(I(t),I
V
(t)) = α
S
i+1, j
if (I(
¯
t
+
1
),I
V
(
¯
t
+
1
)) ∈ I
i+1, j
W
S
(I(t),I
V
(t)) = α
S
i, j−1
if (I(
¯
t
+
1
),I
V
(
¯
t
+
1
)) ∈ I
i, j−1
W
S
(I(t),I
V
(t)) = α
S
i, j+1
if (I(
¯
t
+
1
),I
V
(
¯
t
+
1
)) ∈ I
i, j+1
W
S
(I(t),I
V
(t)) = α
S
i−1, j−1
if (I(
¯
t
+
1
),I
V
(
¯
t
+
1
)) ∈ I
i−1, j−1
W
S
(I(t),I
V
(t)) = α
S
i+1, j+1
if (I(
¯
t
+
1
),I
V
(
¯
t
+
1
)) ∈ I
i+1, j+1
W
S
(I(t),I
V
(t)) = α
S
i−1, j+1
if (I(
¯
t
+
1
),I
V
(
¯
t
+
1
)) ∈ I
i−1, j+1
W
S
(I(t),I
V
(t)) = α
S
i+1, j−1
if (I(
¯
t
+
1
),I
V
(
¯
t
+
1
)) ∈ I
i+1, j−1
and, equivalently, for the weight W
V
(I(t),I
V
(t)). The
bounds crossed and the update of the values for the
case of W
V
(I(t),I
V
(t)) = α
V
i, j
are depicted in Figure 2.
Figure 2: Procedure to change the weights in the cost func-
tion J.
The first segment of the optimal solution defined
over the time interval [0,
¯
t
1
] is then obtained. Taking
ICINCO 2022 - 19th International Conference on Informatics in Control, Automation and Robotics
42

the initial time
¯
t
1
and the initial conditions X(
¯
t
1
), a
new optimal control problem is then defined, different
from the previous one for the choices of the weight
coefficients of the control only. According to the same
procedure, a new switching instant
¯
t
2
can be found,
and so on.
A final time T can be fixed (and then K
1
= 0 in
(30)) or left free. In the first case, it may happen that
there is a switching instant
¯
t
s
< T such that the state
evolution enters the I
0,0
region. In this case, the actual
final time in no more T but
¯
t
s
. In the second case,
¯
t
s
is the final time to be computed. Note that the actual
final time
¯
t
s
cannot be assured a priori, even if T is
defined, since its value depends on the state evolution.
For each iteration, the necessary condition of the
Pontryagin principle to solve the optimal control prob-
lem over each interval can be written as follows, start-
ing from the costate equations
˙
λ =
∂H
∂X
T
where λ(t) has the first order derivative continuous al-
most everywhere and λ(T ) = 0.
The Pontryagin inequality yields for the two con-
trols u
S
(t) and u
V
(t)
1
2
α
S
i j
u
2
S
+ Γ
S
u
S
≤
1
2
α
S
i j
ω
2
S
+ Γ
S
ω
S
(31)
1
2
α
V
i j
u
2
V
+ Γ
V
u
V
≤
1
2
α
V
i j
ω
2
V
+ Γ
V
ω
V
(32)
where
Γ
S
= (λ
1
− λ
2
)(β
SI
SI + β
SI
V
SI
V
) (33)
Γ
V
= (λ
6
− λ
7
)(β
V I
V I + β
V I
V
V I
V
) (34)
and the ω
S
and ω
V
represent any admissible control,
that is any control function satisfying (25).
Due to the convexity, with respect to u
S
, of the
function
1
2
α
S
i j
u
2
S
+ Γ
S
u
S
, and the same for the control
u
V
, the inequalities (31) and (32) yield
u
S
= min
(
U
SM
,max
(
U
Sm
,
−Γ
S
α
S
i j
))
(35)
u
V
= min
(
U
V M
,max
(
U
V m
,
−Γ
V
α
V
i j
))
(36)
The complete optimal control is obtained as the
concatenation of the controls obtained in each single
step.
In next Section 5, a numerical case is illustrated
within the proposed optimal control design frame-
work, defining the optimal switching approaches to
social contact limitations as a consequence of a mas-
sive vaccination campaign. The idea is to show how
the present approach can reproduce the political deci-
sions on increasing or decreasing, at certain times ac-
cording to the epidemic spread, the social containment
constraints; moreover, evidencing the lower contribu-
tion of vaccinated individuals to the epidemic spread
with respect to the not vaccinates ones, and the dif-
ferent impact in terms of public health and hospital
occupancy, the optimal solutions show that different
policies for unvaccinated and vaccinated individuals
can be adopted.
For this particular problem, a set of assumptions
and simplifications are introduced. The vaccinations
are assumed to proceed at a constant rate, the one ad-
missible by the sanitary systems, following the idea
that the entire population aims at being vaccinated;
the contact rate limitation for people vaccinated with
the first dose only, P, is assumed the same as for the
ones not yet vaccinated, S, so that u
P
= u
S
. A further
simplification, not far from reality especially in more
recent times, is to neglect the single dose vaccine us-
age, so setting u
4
= 0.
5 NUMERICAL RESULTS
In this Section, the switching optimal control ap-
proach described in Section 4 is used to generate the
optimal social and individual distancing, specific for
non vaccinated and vaccinated individuals, modulated
according to different level of severity of the epidemic
measured on the basis of the infected individuals, both
among susceptibles and vaccinated ones. With respect
to the cost function (24), the following coefficients are
taken:
K
1
= 10; K
2
= 10; K
3
= 1 (37)
The decomposition of the subspace I ×I
V
is performed
assuming n
1
= n
2
= 4 and, for each of the two com-
ponents, the subinterval thresholds
I
1
= I
V,1
= 5 · 10
4
, I
2
= I
V,2
= 10
6
, I
3
= I
V,3
= 3 · 10
6
(38)
The corresponding n
1
×n
2
weight matrices α
S
and α
V
are chosen as
α
S
= α
V
=
10
10
10
8
10
7
10
6
10
8
10
7
10
6
10
5
10
7
10
6
10
4
10
3
10
6
10
5
10
3
10
(39)
An important choice concerns the transmission
rates β
∗,∗
. Taken β
SI
= 10
−8
as an average value from
literature (Di Giamberardino et al., 2021),(Dan et al.,
2021) and (Diagne et al., 2021), the other rates are
chosen as
β
SI
V
= 0.1β
SI
; β
PI
= 0.8β
SI
;
β
PI
V
= 0.2β
SI
; β
V I
= 0.1β
SI
;
β
V I
V
= 0.02β
SI
; β
BI
= 0.01β
SI
;
β
BI
V
= 0.005β
SI
(40)
Optimal Social Limitation Reduction under Vaccination and Booster Doses
43
The death rates in model (1)–(10) are the same for
all the compartments except for the infected ones, for
which it results higher than the other ones. Values
adopted are
d
S
= d
E
= d
V
= d
E
V
= d
R
= d
P
=
= d
R
V
= d
B
= 2.81 · 10
−5
(41)
and
d
I
= 5 · 10
−3
, d
I
V
= 0.1 ∗ d
I
(42)
For the remaining parameters, the values are assumed
from assessed knowledge on time constant of illness
progression:
r
1
= r
2
= r
3
=
1
270
; k =
1
4
; γ
V
= γ
I
=
1
21
;
(43)
Tables 1 and 2 summarise the values assumed for
the parameter
Table 1: Values of parameters assumed in numerical simu-
lations.
Parameter Value
N 1.69e3
r
1
, r
2
, r
3
1/270
k 1/4
γ
I
, γ
V
1/21
d
S
, d
E
, d
V
, d
E
V
, d
R
, d
P
, d
R
V
, d
B
2.81e-5
d
I
5e-3
d
I
V
0.1d
I
Table 2: Values of the transmission rates β
∗
.
Parameter Value
β
SI
1e-8
β
SI
V
0.1β
SI
β
PI
0.8β
SI
β
PI
V
0.2β
SI
β
V I
0.1β
SI
β
V I
V
0.02β
SI
β
BI
0.01β
SI
β
BI
V
0.005β
SI
The controls u
S
and u
V
are the ones that has to be
optimised. As far as the other seven is concerned, the
following assumptions are made. First of all, the re-
maining containment controls u
P
and u
B
are taken, as
previously said, equal to u
S
and u
V
respectively. The
choice is mainly motivated by the fact that it is very
difficult to impose different everyday life behaviour
to people in slightly similar conditions with respect to
vaccination. Moreover, according to the previous dis-
cussions, the vaccination rates are assumed constant
over the simulation time; their values are u
1
= 0.01,
u
2
= 1/21, u
3
= 0, u
4
= 0.1u
1
and u
5
= 1/120. To
complete the problem set up, the values assumed for
the constraints (25) are U
SM
= U
V M
= 0.95 and U
Sm
=
U
V m
= 0.
Simulation is performed to replicate the situation
in Italy from the beginning of massive vaccination on
January 2021. The goal is to put in evidence how it
would have been possible to relax the containment
measures acting since the earliest times, differently
according to vaccination conditions. The first four
months have been considered.
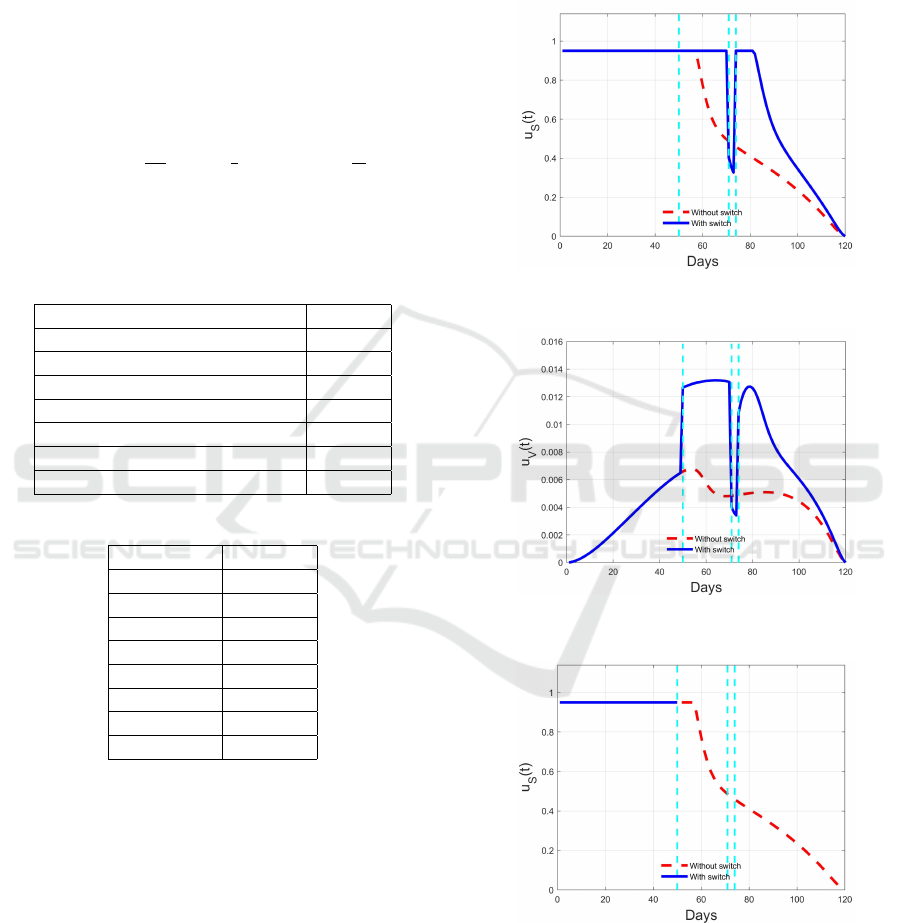
Figure 3: Optimal switching control u
S
(t) (solid blue line)
compared with a non switching solution (dashed red line).
Figure 4: Optimal switching control u
V
(t) (solid blue line)
compared with a non switching solution (dashed red line).
Figure 5: Optimal switching control u
S
(t) over the first time
interval (solid blue line) and the remaining part without
switch (dashed red line).
The resulting optimal switching controls are plot-
ted in Figures 3 and 4 for u
S
(t) and u
V
(t) respectively,
while the sequences of control segments computed in
the iterative procedure are reported in Figures 5–8 for
ICINCO 2022 - 19th International Conference on Informatics in Control, Automation and Robotics
44
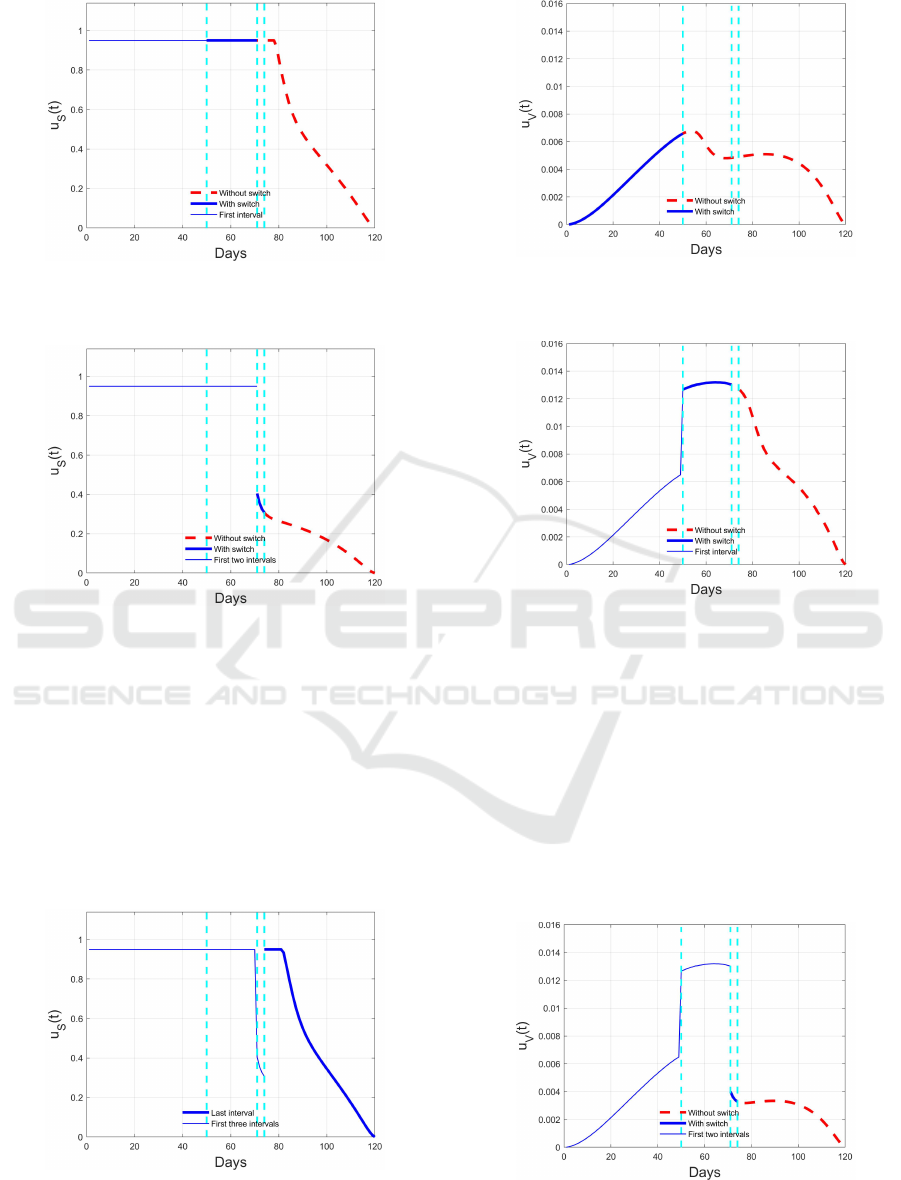
Figure 6: Optimal switching control u
S
(t) over the first two
time intervals (thin and thick solid blue line) and the re-
maining part without switch (dashed red line).
Figure 7: Optimal switching control u
S
(t) over the first
three time intervals (thin and thick solid blue line) and the
remaining part without switch (dashed red line).
u
S
(t) and in Figures 9 for u
V
(t).
The control design procedure is reported step by
step. In Figures 3 and 4 the full optimal controls are
reported as solid blue lines, showing the time switch
instants (
¯
t
1
,
¯
t
2
,
¯
t
3
) = (50,71,74). In the same figure,
the switching solution is compared with the optimal
one obtained keeping the weights W
S
and W
V
constant
over all the integration (dashed red lines).
The above described iterative procedure is put in
Figure 8: Optimal switching control u
S
(t): thin solid blue
line for the already computed segments, thick solid blue
line) for the last segment.
Figure 9: Optimal switching control u
V
(t) over the first
time interval (solid blue line) and the remaining part with-
out switch (dashed red line).
Figure 10: Optimal switching control u
V
(t) over the first
two time intervals (thin and thick solid blue line) and the
remaining part without switch (dashed red line).
evidence by the sequences of picture. Starting from
Figures 5 and 9, the solutions of the first optimal prob-
lem are reported and the corresponding evolutions for
I(t) and I
V
(t) are depicted in Figures 13 and Figures
17. They correspond to the final optimal solution
for the time interval [0,50), evidenced in the Figures,
since at time
¯
t
1
= 50 a switch occurs due to the pas-
sage of I
V
(t) from one region to the one above. The
red dashed lines in these figures represent the evolu-
tion if the switch were not applied. Actually, with the
Figure 11: Optimal switching control u
V
(t) over the first
three time intervals (thin and thick solid blue line) and the
remaining part without switch (dashed red line).
Optimal Social Limitation Reduction under Vaccination and Booster Doses
45
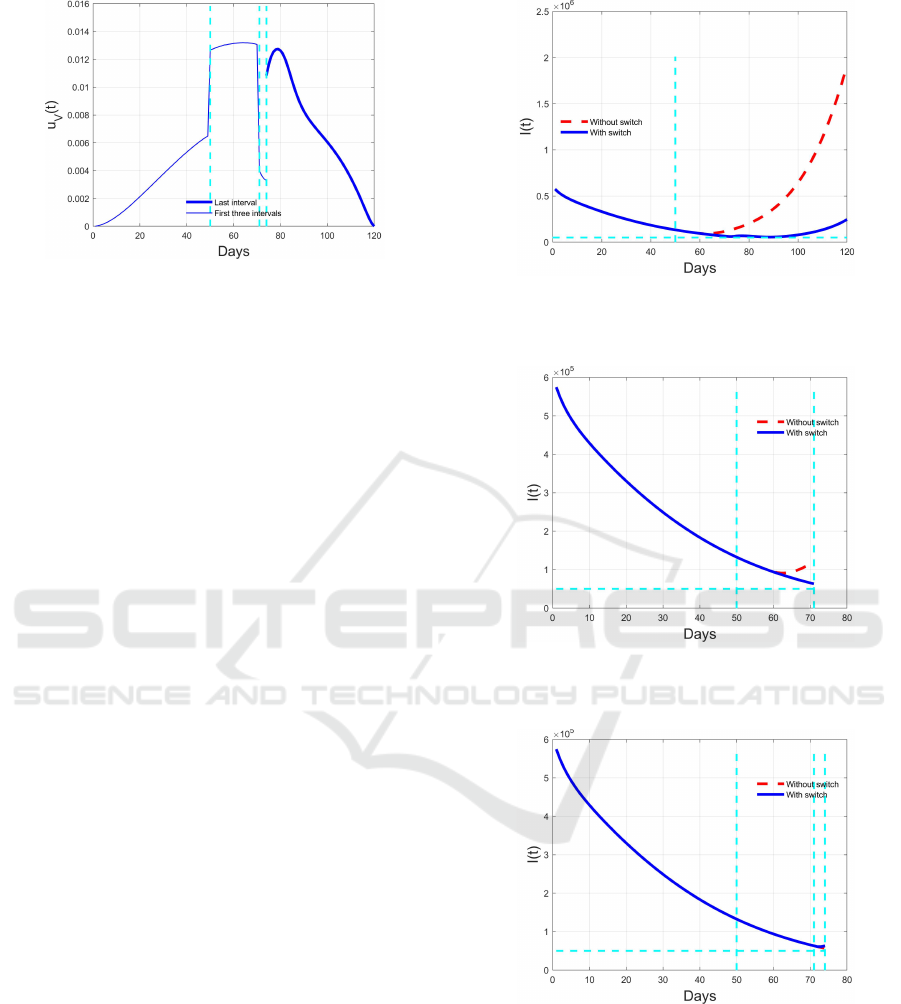
Figure 12: Optimal switching control u
V
(t): thin solid blue
line for the already computed segments, thick solid blue
line) for the last segment.
change of region, the new optimal problem is solved
with different weights for the controls, in particular
smaller than in the previous case due to the more dan-
gerous situation of a greater number of infected indi-
viduals above the fixed threshold.
Figures 6 and 10 show the result of this latter
problem, with the new optimal control depicted from
t = 50 to the end. The segment computed in the pre-
vious step is marked with a thin blue line, the present
segment with a thick blue line and the red dashed line
denotes the remaining part of the solution if a new
switch were not present. The corresponding effect on
the optimal state evolution is reported in Figures 14
and 18 for the two classes of infected individuals. It
can be noted that the introduction of changes in the
weights of the cost function allow to react to a worse
situation with a greater control effort.
At time
¯
t
3
= 74 the higher control action brings
the state variable I
V
(t) to return into the previous, less
dangerous, region of decomposition, so giving a new
switch with a consequent increment of the weights
for the control in the cost function. The segment of
controls in the time interval [
¯
t
2
,
¯
t
3
) = [71,74) are evi-
denced by thick blue solid curves in Figures 7 and 11
(u
S
(t) and u
V
(t) respectively), while the correspond-
ing evolutions of I(t) and I
V
(t) are reported in Figures
15 and 19, with the same notation as for the previ-
ous segment. The reduction of the controls effort after
¯
t
2
produces an increment of infected individuals once
more. This brings to a new switch in t =
¯
t
3
and the
consequent evolution over the last time segment con-
sidered. Figures 8 and 12 report the evolution in such
a time interval for the controls, while Figure 16 and 20
depict the resulting time histories for I(t) and I
V
(t).
6 CONCLUSIONS
In the present paper an optimal control approach with
an available effort planned on the basis of the danger-
Figure 13: I(t) evolution under optimal switching control
(blue solid line) compared with the non switching solution
(red dashed line).
Figure 14: Switching (blue solid line) and non switching
(red dashed line) evolution of I(t) during the first two time
intervals of the algorithm [0,50) and [50,71).
Figure 15: Comparison between switching (blue solid line)
and non switching (red dashed line) evolution of I(t) during
the third time interval [71,74).
ousness of the health conditions is proposed for defin-
ing the individual and social contact limitations after
vaccination in COVID-19 pandemic situation. The re-
sults show how the possibility of allowing a greater,
even more expensive, effort in some severe conditions
while planning a reduction under less serious situa-
tions allow to have effective results with a global con-
ICINCO 2022 - 19th International Conference on Informatics in Control, Automation and Robotics
46
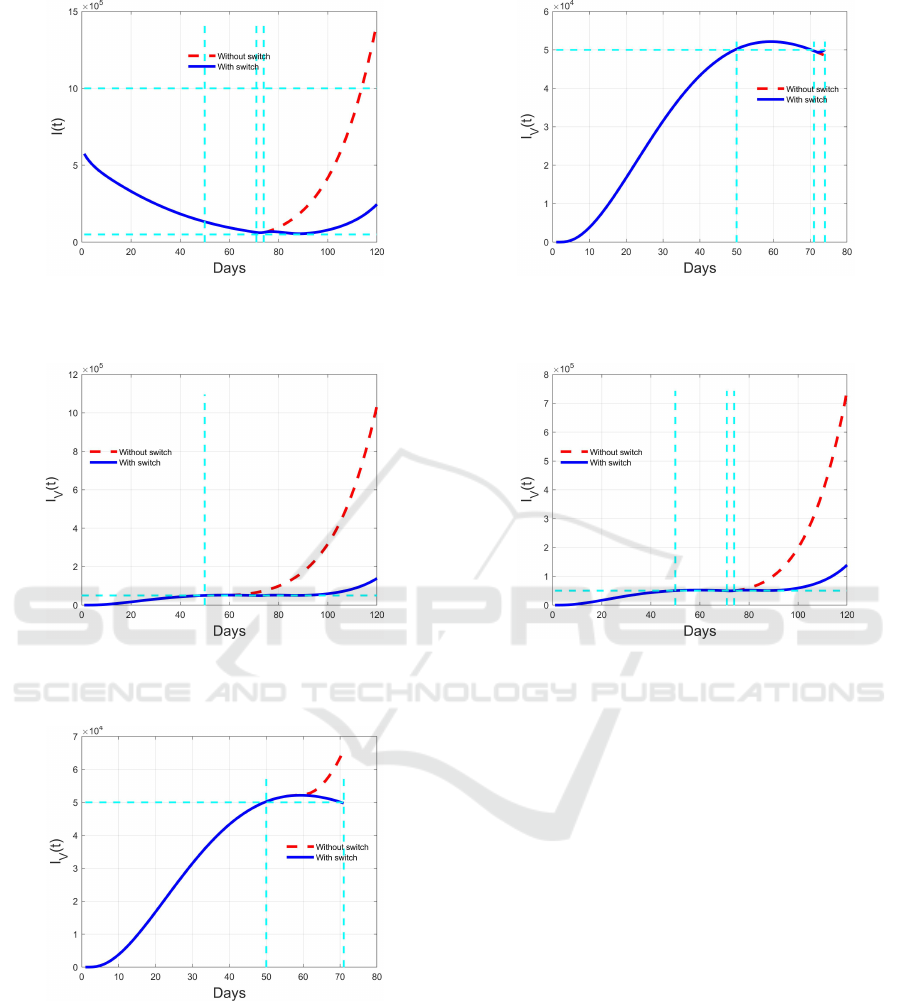
Figure 16: Comparison between switching (blue solid line)
and non switching (red dashed line) evolution of I(t) during
the fourth time interval [74,120).
Figure 17: I
V
(t) evolution under optimal switching control
(blue solid line) compared with the non switching solution
(red dashed line).
Figure 18: Switching (blue solid line) and non switching
(red dashed line) evolution of I
V
(t) during the first two time
intervals of the algorithm [0,50) and [50,71).
tainment of the resources and the costs. In particular,
it has been shown how the restrictions on vaccinated
and not vaccinated individuals would have been dis-
tinguished, reducing them for vaccinated ones since
the beginning of the vaccination campaign. Actually,
the use of specific certifications for entering restau-
rants, theatres and, generally, attending public events
Figure 19: Comparison between switching (blue solid line)
and non switching (red dashed line) evolution of I
V
(t) dur-
ing the third time interval [71,74).
Figure 20: Comparison between switching (blue solid line)
and non switching (red dashed line) evolution of I(t) during
the fourth time interval [74,120).
with high number of participants, adopted in many
countries, is supported by these results.
REFERENCES
Dan, J. M., Mateus, J., Kato, Y., Hastie, K., Yu, E. D., and
Faliti, C. E. (2021). Immunological memory to sars-
cov-2 assessed for up to 8 months after infection. Sci-
ence, 371(6529):1–15.
Di Giamberardino, P. and Iacoviello, D. (2017). Optimal
control of SIR epidemic model with state dependent
switching cost index. Biomedical Signal Processing
and Control, 31.
Di Giamberardino, P. and Iacoviello, D. (2020). A control
based mathematical model for the evaluation of inter-
vention line in epidemic spreads without vaccination:
the covid-19 case study. Submitted to IEEE Journal of
Biomedical and Health Informatics.
Di Giamberardino, P. and Iacoviello, D. (2021). Evaluation
of the effect of different policies in the containment of
epidemic spreads for the COVID-19 case. Biomedical
signal processing and control, 65(102325):1–15.
Optimal Social Limitation Reduction under Vaccination and Booster Doses
47

Di Giamberardino, P., Iacoviello, D., F.Papa, and
C.Sinisgalli (2021). Dynamical evolution of COVID-
19 in Italy with an evaluation of the size of the
asymptomatic infective population. IEEE Journal
of Biomedical and Health Informatics, 25(4):1326–
1332.
Diagne, M. L., Rwezaura, H., Tchoumi, S. Y., and
Tchuenche, J. M. (2021). A mathematical model
of covid-19 with vaccination and treatment. Com-
putational and Mathematical Methods in Medicine,
(1250129):1–16.
Giordano, G., Blanchini, F., Bruno, R., Colaneri, P., Fil-
ippo, A. D., and Colaneri, M. (2020). Modeling the
COVID-19 epidemic and implementation of popula-
tion wide interventions in Italy. Nature Medicine, 26.
Liu, Z., Omayrat, M., and Stursberg, O. (2021). A study
on model-based optimization of vaccination strategies
against epidemic virus spread. In Proceedings of the
18th International Conference on Informatics in Con-
trol, Automation and Robotics - ICINCO,, pages 630–
637. INSTICC, SciTePress.
Olivares, A. and Staffetti, E. (2021). Optimal control-
based vaccination and testing strategies for covid-
19. Computer Methods and Programs in Biomedicine,
211(106411):1–9.
Radulescu, A., Williams, C., and Cavanagh, K. (2020).
Management strategies in a SEIR-type model of
COVID-19 community spread. Nature. Scientific Re-
ports, 21256.
Silva, C. J., Cruz, C., Torres, D. F. M., Munuzuri, A. P.,
Carballosa, A., Area, I., Nieto, J. J., Pinto, R. F., Pas-
sadouro, R., dos Santos, E., Abreu, W., and Mira, J.
(2021). Optimal control of the covid-19 pandemic:
controlled sanitary deconfinement in portugal. Scien-
tific Reports, 11(3451):1–15.
Tang, B., Bragazzi, N. L., Li, Q., Tang, S., Xiao, Y., and Wu,
J. (2020). An updated estimation of the risk of trans-
mission of the novel coronavirus (2019-nCov). Infec-
tious disease modeling, 5.
Van Den Driessche, P. (2017). Reproduction numbers of
infectious disease models. Infectious disease models,
2:288–303.
ICINCO 2022 - 19th International Conference on Informatics in Control, Automation and Robotics
48
